Abstract
Understanding the early factors affecting obesity development in males and females may help to prevent obesity and may lead to the discovery of more effective treatments for those already obese. The Otsuka Long-Evans Tokushima Fatty (OLETF) rat model of obesity is characterized by hyperphagia-induced obesity, due to a spontaneous lack of CCK1 receptors. In the present study, we focused on the behavioral and physiological aspects of obesity development from weaning to adulthood. We examined body weight, feeding efficiency, fat pad [brown, retroperitoneal, inguinal and epydidimal (in males)] weight, inguinal adipocyte size and number, leptin and oxytocin levels, body mass index, waist circumference, and females' estrous cycle structure. In the males, central hypothalamic gene expression was also examined. OLETF rats presented overall higher fat and leptin levels, larger adipocytes, and increased waist circumference and BMI from weaning until adulthood, compared with controls. Analysis of developmental patterns of gene expression for hypothalamic neuropeptides revealed peptide-specific patterns that may underlie or be a consequence of the obesity development. Analysis of the developmental trajectories toward obesity within the OLETF strain revealed that OLETF females developed obesity in a more gradual manner than the males, presenting delayed obesity-related “turning points,” with reduced adipocyte size but larger postweaning fat pads and increased adipocyte hyperplasia compared with the males. Intake decrease in estrus vs. proestrus was significantly less in OLETF vs. Long-Evans Tokushima Otsuka females. The findings highlight the importance of using different sex-appropriate approaches to increase the efficacy of therapeutic interventions in the treatment and prevention of chronic early-onset obesity.
Keywords: adipose tissues, adipocyte hypertrophy, hyperplasia, hyperphagia
the global epidemic of obesity, and associated diseases, is having a major impact on human morbidity, mortality, and quality of life, and it is a major drain on health care resources (27, 50). Only recently has there been a focus on childhood obesity, a phenomenon rising in alarming proportions in both developed and developing countries. It is well known that overweight children tend to become overweight adults, making obesity a perdurable problem that is very difficult to treat. Because early interventions may be more successful in avoiding or improving eventual outcomes (1, 5, 6, 25a, 37, 48), we have attempted, using an animal model, to identify critical time points in the development of several obesity parameters observed during childhood and adolescence that may help identify the disorder before it becomes a health threat.
The prevalence of obesity and overweight in women is consistently higher than in men in many countries around the world (20, 24, 25a). Moreover, it has been suggested that there are sex-specific responses of body fat and weight during periods of energy imbalance (10). For example, female rats that regularly exercise usually continue to gain weight at approximately the same rates as sedentary controls (1) and show an increase in food intake to compensate for the energy expenditure (37). In contrast, males typically experience substantial weight loss and intake decreases with regular exercise (9, 34, 37). There is also a sexually dimorphic response in feeding and appetite-associated hormonal responses to food restriction. Females seem to deal more successfully with energy deficits; because they defend their body weight in a more efficient way than males in response to these challenges (14, 18, 37). While females throughout evolution may have benefited from their greater capacity to store fats (to maintain their reproductive capacity), the eating habits of modern times (high-energy food) may make females more vulnerable to the negative health consequences of overeating and obesity.
The Otsuka Long-Evans Tokushima Fatty (OLETF) rat lacks the CCK1 receptor due to a spontaneous genetic mutation and represents a broadly established model of non-insulin-dependent diabetes mellitus (NIDDM) (29, 30) and hyperphagia-induced obesity (35). CCK is a brain-gut peptide that acts as a peripheral satiety signal (48, 54) and elicits the earlier appearance of the behavioral satiety sequence (21). The development of obesity in this strain is secondary to their hyperphagia (35). Eventually, their chronic overeating induces OLETF males and females to become obese. In parallel, the acquisition of large amounts of body fat might cause the dysregulation of further systems [such as the leptin resistance that OLETF males develop around the age of 8 wk (38)] that may, in turn, worsen the problem. Adult OLETF males are hyperphagic, obese, hyperleptinemic, hyperglycemic, and develop NIDDM late in life (35, 38). When exposed to a high-fat diet, the mean body weight of adult male Long-Evans Tokushima Otsuka (LETO) and female OLETF rats increased at a similar rate to that of male OLETF rats, while female LETO rats did not show increased body weight (26).
It has been suggested that hyperphagia in OLETF rats results from different regulatory deficits (35). The first is the absence of vagal CCK1 receptors and as a consequence, the loss of the short-term satiety signal that should limit meal size. One further deficit contributing to the OLETF's hyperphagia may also be the increased orosensory stimulation associated with dopaminergic deficits (16). Another deficit results from the absence of dorsomedial hypothalamus (DMH) CCK1 receptors, which results in the loss of their inhibitory influence on DMH neuropeptide Y (NPY) expression. The lack of inhibition causes NPY upregulation in the DMH and prevents the obese males from compensating for their overall increased meal size. Adult OLETF males presented upregulation in arcuate nucleus (ARC), proopiomelanocortin (POMC), while young OLETF males presented upregulation of DMH NPY (7). Thus a role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats has been suggested, but the full developmental patterns of hypothalamic gene expression in the OLETF rat have not been reported.
The OLETF rat obesity profile shares many features found in human obesity. These rats do not have a primary deficit in leptin signaling; they only develop leptin and insulin resistance as the disease progresses (29, 30, 38). As in humans, they are susceptible to diet-induced obesity, because when giving them access to a high-fat diet, their otherwise moderate obesity becomes morbid (6). They have also been demonstrated to have increased relative preferences for palatable diets (22), and unlike other rodent obesity models, the OLETF males develop type II diabetes as they become obese (30) and can be normalized under food restriction (7), making them a particularly relevant model. Thus, although the specific deficit that initiates the cascade for obesity development is specific to the OLETF rat, the manifestations of that deficit that results in obesity are very similar to those found both in other rodent models and in human obesity. Moreover, although the primary deficit is in the CCK1 receptor, its absence has consequences on multiple systems through development resulting in a variety of molecular and physiological outcomes. The current study adds to the overall understanding of interactions in development that can produce an obese phenotype and how the obesity feeds back on neural pathways.
Previous studies of young OLETF males and females have shown that specific preobese characteristics can be observed as early as postnatal day (PND) 1 (12, 44–47). Briefly, OLETF males and females are heavier from birth (47), hyperphagic in independent ingestion tests as early as PND 2 (12), consume more milk during the nursing episodes (45), initiate more nursing bouts (44), and present increased % body fat, larger adipocytes, and increased waist circumference as early as PND 7 compared with LETO controls (46). Altogether, these developmental findings have led us to conclude that obesity in this strain starts developing very early in life (maybe even during gestation), as a result of life-long abnormalities in eating behavior.
In continuation to our previous studies, in which we focused on postnatal preobese characteristics of pups genetically predisposed to become obese, the present study was intended to identify time windows during postweaning development, when the obesity becomes more explicit and turning points can be clearly observed through examination of different obesity parameters. Finding such critical time windows may allow targeting future interventions to those sensitive periods, with the hope that they will be more successful in preventing or moderating the disease in the long term. Another focus of interest in the present study was the characterization of patterns of hypothalamic gene expression in the OLETF male rats from PND 7 to adulthood.
MATERIALS AND METHODS
Subjects
OLETF and LETO males and females were raised in the specific pathogen-free facility of the Gonda Brain Research Center at Bar-Ilan University, Ramat-Gan, Israel. The progenitor rats were received as a generous gift from the Tokushima Research Institute, Tokushima, Japan. OLETF and LETO offspring were housed together with their dams and litters until weaning and then in pairs from weaning and on. Polycarbonate cages (18.5 cm height × 26.5 cm width × 43 cm length) were used, with stainless steel wire lids and wood shavings as bedding material. Food (2018S Teklad Global, 5% fat) and water were freely available. The animals were on a 12:12-h light-dark cycle, with lights on at 6:00 AM. Room temperature was maintained at 22 ± 2°C. Newborn litters were culled to 10 pups (minimum 7), with sex distribution kept as equal as possible in each litter. The research protocol was approved by the Institutional Animal Care and Use Committee, and it adhered to Institutional and National regulations and to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Body Weight and Intake
Rats were weighed every 5th day from weaning on PND 22–23 until PND 90 (males) or 120 (females). Intake was assessed daily from pairs of rats starting at the time of weaning (PND 22) between 10 and 12 h. On PND 22, 90, and 120 (only females), the body mass index [BMI = body weight in grams/(body length in cm2)] and waist circumference (WC) were also examined as further obesity parameters. Feeding efficiency was calculated (5-day body weight gain/5-day intake in grams) to assess possible metabolic deficiencies.
Tissue Collection
Five death time points for the males and six for the females were chosen throughout development to sufficiently characterize their development toward obesity. The period from PNDs 22 to 23 was chosen to characterize the weaning period. The time between PND 30 and PND 40 has repeatedly been reported as a developmental period during which no phenotypic differences could be observed between the strains (in males) (8, 9, 36). In previous studies, we did find differences in body weight between the strains in both sexes at this age (47), but only after expanding the number of subjects. It seems that during this period of time, both strains become phenotypically closer, and it was, therefore, of great interest to explore the relevant physiology beneath this (PND 38 and PND 48). PND 65 and PND 90 represent early and midadulthood. We did not distinguish any explicit obesity turning points in the females until PND 90, but the increase in adiposity-related parameters at this last PND age suggested a late turning point in the obese strain. Therefore, a longer follow-up was performed, by delaying time of death until PND 120 in the females. On the experimental days, rats were weighed and euthanized between 11:00 AM and 2:00 PM. Interscapular brown adipose tissue (BAT), retroperitoneal, inguinal (IAT), and epydidimal (EPY, only males) adipose tissues were collected from decapitated animals, weighed, and immediately frozen on dry ice. The calculation of total white fat percentage included only the inguinal and retroperitoneal fat pads, to allow a valid comparison between males and females. Samples were preserved at −80°C until analyzed. The inguinal and retroperitoneal fat pads were chosen as representative of subcutaneous and visceral fat. BAT was studied not only as a fat pad that can be implicated in obesity, but because, in principle, shifts in BAT may underpin different tendencies toward the progression of obesity via changes in thermogenesis. Glucose was assessed from trunk blood immediately after decapitation by a glucometer (Accu-check active, Roche, Germany). Trunk blood for leptin and oxytocin analysis was collected in chilled heparinized Vacutainer tubes coated with EDTA. Plasma was stored at −80°C until assayed.
Plasma Measurements
Plasma leptin and oxytocin levels were assessed using commercial ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Histology
Samples of the IAT were used to characterize adipocyte cell size. Tissues were sectioned to 8 μm on a Cryostat (Leyca) at −35°C and mounted on glass slides. Digital photographs were taken using the ACT1 program, at ×200 magnification. For each inguinal fat pad examined, 10–20 pictures were taken from three different zones of the sample, with at least 100-μm distance between samples. Adipocyte size parameters were derived from 3 to 6 representative cells from each picture, using the public domain National Institutes of Health Scion image program. For each animal, at least 50 cells were analyzed. Representative cells chosen presented a smooth and clear membrane, without surrounding granulations. A similar methodological approach was described elsewhere (31, 57). The estimated number of cells per fat pad was calculated using the average diameter, a density conversion factor (0.915 g/cc), and the mass of the fat pads, as previously described (3, 32).
Estrous Cycle
The estrous cycle of 50- to 80-day-old females was examined daily in the morning. Vaginal cytology samples were collected by introduction and immediate extraction of a small amount of phosphate buffer with a micropipette in the rat's vagina. The stage of the estrous cycle (diestrous 1 or metaestrous, diestrous 2, pro-estrous, or estrous) was determined by examining the appearance and abundance of cells within the vaginal cytology samples. Metaestrous was characterized by leukocytes and clusters of cornified cells; diestrous 2 was characterized by leukocytes and nucleated epithelial cells; proestrous was characterized primarily by nucleated epithelial cells; and estrous was characterized by an abundance of cornified cells. In 5-day cycles, either diestrous 2 or estrous appeared twice. This study was performed on a separate set of animals, to avoid exposing the experimental females to different conditions than the males. Females were maintained under a reversed light-dark cycle. Two to six cycles were analyzed per female, the age of the first estrous was recorded, and food intake was also assessed across the different stages of the estrous cycle.
In Situ Hybridization Determination of Hypothalamic Neuropeptide Gene Expression
In situ hybridization determination was conducted, as preciously described (7, 9). Briefly, coronal sections (14 μm) were cut via a cryostat, mounted on superfrost/plus slides (Fisher Scientific, Fair Lawn, NJ) in a 1:6 series (1:5 series for postnatal days 7 and 15), and even six series of slides per brain (five series for postnatal days 7 and 15) were collected. Sections were fixed with 4% paraformaldehyde and stored at −80°C for later in situ hybridization determination. One series of slides was stained with cresyl violet acetate for allowing sections to be anatomically matched among animals. Sections at levels over the compact subregions of the DMH were taken for determinations of ARC NPY, ARC, POMC, and DMH NPY mRNA expression levels, and sections at levels of the paraventricular nucleus (PVN) were taken for determination of PVN CRF mRNA levels. 35S-labeled antisense riboprobes of NPY, POMC, or CRF were transcribed from rat NPY precursor cDNA, mouse full-length POMC cDNA, or rat CRF precursor cDNA, respectively, by using in vitro transcription systems (Promega, Madison, WI) and purified by Quick Spin RNA columns (Roche, Indianapolis, IN). Frozen tissue sections were allowed to warm to room temperature, treated with acetic anhydride, and incubated in hybridization buffer containing 50% formamide, 0.3 m NaCl, 10 mm Tris/Cl (pH 8.0), 1 mm EDTA (pH 8.0), 1 × Denhardt's solution (Eppendorf, Netheler, Germany), 10% dextran sulfate, 10 mm dithiothreitol, 500 μg/ml yeast tRNA, and 6–8 × 107 cpm/ml of 35S-uridine 5-triphosphate at 55°C overnight. After hybridization, sections were washed three times with 2× saline sodium citrate (SSC), treated with 20 μg/ml RNase A (Sigma, St. Louis, MO) at 37°C for 30 min, and then rinsed in 2× SSC twice at 55°C and washed twice in 0.1× SSC at 55°C for 15 min. Slides were dehydrated in gradient ethanol, air-dried, and exposed with BMR-2 film (Kodak, Rochester, NY) for 1–3 days to obtain the linear range of the autoradiographs for the semiquantification of mRNA levels. All quantification was done from films on which the signals were below saturation levels.
Quantitative analysis of the in situ hybridization was done with National Institutes of Health Scion image software (Bethesda, MD). Autoradiographic images were first scanned on a professional scanner (Epson, Long Beach, CA) and saved in a computer for subsequent analyses with Scion image program using autoradiographic 14C microscales (Amersham, Piscataway, NJ) as a standard. Data for each animal represented a mean of the product of hybridization area × density (background density was subtracted) obtained from four to six sections and was normalized to an average of LETO controls at age of 65 days as 100%. Data from each group were presented as means ± SE.
Statistical Approach
Data were analyzed by repeated-measures 3-way ANOVA with strain and sex as independent variables and age as the repeated measure. Significant interactions were followed by one-way ANOVAs comparing all four groups (male and female OLETF and LETO) at each age separately, followed by post hoc Duncan's multiple range tests (P < 0.05). Developmental trends were examined post hoc, by repeated-measures ANOVA over the ages performed separately in each group, or comparing trends of groups of interest. Post hoc Duncan's multiple range tests between age groups further allowed us to determine within-group developmental turning points on the different measures. The determination of these turning points was thus by a statistical criterion—a significant increase/decrease from one age to the next, within groups, according to Duncan's multiple range test. Between-group comparisons of the age in which a turning point appeared were based on significant group × age interaction effects. Data for NPY, POMC, and CRF mRNA expression levels were similarly examined by two-way (strain × age) ANOVA, followed by Duncan's multiple range tests for developmental trends and pairwise multiple least significant difference comparisons.
RESULTS
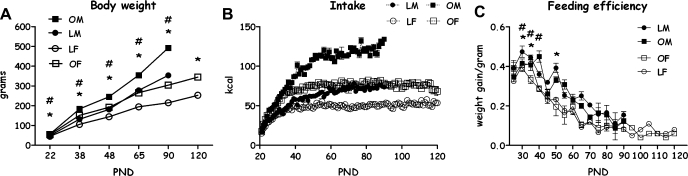
OLETF and LETO male and female rats' weight in grams differed significantly over time, from weaning to PND 90 [F(4,203) = 7.21, P < 0.001 for the strain × sex × age interaction effect; all main effects and two-way effects were also significant]. At all ages and within each sex (including females at PND 120), OLETF rats weighed significantly more than LETO controls (P < 0.001). In addition, the males in both strains presented significantly steeper growth curves than the females [F(4,148) = 31.87, P < 0.001, interaction effect] (Fig. 1A).
Fig. 1.
A: Otsuka Long-Evans Tokushima Fatty (OLETF) and Long-Evans Tokushima Otsuka (LETO) rats' body weight in grams on postnatal day (PND) 22, 38, 48, 65, 90 (both sexes), and 120 (only females). B: OLETF and LETO rats' daily caloric intake from PND 22 until PND 90 (males) and PND 120 (females). At all ages and within each sex, OLETF rats ate significantly more than LETO controls (P < 0.001). In addition, OLETF males ate regularly significantly more than OLETF females starting from PND 32, and LETO males ate significantly more than LETO females starting from PND 38 (P < 0.05). C: OLETF and LETO rats' feeding efficiency from PND 22 to PND 90. Data are presented as means ± SE. OM, OLETF male; LM, LETO male; LF, LETO female; OF, OLETF female. *P < 0.05 for the females; #P < 0.05 for the males (for strain differences at each age); n = 8–18 per group.
Intake in kilocalories of the OLETF and LETO male and female rats differed significantly over time, from weaning to PND 90 [F(13,25) = 14.89, P < 0.001, for the strain × sex × age interaction effect; all main effects and 2-way effects were also significant]. At all ages and within each sex (including females at PND 120), OLETF rats ate significantly more than LETO controls (P < 0.001). In addition, OLETF males ate regularly significantly more than OLETF females starting from PND 32, and LETO males ate significantly more than LETO females starting from PND 38 (Duncan's multiple range tests, P < 0.05; Fig. 1B).
The data on feeding efficiency, presented in Fig. 1C, demonstrated a significant 3-way interaction of strain, sex, and age [F(13,18) = 3.49, P < 0.01]. As can be seen, while females of both strains showed a similar decreasing trend over time, the males of both strains showed frequent fluctuations in their feeding efficiency. Examination of group differences at each 5-day interval shows that between PND 35 and PND 70, females had significantly lower efficiency at most of the time points (Duncan's multiple range test, P < 0.05) Furthermore, focusing on the adolescent age range of PND 30–40 (in which we did not find strain differences in many other measures) showed a significantly greater efficiency in PND 30–35 LETO vs. OLETF rats of both sexes (P < 0.05), while on PND 40, this difference disappeared in the females and was even reversed in the males.
White Fat
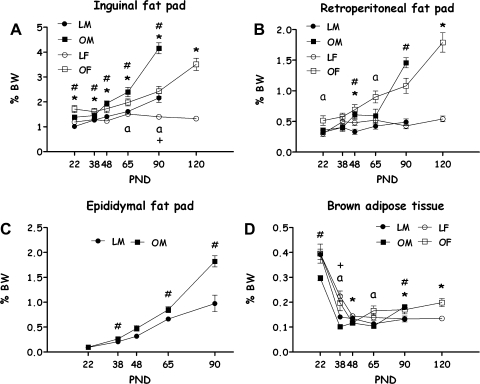
The weight of the white fat pads [expressed as a percentage of the rat's body weight (BW)] was significantly affected by age, strain, and sex [F(4,165) = 3.45, P < 0.01 for strain × sex × age interaction effect; all main effects and two-way interactions were also significant, except for strain × sex]. With the exception of PND 38 (NS), at all other ages and within each sex (including females at PND 120), OLETF rats' fat pads weighed significantly more than LETO fat pads (P < 0.05). In addition, the fat pads of males weighed significantly more than those of females in both strains [F(4,165) = 13.82, P < 0.001] (not shown).
Group differences in different fat pads (expressed as a percentage of BW) are presented in Fig. 2.
Fig. 2.
OLETF and LETO rats' fats [expressed as a percentage of body weight (BW)] on PND 22, 38, 48, 65, 90 (both sexes), and 120 (only females). Inguinal fat pad (A), retroperitoneal fat pad (B), epididymal white fat (only males) (C), and brown adipose tissue (D). Data are presented in means ± SE. *P < 0.05 for the females; #P < 0.05 for the males (for strain differences at each age); aP < 0.05 for sex differences within the OLETF strain; +P < 0.05 for sex differences within the LETO strain; n = 7–12 per group.
Inguinal adipose tissues.
OLETF rats had a greater percentage BW of fat, at all ages, compared with LETOs, in both sexes. In LETO rats, males had more IAT than females only at PND 90. In OLETF rats, females had more IAT than males at weaning. There were no significant sex differences at PND 38 and 48, while on PND 65 and 90, males had significantly larger inguinal fat pads than females (Duncan's multiple range tests P < 0.05).
Retroperitoneal fat pads.
OLETF rats had larger retroperitoneal fat pads than LETOs on PND 48 (in both sexes), on PND 90 (only males), and on PND 120 (only females). On PND 22 and 65, OLETF males presented smaller retroperitoneal fat pads than OLETF females (Duncan's multiple range tests P < 0.05).
Epydidimal fat pads.
OLETF males had significantly more EPY than LETO controls on PND 38, 65, and 90 (Duncan's multiple range tests P < 0.05).
Brown adipose tissues.
OLETF rats had significantly less brown fat than LETOs on PND 22 (only males) and 48 (only females), and significantly more BAT on PND 90 (males and females) and 120 (females) (Duncan's multiple range tests P < 0.05). Females presented significantly more BAT than males on PND 38 in both strains and on PND 65 within the OLETF strain (P < 0.001).
Overall, the turning point at which OLETF male white fat percentage sharply increased (PND 48) was earlier than in LETO males (PND 65). In the males of both strains, fat percentage rapidly increased at an earlier turning point (PND 65) compared with the females (OLETF female's fat increased at PND 90 and even more at PND 120 and LETO females did not show a clear pattern of increase over time). In OLETF males, there was an additional (previous) catch-up growth of fat pads from PND 38 to PND 48, reaching at that point the fat levels of female OLETFs (Duncan's multiple range tests, P < 0.05). Note that regarding some fat pads at particular ages, a relatively large variance may have limited the ability to find significant differences.
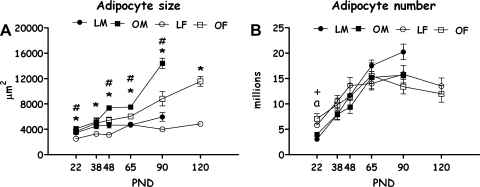
Adipocyte Size and Number
In accordance with the pattern described above, mean inguinal adipocyte size changed over ontogeny differently, from weaning to PND 90, in OLETF compared with LETO rats [F(5,69) = 26.134, P < 0.001], and in male compared with female rats [F(4,69) = 7.40, P < 0.001; the 3-way interaction did not reach significance]. Except for PND 38 (NS) at all other ages and within each sex (including females at PND 120), OLETF rats displayed significantly larger adipocytes than LETO rats (P < 0.05). Examination of Fig. 3A further shows that the turning point at which OLETF male white fat cell size sharply increased over time (PND 48) was much earlier than the sharp increase found in LETO males (PND 90) and in OLETF females (PND 90 and even more on PND 120); LETO females did not show a clear pattern of increase over time beyond a transitory increase in cell size at the time of sexual maturation (PND 65; Duncan's multiple range tests, P < 0.05; see Fig. 3A).
Fig. 3.
Adipocyte size (A) and number (B) of OLETF and LETO males and females on PND 22, 38, 48, 65, 90 (both sexes), and 120 (only females). Data are presented in means ± SE. *P < 0.05 for the females; #P < 0.05 for the males (for strain differences at each age); aP < 0.05 for sex difference within the OLETF strain; +P < 0.05 for sex difference within the LETO strain; n = 4–6 per group.
The calculation of estimated adipocyte number revealed no overall strain differences in hyperplastic trajectory. However, a significant main effect of age [F(4,63) = 44.51, P < 0.001] was found, together with a sex × age interaction [F(4,63) = 3.87, P < 0.01]. Post hoc Duncan's multiple range tests revealed sex differences in the termination age of adipocyte hyperplasia. In the females, the turning point was on PND 48, while in the males, this process continued until PND 65 (P < 0.05) (see Fig. 3B). From the turning points onward, cell number appeared to remain stable within all groups, in accordance with established knowledge of adipocyte development in rats (16).
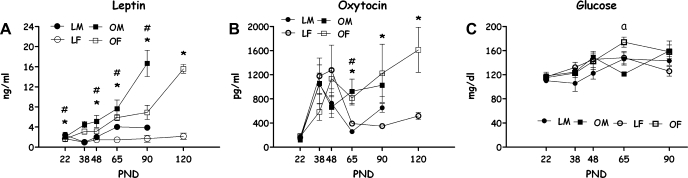
Leptin, Oxytocin, and Glucose
Increases in leptin levels from weaning to PND 90, differed by strain and sex [F(4,83) = 2.54, P < 0.05 for strain × sex × age interaction effect; all main effects and two-way effects were also significant, with one exception: strain × sex, P = 0.07]. Except for females at ages PND 22 and 48, at all other ages and within each sex (including females at PND 120), OLETF rats presented significantly higher leptin levels than LETO rats (P < 0.05). Examination of Fig. 4A further shows that while the turning point at which OLETF and LETO males' showed an increase in leptin levels was similar (PND 65), the magnitude of the increase in the OLETF group was dramatically sharper, with a doubling of values from PND 65 to PND 90. In the females, while LETOs did not increase leptin levels over ontogeny, OLETF females showed a turning point at PND 65, and a doubling of values from PND 90 to PND 120 (Duncan's multiple range tests, P < 0.05).
Fig. 4.
Plasma leptin (A), oxytocin (B), and glucose (C) levels of OLETF and LETO males and females on PND 22, 38, 48, 65, 90 (both sexes), and 120 (only females). Data are presented as means ± SE. *P < 0.05 for the females; #P < 0.05 for the males (for strain differences at each age); aP < 0.05 for sex difference within the OLETF strain; n = 4–6 per group.
OLETF rats displayed higher levels of oxytocin than LETO rats [F(1,81) = 8.25, P < 0.01]; the age main effect was also significant [F(5,81) = 6.26, P < 0.001], as well as the strain × age interaction [F(5,81) = 4.06, P < 0.01]. Until PND 38, no group differences were evident; on PND 48, females appeared to have higher oxytocin levels than males, but this trend was not significant. Differences between the strains in the developmental trajectory of peripheral oxytocin became significant on PND 65 [F(3,15) = 6.96, P < 0.01], were almost significant on PND 90 [F(3,17) = 3.13, P = 0.059] and were significant again on PND 120 [F(1,17) = 8.38, P < 0.01]. In all cases, animals presented a sharp increase in oxytocin levels after weaning, reaching a peak in the males on PND 38 and in the females on PND 48 (Fig. 5 B). After that, both OLETF males and females presented higher levels of oxytocin compared with LETO controls (Duncan's multiple range tests, P < 0.05).
Fig. 5.
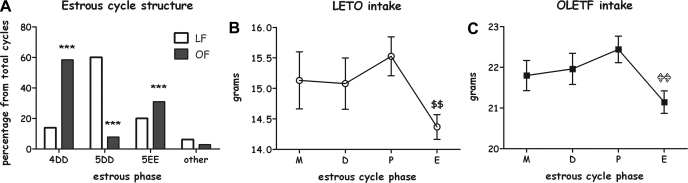
OLETF and LETO females' estrous cycle structure (A). 4DD, 4-day cycle; 5DD, 5-day cycle with double diestrous; and 5EE, 5-day cycle with double estrous. Data are presented in percentages of total cycles examined. B: twenty-four-hour intake across the estrous cycle of LETO females; LETO females displayed an 8.9% decrease in food intake during the estrous phase compared with the proestrous phase of their cycle ($$P < 0.01). C: twenty-four-hour intake across the estrous cycle of OLETF females. Compared with LETO rats, OLETF females displayed a significantly smaller (4.4%) decrease in estrous vs. proestrous food intake (P < 0.05). ***P < 0.001 for strain differences. $$P < 0.01 within the LETO strain (significantly different from the proestrous phase). P < 0.01 within the OLETF strain (significantly different from the proestrous phase). Data are presented as means ± SE; n = 11 LETO, 17 OLETF.
Glucose levels were similar among both strains and sexes throughout development, with one exception: On PND 65, a significant effect was found [F(3,24) = 5.49, P < 0.01], with OLETF females presenting transitory higher levels of glucose than OLETF males. Before and after that, blood glucose levels did not differ (Fig. 5C).
BMI and Waist Circumference
OLETF rats had significantly greater mean BMI scores than LETO controls at weaning and adulthood, in both sexes (Table 1, P < 0.05 for all between-strain comparisons). The strain difference was significantly greater on PND 90 than PND 22 [F(1,52) = 12.18, P < 0.01 for the strain × age interaction].
Table 1.
External obesity measures
| PND | LETO M | SE | OLETF M | SE | LETO F | SE | OLETF F | SE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | ||||||||||||||||
| 22 | 0.37 | 0.01 | 0.41† | 0.01 | 0.38 | 0.02 | 0.42* | 0.01 | ||||||||
| 90 | 0.64§ | 0.02 | 0.78†‡ | 0.01 | 0.54 | 0.02 | 0.69* | 0.01 | ||||||||
| 120 | 0.59 | 0.02 | 0.76* | 0.01 | ||||||||||||
| WC | ||||||||||||||||
| 22 | 6.70 | 0.14 | 8.89† | 0.40 | 6.53 | 0.20 | 8.7* | 0.31 | ||||||||
| 90 | 14.78§ | 0.22 | 17.83† | 0.25 | 13.87 | 0.17 | 17.1* | 0.26 | ||||||||
| 120 | 13.46 | 0.29 | 18.11* | 0.29 | ||||||||||||
Body mass index (BMI) and waist circumference (WC) of Otsuka Long-Evans Tokushima Fatty (OLETF) and Long Evans Tokushima Otsuka (LETO) males and females on postnatal day (PND) 22, 90 (both sexes), and 120 (only females). M, males; F, females. Data are presented in means ± SE.
Significantly different from opposite strain in the females.
Significantly different from opposite strain in the males.
Significantly different from opposite sex in OLETF rats.
Significantly different from opposite sex in LETO rats. All significant differences derived from post hoc Duncan's multiple range tests, P < 0.05; n = 7–12 per group.
OLETF rats had significantly greater mean WC than LETO controls at weaning and adulthood, in both sexes (Table 1, P < 0.001 for all between-strain comparisons). The strain difference was significantly greater on PND 90 than PND 22 [F(1,52) = 6.82, P < 0.05 for the strain × age interaction].
Estrous Cycle and Intake Decrease at Estrus
As shown in Fig. 5A, 80% of the LETO rats' cycles were 5 days long (58% with double-diestrous, 22% with double estrous) and 15% were 4-day cycles. In OLETF rats, we found a significantly different pattern (χ2-test = 59.11, df = 2, P < 0.001): 39% 5-day cycles (8% with double-diestrous, 31% with double estrous), and 58% were 4-day cycles. Longer or shorter cycles were rare (5% in LETO and 3% in OLETF). The age of appearance of the first estrus was significantly earlier in the OLETF females compared with controls [PND 37.1 ± 0.46 for OLETF and 39.67 ± 1.05 for LETO] (P < 0.05).
Representative (most common) types of cycles were chosen for analysis of intake across the estrous cycle: 5-day double diestrous (5DD) and 5-day double estrous (5EE) cycles were chosen for LETOs and 4 day (4DD) and 5 day double estrous (5EE) cycles were chosen for the OLETF females. To make statistical comparisons between LETO and OTLEF rats, the extra day (diestrous 2 or estrous 1) was excluded, and food intake was examined across the remaining 4 days. Strain differences in intake were much larger than between-cycle differences. Therefore, to compare the level of intake decrease in estrous vs. proestrous between the two strains, we calculated the percent decrease in each animal and then compared the strains, thus normalizing the effect of interest from absolute intake levels. As shown in Fig. 5B, LETO females displayed an 8.9% decrease in food intake during the estrous phase compared with the proestrous phase of their cycle (P < 0.01). In contrast, OLETF females displayed a significantly smaller (4.4%) but still significant decrease in estrous vs. proestrous food intake (P < 0.05; Fig. 5C).
Hypothalamic Gene Expression
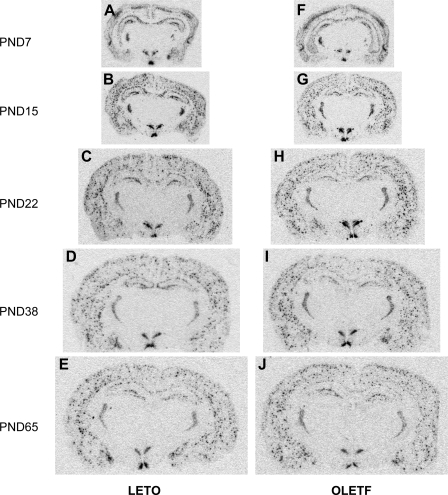
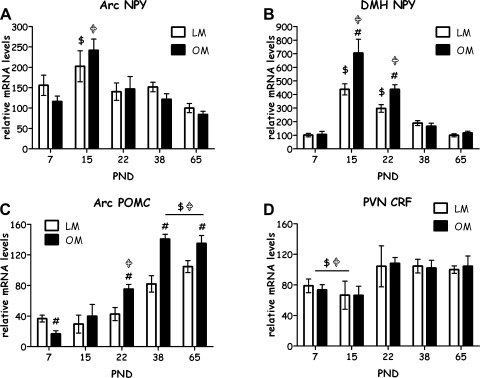
Figure 6 shows images of in situ hybridization of NPY gene expression in the brain in LETO and OLETF rats from PND 7 to young adult at the age of 65 days. Data showed that the distribution of NPY gene expression in the brain areas was similar in both strains (Fig. 7). Within the hypothalamus, NPY was primarily expressed in the ARC and the DMH over the 65-day postnatal period (Fig. 7, A and B). The patterns of the development of NPY gene expression in the ARC and the DMH were similar in both strains. NPY gene expression was strongly detected on PND 7, reached a highest expression level on PND 15, and then gradually decreased from the peak and at the age of 65 days was back to levels similar to those on PND 7 (Figs. 6 and 7, A and B). Within the ARC, NPY gene expression was exclusively localized to the medial part. The two-way ANOVA demonstrated a significant effect of age [F(4,56) = 9.05, P < 0.001], but there was no significant effect of strain and no significant interaction between strain and age (Fig. 7A), indicating that there was no primary deficit in ARC NPY gene expression in OLETF rats. Within the DMH, NPY gene expression was primarily expressed in the compact subregion over the 65-day postnatal period in both strains (Fig. 7B). In addition, NPY gene expression was highly detected in the dorsal part of the DMH on PND 15 and 22 of OLETF rats (Fig. 6, G and H). The two-way ANOVA demonstrated significant effects of strain [F(1,50) = 17.21, P < 0.001], and age [F(4,50) = 79.02, P < 0.001], as well as a significant interaction between strain and age [F(4,50) = 6.95, P < 0.001] (Fig. 7B), implicating an alteration in NPY gene expression in the DMH of OLETF rats. Post hoc comparisons revealed significant elevations of NPY gene expression in the DMH of OLETF rats on PND 15 and 22 compared with LETO rats (Fig. 7B). The developmental pattern of POMC gene expression in the ARC is different from that of ARC NPY. Levels of POMC gene expression were low on PND 7, and its expression levels were gradually increased during the development and reached a high level at PND 38 (Fig. 7C). The two-way ANOVA demonstrated significant effects of strain [F(1,55) = 16.45, P < 0.001], and age [F(4,55) = 40.99, P < 0.001], as well as a significant interaction between strain and age [F(4,55) = 5.84, P < 0.001] (Fig. 7C). Post hoc comparisons further determined that ARC POMC gene expression was significantly lower in OLETF vs. LETO on PND 7, did not differ on PND 15, and was significantly higher in OLETF vs. LETO on PND 22, 38, and 65 (Fig. 7C). CRF gene expression in the PVN was not affected in OLETF rats relative to LETO control during development. Although there was a main significant effect of age on CRF gene expression in the PVN [F(4,34) = 8.22, P < 0.001], there were no significant effects of strain or interaction between age and strain (Fig. 7D). Overall, on PND 7 and 15, CRF gene expression in the PVN was significantly lower than on later PNDs. CRF was slightly reduced in OLETF rats compared with LETO on PND 22, but this reduction did not reach a statistical significance (P < 0.1) (Fig. 7D).
Fig. 6.
Representative pictures of brain slices examined for mRNA levels by in situ hybridization in LETO and OLETF male rats from PND 7 to 65.
Fig. 7.
Relative mRNA levels of hypothalamic neuropeptides in OLETF and LETO males from PND 7 to 65. Data are presented in means ± SE; n = 4–8 per group. A: levels of NPY in the ARC. B: levels of NPY in the DMH. C: levels of POMC in the ARC. D: levels of CRF in the PVN. $P < 0.05 within the LETO strain (significantly different from all other ages);  P < 0.05 within the OLETF strain (significantly different from all other ages); #P < 0.05 for strain differences.
P < 0.05 within the OLETF strain (significantly different from all other ages); #P < 0.05 for strain differences.
DISCUSSION
Most of the research performed on models of obesity has focused on adult males. This is also the case for the OLETF obesity model, in which only few reports can be found on the pups and the females. In the present study, we characterized the development of obesity in the postweaning period in male and female OLETF rats and identified specific physiological and molecular features that, together with the previous behavioral findings, suggest that obesity develops early in life in the OLETF strain in both sexes.
We have previously demonstrated that OLETF males and females are born heavier and present more white fat soon after birth (46). The developmental follow-up conducted here has exposed an abrupt change during and after the period of sexual maturation (PND 90 in the males and PND 120 in the females), especially in the males, where body weight, fats, and adipocyte area sharply increased.
In another rat model of early-onset obesity, early overfeeding of the pups during the suckling period produced a model of hyperplastic obesity that persisted over time (15, 28). In one of those studies, a general pattern of increased cell size in males and increased cell number in females emerged as being the important determinants responsible for the differences in fat depot sizes in the obese group in the long term (15). According to a more recent study by Herrera and Amusquivar (25), the phase of higher hyperplasia in rats occurs between birth and PND 40 and from that time onward, cell number remains more or less constant. From PND 40 until PND 80, adipocyte hypertrophy is the main process responsible for the growth of the fat pads (25). In the present study, the analysis of the obesity-related turning points revealed a similar pattern of fat cell hyperplasia as the one just mentioned, especially in the females. We have recently reported that females present a significantly higher level of adipocyte cell increase in the preweaning period, compared with the males (46). Here, the first weeks postweaning appeared to be a continuation of this pattern, with females presenting more fat cells than males until PND 48; from then on, the cell number in the females remained constant. The pattern of increase in the males was significantly different: While there was no significant increase in cell number during the preweaning period (46), the hyperplasic period started at weaning and lasted about 2 wk longer than in the females. From PND 65 onward, the hyperplasic phase in the males ended, and fat cell number remained constant. Altogether, the pattern of cell number development appeared to be linked to sex rather than strain differences, showing that the females have an earlier onset and earlier endpoint than the males, but the length of the window was about the same, and while the trajectory was different, both sexes reached similar cell numbers at the end of the process.
The similarity between the patterns of increase in inguinal fat pads and fat cell size, together with the lack of significant cell number differences between the strains, suggests that the increase in adipocyte size may be the main reason for the enlargement of the fat depots observed from PND 48 onward in the males and PND 90 onward in the females. Pathogenic adipose tissue is associated with many of the common metabolic diseases, like type II diabetes, hypertension, and dyslipidemia. If adipogenesis is impaired during positive caloric balance, then existing adipocytes must undergo hypertrophy to store the excessive energy. It has been suggested that adipocyte hypertrophy may result as a failure of adipocytes to adequately proliferate (5). During times of positive caloric balance, if energy is stored mainly through lipogenesis and fat cell hypertrophy of existing adipocytes, as opposed to adipogenesis with recruitment and differentiation of new fat cells and fat cell hyperplasia, then this may lead to pathogenic adipose tissue responses that contribute to metabolic disease (4, 5). Presumably, a longer follow-up of these animals might reveal a further hyperplastic phase later in life, a feature also observed in other hyperphagia-induced obesity models such as the Zucker rat (33).
We included in the present study a developmental analysis of plasma oxytocin levels, in following with our recent findings that oxytocin levels varied dramatically over reproductive stages in OLETF but not in LETO female rats, and that in the OLETF rats, fat pads, plasma leptin and oxytocin levels were closely correlated over reproductive stages (57). Oxytocin neurons in the PVN have been suggested to play a vital role in coordinating feeding termination (11, 40, 41, 52) by acting as targets for factors that induce anorexigenic behavior such as CCK (42) and leptin (23, 51). Central and peripheral concentrations of oxytocin are closely related and are elevated in pathological states of energy balance such as obesity (49). Serum oxytocin in humans is approximately fourfold higher in obese compared with control subjects (49) and is significantly elevated in a rodent model of diet-induced obesity (39). Our results appear to be similar to the studies previously performed on obese subjects, since beyond adolescence, the levels of oxytocin in both sexes and strains were overall low in controls and high in adult OLETF rats. Although the mechanisms through which oxytocin exerts its effects on energy balance are still unclear, the present findings imply a further disruption in other systems related to CCK that become dysregulated in the OLETF strain, with the development of obesity and may further contribute to their overall obese phenotype.
One of the main goals of this study was to try and find sensitive time points for future interventions. Despite the OLETF's preobese phenotype from birth, obesity per se appears to develop at different points in the two sexes. For males, the period between weaning and PND 48 appears to be a developmental time in which major changes take place, and the genetic tendency and preobese phenotype transition to explicit obesity. For the females, the identification of a critical transition point is less evident. Their gradual weight/fat gain makes the identification of a specific turning point complicated. Still, sexual maturation precedes the explicit obesity observed from PND 65–120, and while the transitory “normalization” observed on PND 38 appears less dramatic than in the males, it might still be a sensitive point for the females too. It may be the case that interventions during that period of time may help normalize OLETF's otherwise “abnormal” (compared to LETO controls) estrous cycle, which might have some relation to the OLETF females' well-known fertility problems (46, 53).
Analyses of the development of hypothalamic peptide signaling revealed age- and strain-related effects for multiple peptides. Consistent with prior data suggesting a role for DMH NPY in the etiology of the hyperphagia and obesity in the OLETF rat, DMH NPY levels were significantly elevated in OLETF relative to LETO pups beginning on PND 15 and remaining significantly elevated on PND 23. Although expression levels were significantly elevated on PND 38 relative to those on PND 65, these did not differ from those on LETO rats. These data are somewhat consistent with prior work demonstrating significant elevation in OLETF rats postweaning and in response to pair feeding as adults (7). Further support of a role for elevated DMH NPY expression in the hyperphagia and obesity of OLETF rats derives from recent data demonstrating decreases in food intake in body weight in response to AAV-RNAi-mediated knockdown of DMH NPY expression in OLETF rats (56).
Within the arcuate nucleus, the major effects on NPY expression are age related with increased expression in the LETO rats on days 7, 15, and 22 and on days 15 and 22 in the OLETF rats. In contrast, levels of POMC expression are relatively diminished at early ages in both LETO and OLETF rats. With increasing body weight around the time of weaning and postweaning, arcuate POMC expression is significantly elevated in the OLETF rat. This is consistent with what has been seen in adult OLETF rats and may best be viewed as a compensatory response to the increased adiposity and leptin levels in these rats (7). The persistent increase in POMC mRNA (PND 38–65) in OLETF rats may suggest that they are producing more α-MSH to try to defend a “normal” body weight but have become “insensitive” to melanocortin agonism. Alternatively, transcription levels of α-MSH peptide may have changed despite the increase in POMC precursor.
Levels of PVN CRF expression tended to be diminished in the OLETF rats at early developmental time points (PND 15 and 22). These reductions in expression of an anorexigenic peptide may facilitate the increased food intake and weight gain at these developmental time points.
At the time of weaning, OLETF males and females present preobese characteristics, such as high BW, fat mass, adipocyte size, BMI, and WC. Interestingly, besides the significant strain differences in BW at PND 38, the other differences observed between the strains at all other ages were absent at this preadolescent age. Similarly, another study found significant differences in BW between the strains on postnatal week 5 (38). However, others reported a lack of phenotypic differences at this age in males (8, 9, 36). Thus, at this age, strain differences seem to be minimal and elusive.
Even though we have previously reported clear OLETF-LETO differences as early as PND 1, it was of special interest to examine the underlying physiology of the unique postweaning, prepubertal period. We found significant differences in body weight as we previously reported (47); however, we now discovered that body fat percentage on PND 38 did not differ between the strains or the sexes. The analysis of feeding efficiency revealed a possible partial explanation for this: on PND 30–35, LETO males and females showed a different pattern of feeding efficiency from the OLETF rats, showing a more efficient utilization of the food consumed, which might account for a slight and temporary increase in body weight and fat mass. This increased efficiency was not evident in the OLETF strain, where a transitory lack of increase in body fat and adipocyte area was observed. We can only speculate about the reason for this outcome. Probably this transitory change in the physiology of the rats is related to the overall changes taking place at the time of puberty. Moreover, the results of hypothalamic DMH NPY and Arc POMC expression show a sharp decrease in NPY mRNA and a sharp increase in mRNA POMC in OLETF males at this age, further suggesting that critical changes in intake regulation take place around this time point. Taken together, these transitory BW and fat increases in the LETO strain, together with the relatively high variance and lack of increase in body fat observed in the OLETFs resulted in the lack of the significant differences observed on PND 38. However, on PND 40, a sharp increase in feeding efficiency was detected in the OLETF males, which is probably the reason why the next time-point (PND 48) showed renewed significant differences in body fat between the strains. From that point onward, although sharing the same feeding efficiency profile, OLETF males embarked into an evident trajectory toward obesity, as a direct consequence of their hyperphagia. On PND 65 and even more so on PND 90, OLETF males presented obese characteristics accompanied by very large adipocytes and hyperleptinemia.
In contrast to the males, whose feeding efficiency between the strains changed back and forth throughout development, the females of both strains presented almost equal curves of feeding efficiency (besides the PND 30–35 period), and the OLETF females' obesity development seemed more moderate and gradual than that of the males, with no explicit turning points until the time of sexual maturation. LETO females presented no significant changes or turning points during the whole period examined (in contrast to LETO males), further exposing the different developmental patterns males and females undergo over time. Although it is true that beyond the lack of difference on PND 38, OLETF females presented overall higher scores than LETO in all of the obesity parameters examined, their profile of obesity development appeared to be different from that of the males, suggesting that future treatments should use different approaches with male and female subjects.
The intake data presented in this paper closely resemble a previous report (30). We note that despite all of the mentioned turning points observed in the obesity parameters, intake in both sexes hardly increased from around PND 40 onward. Despite the fact that intake in the OLETF strain was about 30–40% higher than LETO controls, no significant caloric increase was found during and after the reported obesity turning points. Thus, one possible explanation might be related to changes in nutrient partitioning; hence, as the rapid growth phase comes to an end, energy becomes more likely to be converted into fat stores than into other tissues. Still, this last statement seems less true for the females, which show relatively late turning points, further implying that deep changes in energy balance take place around the time points discussed that turn their chronic hyperphagia and overweight almost suddenly (especially in the males) into obesity. Another, unstudied possibility is the potential differential loss of calories in feces.
The patterns of estrous cycles of OLETF rats differed from those of LETO controls, with OLETF rats showing, on the average, shorter cycles. Furthermore, OLETF females achieved sexual maturity, as represented by the age of the first estrous and the appearance of the regular cycles, earlier than LETO controls. A previous report from Japan reported OLETF-LETO differences in cycle length, but there the OLETF had longer cycles than controls (53). It is possible that this between-colony difference may be explained by different amounts of phytoestrogens in the “standard” animal diets (13, 43).
Adult females (rats, mice, and women) eat less during the periovulatory part of the ovarian cycle (estrous in rats) than other phases. This effect is under the control of cyclic changes in estradiol secretion (2). Accordingly, control LETO rats showed lower intake levels in estrous vs. proestrous in the current research. Studies that manipulated exogenous and endogenous CCK and estradiol produced converging evidence that the activity of the CCK satiation-signaling pathway is increased by estradiol, and this accounts for the decrease in food intake during estrous (19). Of particular relevance, administration of a selective CCK1 receptor antagonist, devazepide, increased spontaneous food intake during estrus, but not during diestrous (17). We now report that OLETF rats had a significantly attenuated (by ∼50%) estrous-related decrease in feeding compared with LETO controls, a pattern of results resembling the effect of devazepide during estrous in other animals. This finding, given the functional absence of CCK1 receptors in this strain, provides further support for the role of CCK in estrous-induced intake reduction.
Much work has been performed on the organization of central and peripheral mechanisms involved in energy balance during development and also on the effectiveness of early interventions in resetting genetic predispositions toward obesity. All of these suggest that specific changes take place during the postpartum and postweaning period that can be altered because the mechanisms are still developing. We intended to detect those changes by using the turning point strategy. Although by no means do we claim that changes take place at the specific time points we examined, this classification helped us identify time windows in which changes take place and will allow for further examination of the underlying mechanisms mediating the worsening of the obesity-prone phenotype.
Perspectives and Significance
OLETF rats present preobese characteristics during development and develop obesity as a consequence of long-term hyperphagia. Their BMI, WC, percentage of white fat, adipocyte size, and leptin levels are significantly higher than controls from childhood in both sexes and this, together with previous findings on behavioral abnormalities (regarding intake), further establish them as a model of early onset obesity. Ontogenetic patterns of expression of DMH NPY and ARC POMC described suggest that they may play a mediating role in the developing hyperphagia and obesity of OLETF rats. Moreover, a different trajectory toward obesity has been identified between the sexes in the OLETF strain, showing that OLETF females develop obesity in a more gradual manner than the males. Although care should be taken when extrapolating conclusions derived from animal studies to the human condition, the differing pattern of obesity development found in males and females in the present study suggests that the use of sex-specific approaches may be of key importance to make future therapeutic interventions more successful in the treatment and prevention of chronic-early-onset obesity.
GRANTS
This work was supported partly by a grant from the U.S.-Israel Binational Research Foundation (to A. Weller and to T. H. Moran), and partly by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases-RO1 DK57609 (principal investigator: T. H. Moran, subcontract: A. Weller). M. Schroeder and L. Shbiro were supported by President's Fellowships at Bar-Ilan University.
DISCLOSURE
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. K. Kawano of the Tokushima Research Institute (Otsuka Pharmaceutical, Japan) for the generous gift of the Otsuka Long-Evans Tokushima Fatty and Long-Evans Tokushima Otsuka rats and Dr. L. A. Eckel of Florida State University for expert advice on assessing intake over the estrous cycle.
The research reported in this paper was completed as part of the first author's Ph.D. dissertation in the Faculty of Life Sciences at Bar-Ilan University, Ramat-Gan, Israel.
REFERENCES
- 1.Applegate EA, Upton DE, Stern JS. Food intake, body composition and blood lipids following treadmill exercise in male and female rats. Physiol Behav 28: 917– 920, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251– 1263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashwell M, Priest P, Bondoux M, Sowter C, McPherson CK. Human fat cell sizing—a quick, simple method. J Lipid Res 17: 190– 192, 1976 [PubMed] [Google Scholar]
- 4.Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther 4: 871– 895, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 6: 343– 368, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol 293: R55– R63, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol 281: R254– R260, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides 36: 171– 181, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka Long-Evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146: 1676– 1685, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bjorntorp PA. Sex differences in the regulation of energy balance with exercise. Am J Clin Nutr 49: 958– 961, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res 993: 30– 41, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Blumberg S, Haba D, Schroeder M, Smith GP, Weller A. Independent ingestion and microstructure of feeding patterns in infant rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol 290: R208– R218, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HA. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol Sci 51: 236– 244, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. Daily exercise reduces fat, protein and body mass in male but not female rats. Physiol Behav 62: 105– 111, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Cryer A, Jones HM. The development of white adipose tissue. Effect of litter size on the lipoprotein lipase activity of four adipose-tissue depots, serum immunoreactive insulin and tissue cellularity during the first year of life in male and female rats. Biochem J 186: 805– 815, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol 288: R292– R300, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides 20: 451– 456, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Gayle DA, Desai M, Casillas E, Beloosesky R, Ross MG. Gender-specific orexigenic and anorexigenic mechanisms in rats. Life Sci 79: 1531– 1536, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Geary N. Estradiol, CCK and satiation. Peptides 22: 1251– 1263, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Geary N, Lovejoy J. Sex differences in energy metabolism, obesity, and eating behavior. In: Sex Differences in the Brain: From Genes to Behavior: Oxford, UK: Oxford University Press, 2008 [Google Scholar]
- 21.Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature 245: 323– 325, 1973 [DOI] [PubMed] [Google Scholar]
- 22.Hajnal A, De Jonghe BC, Covasa M. Dopamine D2 receptors contribute to increased avidity for sucrose in obese rats lacking CCK-1 receptors. Neuroscience 148: 584– 592, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci 18: 559– 572, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among U.S. children, adolescents, and adults, 1999–2002. JAMA 291: 2847– 2850, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev 16: 202– 210, 2000 [DOI] [PubMed] [Google Scholar]
- 25a.International Obesity Task Force Report by the International Obesity Task Force, Data on global prevalence of adult obesity. www.ioft.org/database/documents/GlobalPrevalenceofAdultObesityOctober2009v2.pdf
- 26.Ishida K, Mizuno A, Murakami T, Shima K. Obesity is necessary but not sufficient for the development of diabetes mellitus. Metabolism 45: 1288– 1295, 1996 [DOI] [PubMed] [Google Scholar]
- 27.James WP. The epidemiology of obesity: the size of the problem. J Intern Med 263: 336– 352, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Johnson PR, Stern JS, Greenwood MR, Zucker LM, Hirsch J. Effect of early nutrition on adipose cellularity and pancreatic insulin release in the Zucker rat. J Nutr 103: 738– 743, 1973 [DOI] [PubMed] [Google Scholar]
- 29.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 24Suppl: S317– S320, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422– 1428, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Yu X, Pan W, Unger RH. Gene expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am J Physiol Endocrinol Metab 282: E1334– E1341, 2002 [DOI] [PubMed] [Google Scholar]
- 32.MacLean PS, Higgins JA, Jackman MR, Johnson GC, Fleming-Elder BK, Wyatt HR, Melanson EL, Hill JO. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 290: R1577– R1588, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Marques BG, Hausman DB, Martin RJ. Association of fat cell size and paracrine growth factors in development of hyperplastic obesity. Am J Physiol Regul Integr Comp Physiol 275: R1898– R1908, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Miyasaka K, Ichikawa M, Kawanami T, Kanai S, Ohta M, Sato N, Ebisawa H, Funakoshi A. Physical activity prevented age-related decline in energy metabolism in genetically obese and diabetic rats, but not in control rats. Mech Ageing Dev 124: 183– 190, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360– 367, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Moran TH, Katz LF, Plata-Salaman CR, Schwartz GJ. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol Regul Integr Comp Physiol 274: R618– R625, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Nance DM, Bromley B, Barnard RJ, Gorski RA. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiol Behav 19: 155– 158, 1977 [DOI] [PubMed] [Google Scholar]
- 38.Niimi M, Sato M, Yokote R, Tada S, Takahara J. Effects of central and peripheral injection of leptin on food intake and on brain Fos expression in the Otsuka Long-Evans Tokushima Fatty rat with hyperleptinaemia. J Neuroendocrinol 11: 605– 611, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Northway MG, Morris M, Geisinger KR, MacLean DB. Effects of a gastric implant on body weight and gastrointestinal hormones in cafeteria diet obese rats. Physiol Behav 45: 331– 335, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptor antagonism blunts the effects of anorexigenic treatments in rats: evidence for central oxytocin inhibition of food intake. Endocrinology 129: 785– 791, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Olson BR, Drutarosky MD, Stricker EM, Verbalis JG. Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am J Physiol Regul Integr Comp Physiol 260: R448– R452, 1991 [DOI] [PubMed] [Google Scholar]
- 42.Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res 569: 238– 248, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res 17: 845– 869, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Schroeder M, Lavi-Avnon Y, Dagan M, Zagoory-Sharon O, Moran TH, Weller A. Diurnal and nocturnal nursing behavior in the OLETF rat. Dev Psychobiol 49: 323– 333, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Schroeder M, Lavi-Avnon Y, Zagoory-Sharon O, Moran TH, Weller A. Preobesity in the infant OLETF rat: the role of suckling. Dev Psychobiol 49: 685– 691, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Schroeder M, Shbiro L, Zagoory-Sharon O, Moran TH, Weller A. Toward an animal model of childhood-onset obesity: follow-up of OLETF rats during pregnancy and lactation. Am J Physiol Regul Integr Comp Physiol 296: R224– R232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder M, Zagoory-Sharon O, Lavi-Avnon Y, Moran TH, Weller A. Weight gain and maternal behavior in CCK1-deficient rats. Physiol Behav 89: 402– 409, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Smith GP. Cholecystokinin and treatment of meal size: proof of principle. Obesity 14Suppl 4: 168S– 170S, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Stock S, Granstrom L, Backman L, Matthiesen AS, Uvnas-Moberg K. Elevated plasma levels of oxytocin in obese subjects before and after gastric banding. Int J Obes 13: 213– 222, 1989 [PubMed] [Google Scholar]
- 50.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 92: 287– 298, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Ur E, Wilkinson DA, Morash BA, Wilkinson M. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology 75: 264– 272, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Verbalis JG, Blackburn RE, Olson BR, Stricker EM. Central oxytocin inhibition of food and salt ingestion: a mechanism for intake regulation of solute homeostasis. Regul Pept 45: 149– 154, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Watanobe H, Yoneda M, Kohsaka A, Kakizaki Y, Suda T, Schioth HB. Normalization of circulating leptin levels by fasting improves the reproductive function in obese OLETF female rats. Neuropeptides 35: 45– 49, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Weller A. The ontogeny of postingestive inhibitory stimuli: examining the role of CCK. Dev Psychobiol 48: 368– 379, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J Neurosci 29: 179– 190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zagoory-Sharon O, Schroeder M, Levine A, Moran TH, Weller A. Adaptation to lactation in OLETF rats lacking CCK-1 receptors: body weight, fat tissues, leptin and oxytocin. Int J Obes (Lond) 32: 1211– 1221, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]