Abstract
Anorexia that accompanies cellular dehydration in rats (DE-anorexia) offers a relatively simple model for investigating the functional organization of neural mechanisms that can suppress feeding during dehydration. Previous studies strongly suggest that the inputs that drive ingestive behavior control neurons in the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA) remain active during DE-anorexia. Here we examine whether these two regions retain their sensitivity to neuropeptide Y (NPY). NPY is an important component in two major feeding-related inputs from the arcuate nucleus and the hindbrain. We found that intake responses to NPY injections in the LHA and PVH were suppressed in DE-anorexia, but the PVH remained less sensitive to the effects of NPY than the LHA in DE-anorexic animals. Indeed the higher dose of NPY (238 pmol) completely overcame shorter periods of DE-anorexia when injected into the LHA but not the PVH. However, the latency to eat after NPY injections remained unchanged from control animals, regardless of NPY dose, injection location, or intensity of anorexia. Furthermore, the onset and size of the strong and rapidly induced compensatory feeding that follows the return of water to DE-anorexic animals was also unaffected by any NPY injections. These data support the hypothesis that DE-anorexia develops as a consequence of the premature termination of regularly initiated meals, which perhaps involves processes that alter the sensitivity of satiety mechanisms downstream to the PVH and LHA.
Keywords: paraventricular nucleus of the hypothalamus, lateral hypothalamic area, satiety, ingestive behavior
energy balance is maintained by regulating energy intake, use, and storage. Negative energy balance typically activates a strong and powerful metabolic response to preserve lean body mass together with central neural circuits that promote energy intake and reduce energy expenditure. The hyperphagia that normally follows caloric deficit restores energy reserves with a degree of relative precision (23, 36, 65). But with anorexia, the drive to increase food intake in the face of negative energy balance is reduced or sometimes eliminated altogether. Although anorexia complicates the prognosis of many clinical conditions, the mechanisms by which appetite and food intake are inhibited are still poorly understood (60).
We have used the anorexia that develops when rats become dehydrated from drinking hypertonic saline (DE-anorexia) as an experimental tool to help elucidate the functional organization of underlying neural networks. DE-anorexia is an important behavioral adaptation that protects fluid homeostasis in the face of a shrinking intracellular fluid compartment (6, 14, 21). Our current model (40, 61, 63) posits that DE-anorexia emerges as a consequence of steadily increasing plasma osmolality, which inhibits the efficacy of behavior control column neurons in the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA) that control ingestive behaviors (53). A fundamental property of this model (63) is that negative energy balance is still capable of engaging those critical inputs to the PVH and LHA that would ordinarily stimulate feeding, including those containing neuropeptide Y (NPY). For example, 2-deoxy-d-glucose will still stimulate corticosterone release in DE-anorexic animals even though the feeding response is markedly suppressed (39). Both responses require catecholaminergic inputs to the PVH (34, 35).
NPY has long been implicated in neural systems that stimulate feeding (12, 25, 46). It is an important component in two major inputs to the PVH and LHA: leptin- and insulin-sensitive neurons that originate in the arcuate nucleus of the hypothalmus and adrenergic inputs from the hindbrain (7, 8, 15, 41, 34). That central administration of NPY can stimulate food intake was noted many years ago (12, 25, 46) and was followed shortly thereafter by the identification of the PVH and LHA as important sites of action (29, 46–48, 51). More recent studies have pointed toward a more complex role for NPY in organizing feeding behavior than is apparent when only the total amount of food consumed is measured (45, 66).
Our current model of DE-anorexia (63) predicts that despite increased arcuate nucleus of the hypothalmus Npy expression (64), DE-anorexic animals will exhibit reduced sensitivity to NPY when administered to the PVH or LHA. To determine how NPY-sensitive elements are affected during DE-anorexia, we injected 300 nl of NPY into either the PVH or LHA in a two-dose design. We then measured the latency to eat and the amount of food eaten every hour for 4 h. Finally, we measured the latency to eat and the amount eaten for 1 h following the return of drinking water to determine whether NPY-stimulated feeding in DE-anorexic animals interacted with the compensatory hyperphagia evident after the animals resumed drinking water (58).
MATERIALS AND METHODS
Animals and Procedures
Adult male Sprague-Dawley rats (240–260 g) obtained from Harlan Laboratories were individually housed in suspended Plexiglas cages with sanitized wood chips. They were maintained in a temperature-controlled room (20–22°C) on a 12:12-h light-dark schedule with lights on at 0600. Rats were provided continuous access to food (Teklad rodent diet no. 8604) and water throughout the experiment, except where stated. For some animals, drinking water was replaced with 2.5% saline for up to 5 days. Body weights and food intake were measured daily throughout the experiment. All procedures were approved by the University of Southern California Institutional Animal Care and Use Committee.
Surgical Procedures
Rats were handled daily for ∼5 days before any surgical intervention and daily, thereafter. Guide cannulas were fabricated from 26-gauge stainless steel tubing and occluded with a removable stainless steel obturator. For stereotaxic implantation of the guide cannulae, rats were anesthetized with an injection (100 μl/kg im) of ketamine/xylazine/acepromazine cocktail solution (5-ml ketamine HCl, 100 mg/ml; 2.5 ml xylazine, 20 mg/ml; 1 ml acepromazine, 10 mg/ml; 1.5 ml 0.9% saline solution). The skull was exposed and trephined at the implantation site by using flat-skull coordinates of 1.8 mm caudal to bregma, 0.2 mm from midline, and 6.5 mm ventral to the dura for injections aimed at the central portion of the PVH. Coordinates were 2.0 mm caudal to bregma, 1.5 mm from midline, and 6.9 ventral to dura for injections aimed at the LHA. Guide cannulae were lowered to the desired site and anchored to the skull with dental acrylic and stainless steel screws. The skin incision was then sutured. Rats were given an intramuscular injection of analgesic (Banamine HCl, 2.5 mg/ml, 100 μl/kg), allowed to recover from anesthesia, and then returned to a clean home cage. Behavioral testing began 7–9 days postsurgery when body weight and food intake had returned to presurgical values.
Experimental Design
Injections.
All injections were given between 0900–1000 in equal volumetric doses of 300 nl over 60–120 s. The obdurator was removed and replaced with a 33-gauge stainless steel injection cannula attached by polyethylene tubing to a 1-μl Hamilton syringe. The injection cannula extended 1.5 mm past the tip of the guide cannula to facilitate the entry of either NPY or artificial cerebrospinal fluid (aCSF) into the parenchyma. The movement of a small bubble in the calibrated infusion line was used to verify injectate delivery. The system was also tested before and after each injection to ensure that it was free of leaks or plugs.
The injection cannula was removed immediately following the injectate delivery, the obturator replaced into the guide cannula, and the rat returned to its home cage for feeding tests with water or 2.5% saline to drink and a premeasured amount of rat chow.
Feeding measurements.
The latency to begin feeding was measured immediately following injections. Food consumption was measured for each animal to the nearest 0.1 g by weighing food and crumbs remaining in cages each hour for the 4 h following injections. In addition to the 4-h feeding tests, 24-h food intake and body weights were monitored throughout the experiment.
Experimental timeline.
Rats were first injected with aCSF into the PVH or the LHA to obtain baseline measurements (day 0). The PVH or the LHA was injected 2 days later (day 2) with either 119 pmol (0.5 μg) or 238 pmol (1.0 μg) human/rat NPY (Peninsula Laboratories, Belmont, CA) dissolved in aCSF. These doses were chosen to incorporate the most dynamic part of the NPY dose/food intake response curve evident during the first 4 h following administration (47). Equal numbers of rats were tested with NPY first to ensure a counterbalanced design. Rats were then matched for body weight and placed into two groups that were maintained with either drinking water (euhydrated group; EU group) or 2.5% saline (DE group). The DE group was given 2.5% saline to drink 2 days after the first NPY injection (day 4).
After 3 days of DE (day 7) when nocturnal food intake was ∼50% of pre-DE intake, rats were again tested for their feeding response to injection into the PVH or the LHA of either aCSF or the same dose of NPY given previously. Each EU animal was given a second dose of NPY directly following that of its weight-matched DE partner. We also determined whether a longer period of DE would suppress intake responses to a dose of NPY that was ineffective at 3 days. To do this, a separate group of animals was tested with 0 pmol (aCSF) or 119 pmol NPY injections into the LHA after 5 days of DE (day 9) when nocturnal food intake was ∼30% of EU controls. We have previously shown that plasma osmolality is increased by ∼6% by 3 days of DE and does not increase further by 5 days of DE (61, 63).
Finally, to test whether the feeding that results when DE rats drink water again (58, 61) remained intact following NPY injections, their 2.5% saline was replaced with water immediately after the 4-h food test. Cumulative food intake was then measured for the hour immediately following the resumption of drinking water.
Histological Analysis
The day following the final behavioral tests, animals were injected through the guide cannula with 300 nl of a 1% solution of bromophenol blue (16). Ten minutes later animals were anesthetized with halothane and rapidly decapitated. Brains were removed and immersion-fixed for 3 days in ice-cold PBS 4% paraformaldehyde. Two adjacent single series of 40-μm frozen coronal sections were cut through the injection site. For observation of the bromophenol blue dye injection sites, one series was mounted onto slides and immediately coverslipped, while the second series was mounted and stained with thionin for clear observation of structures adjacent to injection sites. Injection sites were localized by using landmarks identified with the Swanson Brain Atlas (54).
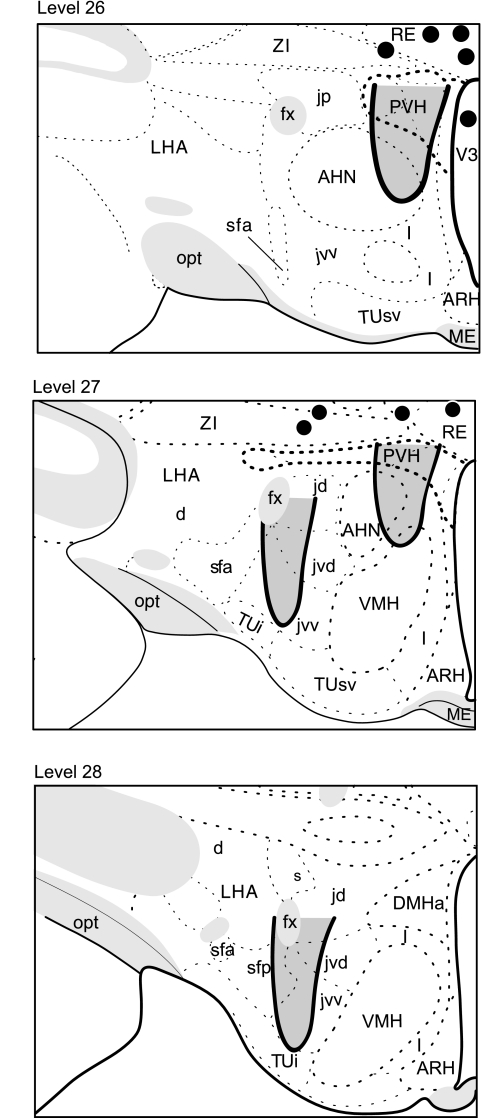
The injection site was mapped microscopically using the areas of dye and tissue damage as markers of the injection. aCSF or NPY-targeted areas were considered to be located along the injection track between the most ventral point of the spread of bromophenol blue and visibly damaged tissue, and the ventral edge of the guide cannula. The location of representative injection sites is illustrated in Fig. 1.
Fig. 1.
Representative locations and extent of the paraventricular nucleus of the hypothalamus (PVH)- and lateral hypothalamic area (LHA)-targeted CSF or neuropeptide Y (NPY) injections are indicated by the shaded areas enclosed by the black lines on levels 26–28 of Swanson's rat brain atlas (54). Nine animals had injections outside target areas, and the locations of their most ventral aspects are marked by black dots. NPY injected at these locations had no effect on food intake. AHN, anterior nucleus of the hypothalamus; ARH, arcuate nucleus of the hypothalamus; d, dorsal region of the LHA; DMHa, dorsomedial hypothalamic nucleus, anterior part; fx, fornix; jd, juxtadorsomedial region of the LHA; jp, juxtaposterior region of the LHA; jvd, juxtadorsomedial region, dorsal zone of the LHA; jvv, juxtadorsomedial region, medial zone of the LHA; l, internuclear zone, hypothalamic periventricular region; ME, median eminence; opt, optic tract; RE, nucleus reunions; s, suprafornical region of the LHA; sfa, suprafornical region, anterior zone, of the LHA; sfp, suprafornical region, posterior zone, of the LHA; TUi, tuberal nucleus, intermediate part; TUsv, tuberal nucleus, subventricular part; V3, third ventricle; VMH, ventromedial nucleus of the hypothalamus; ZI, zona incerta. (modified from Ref. 54. Please refer to Ref. 54 to identify the unlabeled anatomical regions.)
The assignment of animals into PVH or LHA groups was based on the histologically derived location of their injection site. Figure 1 shows that injections aimed at the PVH usually encompassed its dorsal, medial, and ventral parvicellular parts, as well as medial portions of the lateral parvicellular part. PVH-targeted injections also usually involved the dorsal parts of the anterior hypothalamic nucleus. Injections aimed at the LHA encompassed its juxtaventromedial region and the dorsal and posterior zones of the subfornical region of the LHA (see Refs. 54 and 55 for definitions of the subdivisions of the PVH and LHA). Nine animals had injection sites that were located in either the zona incerta or nucleus reuniens, which were dorsal to the target areas in the PVH or LHA (Fig. 1). The food intake of these animals after NPY injections (average 4-h intake after injection 3.6 ± 0.6 g) was not significantly different from controls. Animals with ectopic placements were discarded from further analysis.
Statistical Analysis
Data are expressed as means ± SE. Differences in the latency and intake responses to NPY before and during DE were analyzed using a one-way ANOVA with repeated-measures. Results from EU and DE animals were compared using a 2×2 repeated-measures ANOVA. Bonferroni's pairwise multiple comparisons test was used to evaluate individual differences, with critical level for significance set at P < 0.05.
RESULTS
Feeding Responses to Injections of NPY into the Region of the PVH
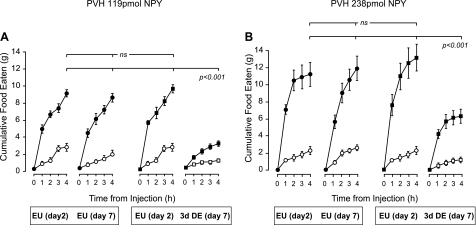
After injecting either 119 pmol (Fig. 2A) or 238 pmol (Fig. 2B) of NPY to the PVH, EU animals ate significant amounts of food in the 4 h following injection (P < 0.001) compared with the eating response after injection of vehicle. Figures 2 and 3 also show that 3 days of DE significantly reduced the ability of NPY to elicit food intake, regardless of dose.
Fig. 2.
Mean ± SE cumulative 4-h food intake in animals given two injections of vehicle (white symbols), or 119 pmol NPY (A; black symbols), or 238 pmol NPY (B; black symbols) given 5 days apart (day 2 and day 7) and targeted to the PVH. Animals were either maintained on drinking water for the duration of the experiment [euhydrated animals (EU)], or maintained on drinking water (EU) for 2 days and then given 2.5% saline to drink [dehydrated (DE)]. The second NPY injection was given on day 7, 3 days after the initiation of drinking 2.5% saline. The main finding is that food intake is significantly suppressed when NPY is injected in the region of the PVH of DE animals. ns, Not significant. See text for the detailed statistics.
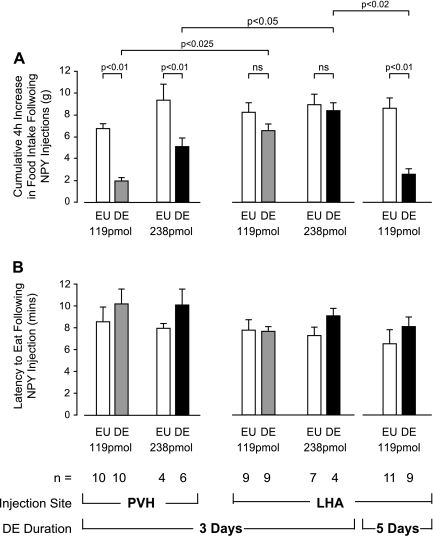
Fig. 3.
A: mean +SE cumulative 4-h food intake for animals injected with 119 pmol or 238 pmol of NPY into the PVH or LHA of EU or animals given 2.5% saline to drink for 3 or 5 days. Data are expressed as the increase in cumulative food intake above mean values from animals injected with saline in the same regions. B: mean +SE latency (minutes) to eat following 119 pmol or 238 pmol NPY injections into either the PVH or LHA. Injections were given to EU animals on day 7 or day 9 of the experiment or to animals given 2.5% saline to drink (DE) for 3 (day 7) or 5 (day 9) days. There were no significant differences in the latency to eat between any treatment group.
Two-way repeated-measures ANOVA revealed a main effect of DE (F[df 1,14] = 72.39, P < 0.001) and an interaction effect between NPY and DE (F[df 1,14] = 95.03; P < 0.001). Food consumption appeared to be partially rescued in DE animals when the dose of NPY was increased to 238 pmol (Figs. 2B and 3), but the main effect of DE and the interaction effect between DE and NPY both remained significant (F[df 1,14] = 27.14 and 23.6, respectively; P < 0.001 for both).
All DE animals ate similar amounts of food when their drinking water was returned, regardless of whether they had been injected with aCSF or NPY into the PVH (Table 1).
Table 1.
DE animals all ate similar amounts of food when their drinking water was returned, regardless of whether they had been injected with artificial cerebrospinal fluid or NPY into the PVH
| Region | Treatment | Amount of Food, g |
|---|---|---|
| PVH | DE CSF | 6.5±0.5 |
| 3 days DE 119 pmol NPY | 7.3±0.3 | |
| DE CSF | 6.2±0.4 | |
| 3 days DE 238 pmol NPY | 6.8±0.3 | |
| LHA | DE CSF | 6.3±0.4 |
| 3 days DE 119 pmol NPY | 5.6±0.6 | |
| 5 days DE 119 pmol NPY | 7.0±0.5 | |
| DE CSF | 6.1±0.2 | |
| 3 days DE 238 pmol NPY | 6.4±0.4 |
Values are means ± SE of the amount of food eaten (g) in the 60 min following the return of water to animals given 2.5% saline to drink (DE) for 3 or 5 days, and injected with vehicle (CSF) or neuropeptide Y (NPY) into the paraventricular nucleus of the hypothalamus (PVH) or lateral hypothalamic area (LHA).
Feeding Responses to Injections of NPY into the LHA
Injection of NPY to the LHA after 3 days of DE.
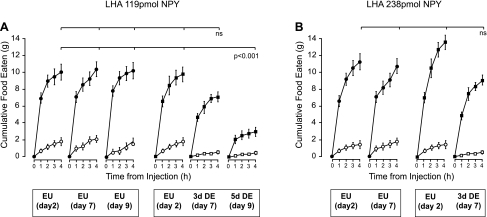
After injection of either 119 pmol or 238 pmol of NPY into the LHA, all EU animals ate significant amounts of food in the 4 h following injection compared with the intake response after injection of vehicle (Figs. 3A and 4). When the injections of 119 pmol or 238 pmol NPY were repeated 3 days later, both DE and EU animals demonstrated a significant intake response (Figs. 3A and 4). There was a significant main effect of DE on eating after the lower dose of NPY (F[df 1,12] = 94; P < 0.05. Fig. 4A), but no significant interaction effect. DE did not affect the ability of NPY to elicit food intake as revealed by a nonsignificant main effect or interaction effect of DE after 238 pmol NPY to the LHA (Figs. 3A & 4B). Figure 3A also shows that both doses of NPY injected in the LHA of DE animals elicited significantly greater food intake 4 h later than those in the PVH. This regional difference was not apparent in EU animals.
Fig. 4.
Mean +SE cumulative 4-h food intake in animals given two injections of vehicle (white symbols), or 119 pmol NPY (A; black symbols), or 238 pmol NPY (B; black symbols) given 5 days apart (day 2 and day 7) and targeted to the LHA. A third group of animals was injected with two injections of vehicle (A; white symbols) or 119 pmol NPY (A; black symbols) given 7 days apart (day 2 and day 9). Animals were either maintained on drinking water for the duration of the experiment (EU), or maintained on drinking water (EU) and then given 2.5% saline to drink (DE). The second NPY injection was given on day 7 or day 9, 3 or 5 days after the initiation of drinking 2.5% saline. The main finding was that food intake returned to control levels when NPY was injected in the region of the LHA of DE animals showing moderate anorexia, but remained significantly suppressed in animals with a longer period of DE and more pronounced anorexia. See text for the detailed statistics.
Table 1 shows that all DE animals exhibited a robust feeding response after their drinking water was returned, regardless of whether they had been given aCSF or NPY. These values did not differ from those of PVH-injected animals when given water to drink.
Injection of NPY to LHA after 5 days of DE.
Figures 3A and 4A show that food intake 4 h after a 119 pmol injection of NPY was significantly suppressed when DE was extended out to 5 days. Two-way repeated-measures ANOVA revealed a main effect of DE (F[df 1,17] = 25.13; P < 0.001) and an interaction effect between NPY and DE (F[df 1,17] = 20.93; P < 0.001).
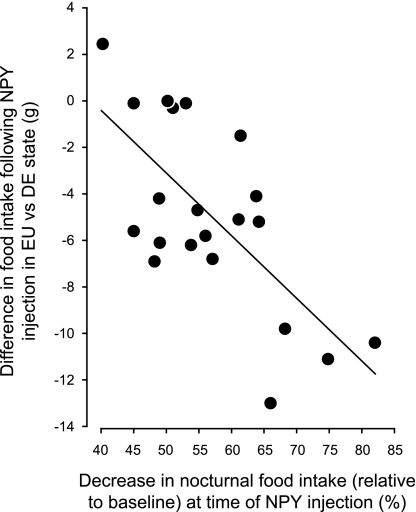
Figure 5 shows the significant correlation (r2 = 0.481; F[df 1,20] = 17.63; P < 0.0005) between the intensity of spontaneous anorexia (measured by the degree of suppression of nocturnal food intake from the EU) and the inhibition of the feeding response after a 119-pmol NPY injection in the LHA following 3 (n = 10) or 5 (n = 11) days of drinking hypertonic saline.
Fig. 5.
Correlation between the development of spontaneous anorexia (expressed as the %reduction in nocturnal food intake) of animals given 2.5% saline to drink (DE) and the inhibition of the feeding response 4 h after 119 pmol NPY injection to the LHA. The feeding response was expressed as the difference between the amount of food intake after the first injection of NPY given when animals were drinking water (EU) and the amount of food intake after the second injection of NPY given either after 3 or 5 days of drinking 2.5% saline (DE). See text for the detailed statistics.
As with all other DE animals, all 5-day DE animals ate significant amounts of food when water was returned, regardless of whether they had been given aCSF or NPY (Table 1).
Latencies to eat after injections of NPY into the PVH and LHA.
The latency to begin eating was between 7.5 and 10 min for all animals injected with NPY, regardless of injection site, hydration state, or the dose of NPY administered (Fig. 3B). There were no significant differences between the latencies to eat (F[df 17,125] = 1.466; P = 0.12) when 119 pmol or 238 pmol of NPY was injected into the LHA or the PVH in EU and DE animals (Fig. 3B).
DISCUSSION
Our results demonstrated three main points regarding NPY sensitivity in DE-anorexic rats. First, all animals began to eat with the same latency after NPY injections regardless of whether they were anorexic. Second, the magnitude of the food intake response of DE-anorexic rats to central NPY injections depended on the location of the injection and the severity of anorexia: NPY was significantly less effective at eliciting food intake when it was injected in the PVH than the LHA of animals at similar states of DE-anorexia. Furthermore, as DE-anorexia became more severe, the ability of NPY injected into the LHA to increase food intake became progressively blunted. Finally, all animals had a robust feeding response when drinking water was returned regardless of whether they had received a NPY or aCSF injection or whether or not they had previously eaten in response to NPY.
Before considering the significance of these results for models of DE-anorexia, it is worth briefly reviewing NPY's known effects on feeding behavior. Although initial studies clearly identified its strong orexigenic effects (12, 25, 46, 48), a more complex role for NPY has since emerged than the one apparent from experiments where only the amount of food consumed is measured (66). This is illustrated by three observations. First, NPY does not simply stimulate the consummatory phase of feeding behavior (4, 43). Instead NPY not only helps direct the appetitive phase toward a food source (2, 13), but also delays the termination phase of feeding by reducing the inhibition generated by postingestive signals (4, 27). In this regard, it is noteworthy that, in contrast to satiated animals, NPY is ineffective at potentiating the food intake that follows an overnight fast when endogenous satiety signals are presumably very weak (32). Some studies also suggest that NPY can sometimes generate aversive responses and actually suppresses food intake (2, 42, 44). Second, the latency to begin feeding following central NPY administration is 8–10 min (47), which is consistent with a requirement for the interaction of NPY mechanisms with other neural systems before behavior is eventually modified (26, 37). In contrast, direct manipulations of amino acid-derived neurotransmitter systems stimulate food intake much more quickly (24, 49, 52). Third, central NPY administration also increases neuroendocrine and autonomic motor responses, including glucocorticoid, insulin, and glucagon secretion (1, 18, 20, 57), as well as gastric and colonic motility (28, 56). Collectively, these results demonstrate that in addition to increasing food intake, NPY engages a broad set of motor responses that coordinate energy metabolism during and after feeding.
The way our study was designed allows us to address in broad terms whether the appetitive, consummatory, or termination phases of NPY-stimulated feeding behavior were differentially impacted during DE-anorexia. We measured the latency to begin eating as a comparative measure of NPY's effects on appetitive phase potency. We reasoned that if the processes that initiate the foraging and approach aspects of meal preparation had been inhibited by drinking hypertonic saline, then the latency to begin eating would be significantly longer than in EU controls. This was not the case. Regardless of hydration state or injection site, animals began to eat between 7 and 9 min after NPY injection, which is consistent with that reported by Stanley and Leibowitz (47). The fact that the latency to eat was unaffected in DE-anorexia suggests that it does not arise from a broad inhibition of motor control components in the appetitive phase. This interpretation is supported by our findings that the number of meals initiated during spontaneous feeding is largely unaffected by drinking hypertonic saline and that the latency to begin feeding following injections of muscimol into the shell of the nucleus accumbens is also unchanged in DE-anorexic animals (30, 31).
In contrast to the latency to feed, drinking hypertonic saline significantly altered the amount eaten following NPY injections. Food consumed was reduced during the test period following all NPY injections in the PVH as well as the smaller dose injected in the LHA of animals with more advanced DE-anorexia. In light of the suggestion (4, 27) that NPY increases food intake partly by suppressing postingestive satiation, i.e., delays the onset of the termination phase, it seems reasonable to suggest that an important contributory mechanism in DE-anorexia is one that counteracts the satiety-suppressive effects of NPY. Interestingly, detailed meal pattern analyses of spontaneous feeding by DE-anorexic rats also reveals a significant reduction in meal duration but not meal number, suggesting that advancing the onset of meal termination is a significant factor in reducing overall food intake in these animals (31).
Although the feeding response was strongly suppressed in 3-day DE animals when the lower dose of NPY was injected toward the PVH, the same dose continued to stimulate food intake when placed in the LHA. In terms of suppressing the ability of NPY to increase food intake, drinking hypertonic saline therefore targets PVH-dependent mechanisms more effectively than those in the LHA, which is consistent with the greater sensitivity of the LHA to NPY reported in EU animals (51). Similar data have been shown in other models of anorexia. The feeding responses of anorexic tumor-bearing rats, senescent rats, and rats with obstructive cholestasis are all attenuated following intraventricular or hypothalamic NPY injections (5, 10, 33).
We found that the higher dose of NPY in the LHA almost entirely rescues the feeding response in moderately anorexic animals. Importantly, this shows that DE-anorexic animals can and will eat if appropriately stimulated, and is consistent with the notion that drinking hypertonic saline does not significantly compromise the consummatory phase of NPY-stimulated feeding behavior. But over time, as DE-anorexia becomes well established, some animals had a strikingly suppressed feeding response to the lower dose of NPY in the LHA compared with when they were tested in the EU state, or to animals tested after, with a shorter duration of DE. Furthermore, while the LHA of DE-anorexic rats remains sensitive to NPY longer than the PVH, drinking hypertonic saline will eventually inhibit the feeding responses to LHA-targeted NPY injections. Thus, we found a significant correlation between the intensity of anorexia and the ability of the lower dose NPY to stimulate feeding when injected into the LHA.
The mechanisms underlying the differential sensitivity of the PVH and LHA remain unclear. Y1 and Y5 receptor subtypes have been most closely linked to the orexigenic actions of NPY (19, 50), and local differences in the physiology of these receptors would seem a reasonable explanation. But detailed analyses of Y receptor number, distribution, or kinetics are currently unavailable in the critical regions of the PVH and LHA that could implicate these variables in the differences in NPY efficacy of these two regions. Interestingly, however, reports show that some anorexic stimuli alter NPY Y1 receptor expression in the hypothalamus (9, 11, 67).
We have previously reported that DE-anorexic animals show a strong and stereotypically organized bout of feeding after drinking water (58, 61). Interestingly, we now find that all DE-anorexic animals eat the same amount of food (6 to 7g) after drinking water, regardless of whether they had previously received an injection of NPY or saline where that injection was given or whether or not animals had eaten following the NPY injection. In some instances, where the larger NPY dose was injected into the LHA, animals ate a total of more than 15 g of food in the 4-h + 1-h test period after NPY followed by the return of drinking water. The fact that previous NPY treatment did not augment subsequent water-stimulated feeding, shows that there was no residual orexigenic activity. This finding is consistent with previous data showing that local levels of exogenously applied NPY are significantly reduced within 4 h of injection (51) and that endogenous extracellular NPY levels can show rapid fluctuations (22). Furthermore, the fact that water-stimulated feeding continued unabated in all DE-anorexic animals shows that any postingestive satiety signals generated by NPY-stimulated feeding were ineffective at the time water was returned. The mechanisms by which anorexia is reversed by drinking water are not entirely clear, but existing data are consistent with a model where stimulatory networks are disinhibited (61) to promote eating behavior quickly, powerfully, and, as we now show, with little regard for any previous satiating effects of food.
Perspectives and Significance
Anorexia is a term that encompasses the profound and dangerous loss of appetite and reduced feeding that complicates the outcome of many important clinical conditions, including cancer, AIDS, anorexia nervosa, and chronic inflammatory bowel syndrome. Few neural systems-level models have been proposed that account for the marked reductions in food intake that characterize the various types of anorexia.
We have previously suggested that with DE-anorexia (a physiologically important adaptation to cellular dehydration) the downstream effectors from ingestive behavior control neurons in the PVH and LHA are compromised. On the other hand, the drive within those NPY-containing neurons originating in the arcuate nucleus of the hypothalmus and the hindbrain that project to the PVH and LHA remains intact (63). We now provide further support for this model by showing that NPY injections in the LHA and, particularly the PVH, are less effective in DE-anorexic animals than when they are challenged in the EU state. Furthermore, the fact that the latency to eat following NPY injections is unchanged in DE-anorexia shows that meal initiation is unaffected during anorexia, whereas meals are prematurely terminated perhaps because of the increased sensitivity and efficacy of satiety mechanisms.
To elucidate the mechanisms responsible for DE-anorexia, these results now highlight the importance of investigating the interactions between networks that stimulate feeding and those responsible for transducing satiety signals to terminate meals.
GRANTS
This study was supported by National Institute of Mental Health Grants MH-066168 (to A. G. Watts) and MH-067392 (to D. Salter-Venzon).
ACKNOWLEDGMENTS
Present address of D. Salter-Venzon: SalNutrilite Health Institute, 5600 Beach Blvd., PO Box 5940, Buena Park, CA 90622-5940.
REFERENCES
- 1.Abe M, Saito M, Ikeda H, Shimazu T. Increased neuropeptide Y content in the arcuato-paraventricular hypothalamic neuronal system in both insulin-dependent and non-insulin-dependent diabetic rats. Brain Res 539: 223– 227, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Ammar AA, Nergårdh R, Fredholm BB, Brodin U. Intake inhibition by NPY and CCK-8: a challenge of the notion of NPY as an “Orexigen”. Behav Brain Res 161: 82– 87, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Södersten P. NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol 278: R1627– R1633, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Baird JP, Gray NE, Fischer SG. Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci 120: 937– 951, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Blanton CA, Horwitz BA, Blevins JE, Hamilton JS, Hernandez EJ, McDonald RB. Reduced feeding response to neuropeptide Y in senescent Fischer 344 rats. Am J Physiol Regul Integr Comp Physiol 280: R1052– R1060, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bolles RC. The interaction of hunger and thirst in the rat. J Comp Physiol Psychol 54: 580– 584, 1961 [DOI] [PubMed] [Google Scholar]
- 7.Broberger C, De Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol 402: 460– 474, 1998 [PubMed] [Google Scholar]
- 8.Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology 70: 295– 305, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Broberger C, Johansen J, Brismar H, Johansson C, Schalling M, Hökfelt T. Changes in neuropeptide Y receptors and pro-opiomelanocortin in the anorexia (anx/anx) mouse hypothalamus. J Neurosci 19: 7130– 7139, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chance WT, Balasubramaniam A, Thompson H, Mohapatra B, Ramo J, Fischer JE. Assessment of feeding response of tumor-bearing rats to hypothalamic injection and infusion of neuropeptide Y. Peptides 17: 797– 801, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Chance WT, Xiao C, Dayal R, Sheriff S. Alteration of NPY and Y1 receptor in dorsomedial and ventromedial areas of hypothalamus in anorectic tumor-bearing rats. Peptides 28: 295– 301, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427– 429, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 289: R29– R36, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dicker SE, Nunn J. The role of antidiuretic hormone during water deprivation in rats. J Physiol (London) 136: 235– 248, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402: 442– 459, 1998 [PubMed] [Google Scholar]
- 16.Feldberg W, Fleishhauer K. Penetration of bromophenol blue from the perfused cerebral ventricles into the brain tissue. J Physiol (London) 150: 451– 462, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Füzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-Y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology 148: 5442– 5450, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Ghibaudi L, Hwa JJ. Selective activation of central NPY Y1 vs. Y5 receptor elicits hyperinsulinemia via distinct mechanisms. Am J Physiol Endocrinol Metab 287: E706– E711, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL, Schaffhauser AO, Whitebread S, Hofbauer KG, Taber RI, Branchek TA, Weinshank RL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 382: 168– 171, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hanson ES, Dallman MF. Neuropeptide Y (NPY) may integrate responses of hypothalamic feeding systems and the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 7: 273– 279, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Hsaio S. Saline drinking effects on food and water intake in rats. Psychol Rep 21: 1025– 1028, 1967 [DOI] [PubMed] [Google Scholar]
- 22.Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA 88: 10931– 109351991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr 127: 1875S– 1883S, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull 21: 905– 912, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides 5: 1025– 1029, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Levine AS, Grace M, Billington CJ. The effect of centrally administered naloxone on deprivation and drug-induced feeding. Pharmacol Biochem Behav 36: 409– 412, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Lynch WC, Hart P, Babcock AM. Neuropeptide Y attenuates satiety: evidence from a detailed analysis of patterns ingestion. Brain Res 636: 28– 34, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Monnikes H, Tebbe J, Bauer C, Grote C, Arnold R. Neuropeptide Y in the paraventricular nucleus of the hypothalamus stimulates colonic transit by peripheral cholinergic and central CRF pathways. Neurogastroenterol Motil 12: 343– 352, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol Regul Integr Comp Physiol 252: R599– R609, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Neuner CM, Watts AG. Effects of GABA in the nucleus accumbens shell on ingestive behavior after dehydration-anorexia (Abstract). Appetite 46: 372, 2006 [Google Scholar]
- 31.Neuner CM, Lorenzen SM, Compton DS, Watts AG. Dual analysis of feeding and drinking patterns during and following dehydration anorexia. In: 2008 Neuroscience Meeting Planner (783.28). Washington, DC: Society for Neuroscience, 2008 [Google Scholar]
- 32.Parikh R, Marks JL. Metabolic and orexigenic effects of intracerebroventricular neuropeptide Y are attenuated by food deprivation. J Neuroendocrinol 9: 789– 795, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Rioux KP, Le T, Swain MG. Decreased orexigenic response to neuropeptide Y in rats with obstructive cholestasis. Am J Physiol Gastrointest Liver Physiol 280: G449– G456, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 432: 197– 216, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357– 1367, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31– 35, 1979 [DOI] [PubMed] [Google Scholar]
- 37.Rudski JM, Grace M, Kuskowski MA, Billington CJ, Levine AS. Behavioral effects of naloxone on neuropeptide Y-induced feeding. Pharmacol Biochem Behav 54: 771– 777, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Sahu A, Kalra PS, Kalra SP. Food deprivation, and ingestion induce reciprocal changes in neuropeptide Y. Concentrations in the paraventricular nucleus. Peptides 9: 83– 86, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Salter D, Watts AG. Differential suppression of hyperglycemic, feeding, and neuroendocrine responses in anorexia. Am J Physiol Regul Integr Comp Physiol 284: R174– R182, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Salter-Venzon Watts DS, AG. The role of hypothalamic ingestive behavior controllers in generating dehydration anorexia: a FOS mapping study. Am J Physiol Regul Integr Comp Physiol 295: R1009– R1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241: 138– 153, 1985 [DOI] [PubMed] [Google Scholar]
- 42.Sederholm F, Ammar AA, Södersten P. Intake inhibition by NPY: role of appetitive ingestive behavior and aversion. Physiol Behav 75: 567– 575, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Seeley RJ, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol Regul Integr Comp Physiol 268: R423– R427, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Sipols AJ, Brief DJ, Ginter KL, Saghafi S, Woods SC. Neuropeptide Y paradoxically increases food intake yet causes conditioned flavor aversions. Physiol Behav 51: 1257– 1260, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Södersten P, Nergårdh R, Bergh C, Zandian M, Scheurink A. Behavioral neuroendocrinology and treatment of anorexia nervosa. Front Neuroendocrinol 29: 445– 462, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci 35: 2635– 2642, 1984 [DOI] [PubMed] [Google Scholar]
- 47.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 82: 3940– 3943, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanley BG, Chin AS, Leibowitz SF. Feeding and drinking elicited by central injection of neuropeptide Y: evidence for a hypothalamic site(s) of action. Brain Res Bull 14: 521– 524, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Stanley BG, Ha LH, Spears LC, Dee MG., II Lateral hypothalamic injections of glutamate, kainic acid, d,l-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-d-aspartic acid rapidly elicit intense transient eating in rats. Brain Res 613: 88– 95, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13: 581– 587, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res 604: 304– 317, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17: 4434– 4440, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res 886: 113– 164, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Swanson LW. Brain Maps: Structure of the Rat Brain. A Laboratory Guide with Printed and Electronic Templates for Data, Models and Schematics ( 3rd ed.). Amsterdam: Elsevier, 2004 [Google Scholar]
- 55.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett 387: 80– 84, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Tebbe JJ, Mronga S, Schafer MK, Ruter J, Kobelt P, Monnikes H. Stimulation of neurons in rat ARC inhibits gastric acid secretion via hypothalamic CRF1/2- and NPY-Y1 receptors. Am J Physiol Gastrointest Liver Physiol 285: G1075– G1083, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Wahlestedt C, Skagerberg G, Ekman R, Heilig M, Sundler F, Hakanson R. Neuropeptide Y (NPY) in the area of the hypothalamic paraventricular nucleus activates the pituitary-adrenocortical axis in the rat. Brain Res 417: 33– 38, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Watts AG. Dehydration-associated anorexia: development and rapid reversal. Physiol Behav 65: 871– 878, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav 37: 261– 283, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Watts AG, Salter D. Neural mechanisms of anorexia. In: Handbook of Behavioral Neurobiology: Neurobiology of Food and Fluid Intake, edited by Stricker E, Woods S. ( 2nd ed.). New York: Kluwer Academic/Plenum Publishers, 2004, vol. 14, p. 383– 420 [Google Scholar]
- 61.Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol 502: 768– 782, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Watts AG, Kelly AB, Sanchez-Watts G. Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci 109: 1146– 1157, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Watts AG, Salter DS, Neuner CM. Neural network interactions and ingestive behavior control during anorexia. Physiol Behav 91: 389– 396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci 19: 6111– 6121, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weigle DS. Appetite and the regulation of body composition. FASEB J 8: 302– 310, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Woods SC, Figlewicz DP, Madden L, Porte D, Jr, Sipols AJ, Seeley RJ. NPY and food intake: discrepancies in the model. Regul Pept 75–76: 403– 408, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Xu B, Kalra PS, Moldawer LL, Kalra SP. Increased appetite augments hypothalamic NPY Y1 receptor gene expression: effects of anorexigenic ciliary neurotropic factor. Regul Pept 75–76: 391– 395, 1998 [DOI] [PubMed] [Google Scholar]