Abstract
Considerable data show that the vestibular system contributes to blood pressure regulation. Prior studies reported that lesions that eliminate inputs from the inner ears attenuate the vasoconstriction that ordinarily occurs in the hindlimbs of conscious cats during head-up rotations. These data led to the hypothesis that labyrinthine-deficient animals would experience considerable lower body blood pooling during head-up postural alterations. The present study tested this hypothesis by comparing blood flow though the femoral artery and vein of conscious cats during 20–60° head-up tilts from the prone position before and after removal of vestibular inputs. In vestibular-intact animals, venous return from the hindlimb dropped considerably at the onset of head-up tilts and, at 5 s after the initiation of 60° rotations, was 66% lower than when the animals were prone. However, after the animals were maintained in the head-up position for another 15 s, venous return was just 33% lower than before the tilt commenced. At the same time point, arterial inflow to the limb had decreased 32% from baseline, such that the decrease in blood flow out of the limb due to the force of gravity was precisely matched by a reduction in blood reaching the limb. After vestibular lesions, the decline in femoral artery blood flow that ordinarily occurs during head-up tilts was attenuated, such that more blood flowed into the leg. Contrary to expectations, in most animals, venous return was facilitated, such that no more blood accumulated in the hindlimb than when labyrinthine signals were present. These data show that peripheral blood pooling is unlikely to account for the fluctuations in blood pressure that can occur during postural changes of animals lacking inputs from the inner ear. Instead, alterations in total peripheral resistance following vestibular dysfunction could affect the regulation of blood pressure.
Keywords: blood flow patterning, venous return, cardiac output, orthostatic hypotension
head-up body rotations in humans or animals typically result in some pooling of blood in the periphery and a resulting reduction in return of blood to the heart. Because cardiac output is directly related to cardiac preload (Starling's law of the heart) (27), cardiac output tends to decrease during head-up movements (25). Furthermore, cardiac output and peripheral vascular resistance determine systemic blood pressure, such that the sympathetic nervous system must produce a rapid net increase in peripheral resistance by inducing vasoconstriction at the onset of head-up body rotations to maintain stable blood pressure (7). Arterial baroreceptor mechanisms play an important role in regulating peripheral vasoconstriction during postural alterations (23). In addition, there is considerable evidence from studies in animals (6, 8, 10, 11, 21, 22, 32) and humans (1, 4, 12, 23, 26, 28, 30) that the vestibular system also participates in triggering increases in vasomotor activity during movements that promote peripheral blood pooling.
Experiments conducted on anesthetized (13, 15) and conscious (32) cats demonstrated that the vestibular system influences on different vascular beds are not equivalent. Electrical stimulation of vestibular afferents produces opposite changes in the activity of sympathetic nerve fibers innervating arterioles in skeletal muscle of the upper and lower body (15) and reciprocal changes in forelimb and hindlimb blood flow (13). Head-up rotations of conscious cats elicit vasoconstriction, as well as a reduction in arterial blood flow, in the forelimb and hindlimb (32). However, after vestibular inputs were removed by cutting the VIIIth cranial nerves, the hindlimb vasoconstriction accompanying head-up tilts was attenuated, although the forelimb vasoconstriction was unaltered (32). The inability to rapidly increase lower body vascular resistance during head-up rotations has been presumed to lead to an accumulation of blood in the hindlimbs and a reduction in venous return to the heart (32), which would account for the decrease in blood pressure that commonly occurs during head-up tilts in cats lacking labyrinthine inputs (10, 11). However, whether vestibulosympathetic reflexes serve to maintain adequate venous return to the heart during postural alterations has not been directly tested, and the present study was carried out to test this hypothesis.
A number of methods, including plethysmography, can be used to monitor accumulation of blood in the limbs. In the present study, however, we elected to estimate blood accumulation in the hindlimb by comparing blood flow simultaneously determined for the femoral artery and vein during the course of head-up body rotations. These measurements allowed us to determine whether increases in arterial flow to the limb were matched by increased venous outflow as body position was altered. The temporal relationship between posturally related changes in arterial perfusion to and venous outflow from the limb could be more accurately established by blood flow measurements than by another method. To our knowledge, this is the first study that collected such data from conscious animals. Our experiments further determined whether the changes in venous and arterial blood flow during head-up tilts were altered after the elimination of labyrinthine signals.
METHODS
All experimental procedures conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals,” as well as the National Research Council Guide for the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Data were collected from seven purpose-bred adult female cats (Liberty Research, Waverly, NY). Animals were spayed before they were included in this study to eliminate cyclic changes in hormonal levels.
Surgical procedures.
Two recovery surgeries were required for each animal. Both surgeries were performed in a dedicated operating suite with the use of aseptic procedures. Animals were initially anesthetized with an intramuscular injection of ketamine (20 mg/kg) and acepromazine (0.2 mg/kg). Subsequently, an endotracheal tube and intravenous catheter were inserted. Anesthesia was maintained using 1–2% isoflurane vaporized in O2, so that limb withdrawal reflexes were absent and heart rate was stable. During the longer initial surgery to implant instrumentation, animals were typically ventilated such that end-tidal CO2 was maintained near 4%. A saline solution was infused intravenously to replace fluid loss during the surgery. A heating pad and heat lamp were used to maintain core temperature near 38°C. After each surgery, animals received antibiotics (amoxicillin, two 50-mg oral doses per day) for 10 days. For 72 h after the first surgery, analgesia was provided through the transdermal delivery of 25 μg/h of fentanyl (Janssen Pharmaceutical Products, Titusville, NJ). After the second surgery, 3 mg/kg of ketoprofen (a nonsteroidal anti-inflammatory drug with analgesic properties) was administered intramuscularly every 12 h for 3 days.
During the first surgery, a fixation plate was mounted on the skull, and perivascular probes (PS series, Transonic Systems, Ithaca, NY) were placed around the femoral artery and vein of each leg and secured in place using sutures. We attempted to place the probes as proximal as possible within the leg, although there was some variability in the placement to circumvent collateral vessels to the parent artery and vein. The cable from each probe was routed subcutaneously, and dental cement was used to attach the connector to the skull behind the fixation plate. Animals recovered for ≥3 wk after this surgery before data collection was initiated.
A second surgery was performed after initial data collection was complete to eliminate vestibular inputs. The tympanic bulla on each side of the skull was opened using a ventrolateral approach to expose the cochlea. A drill was used to remove temporal bone near the base of the cochlea, thereby producing a labyrinthectomy that rendered the vestibular apparatus dysfunctional. This procedure also provided access to the portion of the VIIIth cranial nerve within the internal auditory canal, which was transected under microscopic observation. Thus two independent lesions affecting the vestibular system were made on both sides to ensure that vestibular inputs were eliminated. In no case did nystagmus or a tonic deviation in eye position occur after the surgery, suggesting that the peripheral lesions were complete. Furthermore, postmortem histological examinations, performed as part of our previous studies utilizing this surgical method, revealed that it is always completely effective in eliminating vestibular inputs (2, 3, 11). To ensure that animals received proper hydration and nutrition during the postsurgical period, ∼100 ml of saline solution were administered intravenously or subcutaneously each day, and animals were fed by hand until the spontaneous consumption of food and water returned to prelesion levels (which required ≤1–3 days). After all data recording was completed ∼1 mo after the vestibular lesions, animals were deeply anesthetized with an intramuscular injection of 20 mg/kg ketamine and 0.2 mg/kg acepromazine, followed by an intraperitoneal injection of 40 mg/kg pentobarbital sodium, and killed by transcardial perfusion with saline. An autopsy was performed to ensure that the perivascular probes remained secured to vessels.
Data collection procedures.
The animals were trained over a period of ≥3 wk to remain sedentary, with the limbs fully extended, during whole body head-up tilts at an amplitude of 20°, 40°, or 60°. The acclimation procedures used in this study were identical to those employed in previous experiments (10, 11, 21, 31, 32). During recording sessions, a jacket with attached Velcro straps was placed around the torso; the straps were secured to the sides of the tilt table to prevent the position of the animal from shifting during tilting. The head was immobilized by insertion of a screw into the fixation plate secured on the skull. Extension cables were used to link the head-mounted perivascular probe connectors to perivascular flowmeter modules (model TS420, Transonic Systems).
Data were collected during 30- to 45-min recording sessions. All trials were conducted in a darkened room, and cardboard panels were placed around the animal's head to ensure that no visual cues were available to indicate the onset or magnitude of rotations. Head-up tilts of 20°, 40°, and 60° amplitudes were randomly used throughout the recording sessions, so that animals could not anticipate the amplitude of the next rotation. Tilts persisted for ∼45 s and were separated by ≥45 s. The tilt table was rotated manually and secured in one of three predetermined tilt positions with use of a locking device. Rotations from the Earth-horizontal to the head-up position were performed rapidly, at a velocity of ∼30°/s at all three amplitudes, to produce a sudden orthostatic challenge. Data collected during tilts in which animals vocalized or failed to remain stationary were discarded. However, since data collection was not initiated until animals were well acclimated to the experimental protocol, the vast majority of trials yielded usable data. After baseline recordings were performed over ∼2 mo, vestibular inputs were surgically eliminated; data recording sessions resumed on the day after the surgery and continued for ∼1 mo. Experimental sessions were typically conducted 5 days/wk but were performed daily for the 1st wk after vestibular lesions.
Data recording and analysis.
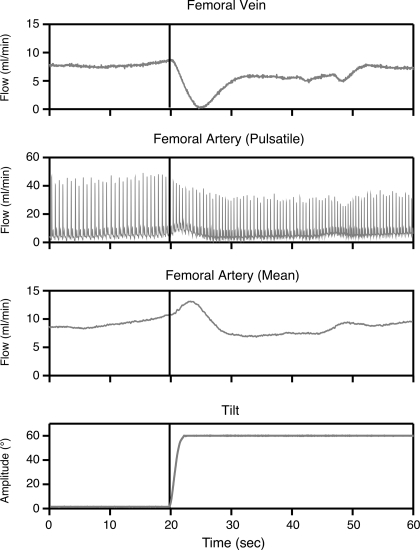
Pulsatile and mean volume flow outputs from the flowmeter modules were sampled digitally at 100 Hz using a data collection system (1401-plus, Cambridge Electronic Design, Cambridge, UK) interfaced with a computer (Macintosh G4, Apple Computer, Cupertino, CA). A recording of tilt table position supplied by a potentiometer was also sampled at 100 Hz. Examples of the data recorded are shown in Fig. 1.
Fig. 1.
Blood flow responses to head-up tilt. Mean femoral vein blood flow (top trace) was obtained from a flow module (model TS420, Transonic Systems); pulsatile and mean femoral artery blood flow traces are also shown. Bottom trace is a recording of table position provided by a potentiometer.
The Spike-2 software package (Cambridge Electronic Design) was used for data analysis. Mean venous and arterial blood flow were determined for the following 2-s time intervals: 15–17 and 3–5 s before the onset of each tilt and 0–2, 2–4, 4–6, 6–8, 8–10, 18–20, 28–30, and 38–40 s after each tilt was initiated. For each tilt, we also calculated the percent difference in venous and arterial blood flow at all time periods relative to baseline (the value ascertained at 3–5 s before the rotation commenced). Instantaneous blood accumulation for each time period was then determined by subtraction of the percent difference from baseline in venous blood flow from the percent difference from baseline in arterial blood flow. As such, the instantaneous blood accumulation values reflected the effects of head-up reorientations of the body axis on the balance of blood flow into and out of the limb at a particular time. Statistical analyses considered the effects of rotation amplitude and vestibular lesions on venous and arterial blood flow for each time period relative to the onset of tilts. In previous studies, we reported that compensation for the effects of vestibular lesions on regulation of blood pressure during postural alterations occurred after 1 wk (11, 32). For this reason, data recorded in the first 7 days after the removal of labyrinthine inputs and in subsequent weeks were considered as separate groups during analyses.
Statistical analyses were performed using SPSS version 16 software (SPSS, Chicago, IL). Statistical significance was set at P < 0.05, and pooled data are presented as means ± SE. The main effects of tilt amplitude on arterial and venous blood flow and instantaneous limb blood accumulation for each animal were determined with the use of multivariate ANOVA. These analyses, in conjunction with Bonferroni's post hoc tests, were used to identify significant differences at each time point. Similar analyses were conducted for each animal to determine the effects of vestibular lesions on arterial and venous blood flow and instantaneous blood accumulation at different times relative to tilt onset. In addition, the mean data from each animal were included in a two-way ANOVA that compared percent changes from baseline in arterial and venous blood flow and instantaneous blood accumulation at different time points. Bonferroni's post hoc tests then established whether data varied in accordance with tilt amplitude or time subsequent to removal of vestibular signals.
RESULTS
Flow recordings for a vessel were assumed to be stable if the acoustic signal transmission indicated by the flowmeter was >60% and did not vary during the course of tilts. In all cases where signal transmission was stable throughout the experiment, the postmortem autopsy revealed that the perivascular probe was firmly attached to the target vessel and surrounded with connective tissue that infiltrated the implantation site. However, in every animal, two or more probes became dislodged from vessels before the end of the data collection period. Such displacement of the probes was obvious, inasmuch as the acoustic signal transmission fell to 0%, and the probes were subsequently noted to be detached from the vessels during the autopsy. Probe detachments from the target vessels usually occurred within a few weeks after implantation, and all data collected for the vessel were discarded.
In animals 1–5, stable recordings were obtained from a femoral artery and vein throughout the course of the experiment. In animals 1, 3, 4, and 5, the two vessels were located in the same hindlimb; in animal 2, stable recordings were obtained only from the left femoral vein and the right femoral artery. Although the venous and arterial data in animal 2 were collected from different limbs, we assumed that parallel events occurred on each side and still calculated blood accumulation values for this case. In animals 6 and 7, data were obtained from only one vessel (the left femoral artery in animal 6 and the left femoral vein in animal 7); it was thus impossible to estimate hindlimb blood accumulation for these cats.
Mean femoral artery and vein blood flow values obtained for each animal before the onset of tilts are shown in Table 1. These data, particularly femoral artery flow values, varied considerably between cats. The variability is partly due to the fact that we placed the perivascular probes more distally in some cases than in others, as required to circumvent large collateral vessels of the proximal femoral artery. Because of the variability in baseline flow values between animals, for each tilt we calculated the percent change in flow that occurred relative to the value ascertained at 3–5 s before the onset of rotation. These relative values to baseline flow levels were used for subsequent calculations.
Table 1.
Average baseline blood flow in vestibular-intact cats
| Blood Flow, ml/min |
||
|---|---|---|
| Animal No. | Femoral artery | Femoral vein |
| 1 | 14.8±0.2 | 14.1±0.3 |
| 2 | 12.2±0.2 | 11.3±0.2 |
| 3 | 20.5±0.2 | 14.0±0.1 |
| 4 | 6.2±0.1 | 13.0±0.1 |
| 5 | 7.8±0.3 | 8.9±0.2 |
| 6 | 14.1±0.1 | |
| 7 | 9.3±0.1 | |
Values are means ± SE.
Effect of tilt amplitude on blood flow into and out of the hindlimb.
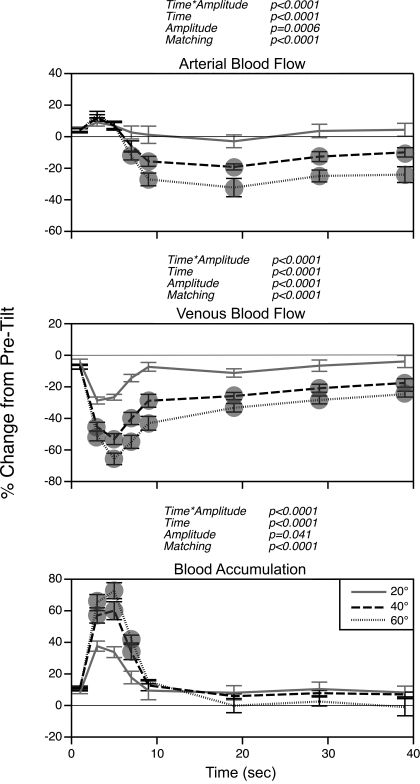
Figure 2 shows the effects of tilt amplitude on blood flow through the femoral artery and vein. The data in Fig 2 represent the average of the mean values obtained for each animal. Femoral artery blood flow increased slightly relative to baseline levels at the onset of head-up tilts but then declined sharply. After ∼10 s following the onset of rotations, femoral artery blood flow stabilized at ∼20% less than baseline values during 40° tilts and at ∼30% less than baseline values during 60° tilts. A two-way ANOVA verified that the alteration in femoral artery blood flow was greater during 40° and 60° head-up rotations than during 20° tilts.
Fig. 2.
Average changes in femoral artery (top trace) and vein (middle trace) blood flow during 20°, 40°, and 60° head-up tilts before removal of vestibular inputs. Bottom trace: instantaneous blood accumulation at each time period, determined by subtraction of percent difference from baseline in venous blood flow from percent difference from baseline in arterial blood flow. Symbols designate changes in blood flow and blood accumulation elicited by 40° and 60° tilt that were significantly different from those resulting from 20° tilt. For points where no symbols are present, significant differences were not detected. Error bars, SE. P values reflect effects on results of the following parameters: interaction of time from tilt onset and tilt amplitude (time * amplitude), time from tilt onset (time), tilt amplitude (amplitude), and matching of subjects (matching).
The rotations also produced alterations in blood flow through the femoral vein, although the temporal dynamics were considerably different from those for the femoral artery. Femoral vein blood flow dropped sharply just after the initiation of table movement and reached its lowest level 4–6 s after the onset of the rotation. At that time, average femoral vein blood flow was 27%, 53%, and 66% lower than baseline levels during 20°, 40°, and 60° rotations, respectively. A two-way ANOVA indicated that the rapid tilt-elicited decreases in femoral vein blood flow were larger during 40° and 60° head-up tilts than during 20° tilts. In addition, the initial drop in venous blood flow was larger during 60° rotations than during 40° table movements. After the body axis was oriented head-up for a short period, venous blood flow rebounded toward baseline levels. By 20 s after the onset of rotations, venous blood flow was only 26% lower than baseline values during 40° tilts and only 33% lower during 60° tilts.
Also shown in Fig. 2 is the difference between the alterations in blood flow through the femoral artery and vein at different times after the onset of rotations. Just after the initiation of tilts, arterial perfusion of the hindlimb increased slightly, while venous return from the limb decreased sharply. As a consequence, the instantaneous blood accumulation in the leg was substantial. However, at ∼10 s after the repositioning of the body axis, venous flow returned toward baseline levels, whereas arterial perfusion of the limb declined as vasoconstriction occurred. The mean alterations in arterial and venous flow were almost perfectly balanced during 60° tilts, such that little additional blood accumulated in the hindlimbs after the body axis was positioned head-up for >10 s.
Effect of elimination of vestibular inputs on blood flow to and from the hindlimb.
After the effects of head-up postural alterations on relative blood flow through the femoral artery and vein were established over ∼2 mo, vestibular inputs were eliminated through a combined labyrinthectomy and vestibular neurectomy. The vestibular lesions did not result in any systematic statistical changes in baseline blood flow through the femoral artery or vein. After removal of labyrinthine inputs, femoral artery blood flow increased an average of 12 ± 11% during the 1st wk but was only 1 ± 12% greater than prelesion values during the subsequent weeks of the survival period. Baseline femoral vein blood flow increased 1 ± 9% during the 1st wk after the elimination of vestibular signals but decreased to 8 ± 9% lower than prelesion values during the subsequent weeks.
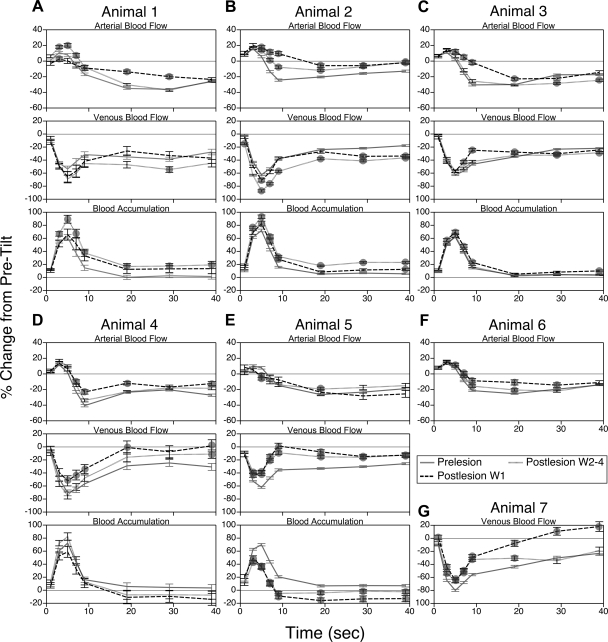
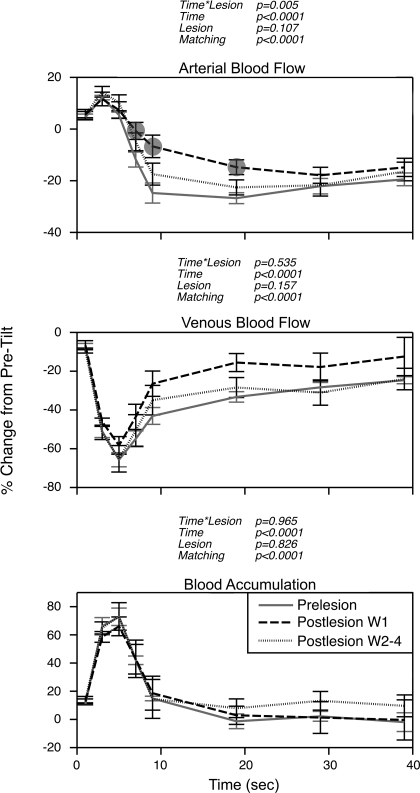
Effects of removal of vestibular inputs on alterations in femoral artery and vein blood flow during 60° head-up tilts for each animal are shown in Fig. 3, and Fig. 4 indicates which of the differences illustrated in Fig. 3 were statistically significant. Pooled data for all animals are shown in Fig. 5. During the 1st wk after vestibular lesions, the decrease in femoral artery blood flow that ordinarily occurred at 7–20 s after the onset of head-up rotations was attenuated in every case, except in animal 5 (Fig. 3E). This reduction of arterial vasoconstriction was also evident from the pooled data for all animals (Fig. 5). Subsequent to the first postlesion week, the vasoconstrictor responses in most animals were similar to those in vestibular-intact animals. In contrast, the perturbation in venous return during rotations was less severe in the majority of cats after removal of vestibular inputs. In other words, elimination of labyrinthine signals typically facilitated venous return from the hindlimb. Only in animals 1 and 2 was the decline in hindlimb venous return at the onset of head-up tilts exacerbated after removal of labyrinthine inputs (and this effect was statistically significant only in animal 2). However, the effects on venous outflow in these two animals were not evident until after the 1st wk following the vestibular lesions, when the deficits in vasoconstriction were mainly resolved.
Fig. 3.
Effects of removal of vestibular inputs on changes from pretilt levels in femoral artery and vein blood flow and hindlimb blood accumulation at different times during 60° head-up rotation. A–G: responses recorded from each animal. Prelesion, data recorded before vestibular lesion; Postlesion W1, values ascertained during the 1st wk after vestibular neurectomy; Postlesion W2–4, responses recorded subsequently. Symbols designate postlesion changes in blood flow or accumulation during tilts that were significantly different from those determined when vestibular inputs were present; relative significance of differences is clarified in Fig. 4. For points where no symbols are present, significant differences were not found. Error bars, SE.
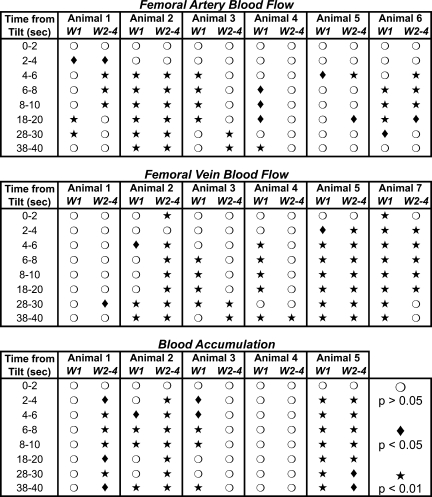
Fig. 4.
Relative significance of differences in postlesion blood flow and hindlimb blood accumulation from prelesion values in each animal. Symbols indicate whether mean femoral artery and vein blood flow and hindlimb blood accumulation determined for the 1st wk (W1) and subsequent weeks (W2–4) after removal of vestibular inputs differed significantly from prelesion values. Mean data are plotted in Fig. 3.
Fig. 5.
Average effects of removal of vestibular inputs on femoral artery and vein blood flow and hindlimb blood accumulation relative to pretilt baseline values. Symbols designate postlesion changes in blood flow and blood accumulation during tilt that were significantly different from those recorded when vestibular inputs were present. For points where no symbols are present, significant differences were not found. Error bars, SE. P values reflect effects on results of the following parameters: interaction of time from tilt onset and lesion state (time * lesion), time from tilt onset (time), lesion state (lesion), and matching of subjects (matching).
Despite the effects of vestibular lesions on hindlimb vasoconstriction, instantaneous blood accumulation in the limb (the difference between the change from baseline in arterial inflow and venous outflow at different time points) was not appreciably exacerbated by removal of the labyrinthine inputs. This is apparent from data of individual animals (Fig. 3), as well as from considerations of pooled data from all animals (Fig. 5). During the 1st wk after removal of vestibular inputs, blood flow into the hindlimb through the femoral artery was 11, 18, and 12% greater at 7, 9, and 19 s, respectively, after the onset of tilts than when labyrinthine inputs were present. At the same time points, venous return was 11, 17, and 18% higher in the 1st wk after the vestibular lesions. This analysis confirms that the increase in arterial perfusion of the hindlimb during tilts was equalized by an increase in venous outflow, and instantaneous blood accumulation was nearly identical before and after vestibular lesions.
DISCUSSION
Our findings validate previous data showing that removal of vestibular inputs results in a decrement in the vasoconstriction that ordinarily occurs in the hindlimb during head-up tilts (32). These observations, combined with findings that vestibular lesions result in perturbations in blood pressure during head-up rotations (10, 11), led to the hypothesis that loss of labyrinthine signals results in considerable lower body blood pooling during postural alterations that promote orthostasis (32). The present study did not support this notion but revealed that venous outflow from the hindlimb was facilitated in most animals during the impaired vasoconstriction. In other words, the increased arterial perfusion of the hindlimb was ordinarily matched by higher venous return. Why vestibular lesions should lead to augmented venous outflow from the lower body during postural alterations is unknown, although it is likely that changes in skeletal muscle tone account for this finding (19). Postural reflexes of the limbs are profoundly affected by removal of vestibular signals, inasmuch as animals with no labyrinthine inputs must rely on proprioceptive information to trigger muscle contractions (16). As such, the effects of vestibular lesions on alterations in limb muscle tone and intramuscular pressure during righting reflexes could modify venous drainage from the lower body.
The present experiments also provided insights about the effects of head-up passive movements on the temporal dynamics of blood flow into and out of the hindlimb. Venous drainage from the hindlimb dropped considerably at the onset of head-up tilts but then rapidly recovered. For example, at 5 s after the animals began a 60° head-up rotation, venous return was 66% lower than when the animals were prone. However, after the animals were maintained in the head-up position for another 15 s, venous return was just 33% lower than before the tilt commenced. At the same time point, arterial perfusion of the limb was 32% lower than baseline values, such that the decrease in blood flow out of the limb was precisely matched by a reduction in blood reaching the limb. The factors leading to this precise bidirectional balancing of blood movement are unknown and are likely multifactorial. The present and prior results show that vestibular signals (13, 15, 32) augment baroreceptor reflexes (23) by adjusting arterial perfusion of the hindlimbs during postural changes. However, other inputs likely also participate, explaining why deficits in hindlimb vasoconstriction dissipate over time following vestibular lesions. There is evidence that inputs from muscle proprioceptors and cutaneous mechanoreceptors are processed along with signals from the inner ear by vestibular nucleus neurons and that these nonlabyrinthine inputs are amplified after vestibular lesions to permit a determination of body position in space (18, 33). Mechanoreceptors in the walls of limb veins could provide signals to the central nervous system reflecting blood pooling during postural alterations (5, 17). It is unclear whether signals from venous afferents can induce hindlimb arterial vasoconstriction or venoconstriction, although they have been suggested to trigger skeletal muscle pumping when blood accumulates in veins (29). Further research is required to decipher the identity and relative roles of the multiple systems that serve to balance arterial inflow and venous outflow from the dependent limbs during movements that promote orthostasis.
The observation that removal of labyrinthine inputs fails to produce increased peripheral blood pooling during postural alterations indicates that another etiology must account for the disturbances in blood pressure that accompany head-up rotations following vestibular lesions (10, 11). Baseline heart rates increase after elimination of signals from the inner ear (11); since cardiac output is the product of heart rate and cardiac preload, it seems unlikely that vestibular lesions promote attenuated cardiac output during postural alterations. A caveat is that removal of vestibular inputs results in a significant increase in the tonic activity of the diaphragm and abdominal muscles (3), which could affect abdominal and thoracic cavity pressures and, potentially, alter venous return to the heart. Nonetheless, it seems improbable that impairment of movement of blood through the abdominal cavity could occur without pooling of blood in the lower limbs (19) and, thus, was presumably not a factor in this study. Stimulation of vestibular afferents affects the activity of vasoconstrictor efferents innervating muscle and visceral blood vessels (14, 15, 26). Thus loss of vestibulosympathetic reflexes could lead to alterations in vasoconstriction throughout the body resulting in deficits in regulation of total peripheral resistance. This hypothesis remains to be tested. It has been suggested that loss of vestibular system receptors in the elderly and the resulting effects on vasoconstriction contribute to the susceptibility of this population to orthostatic hypotension (20, 24). Another study demonstrated that age-related orthostatic intolerance is mainly due to attenuated peripheral vasoconstriction (9). These observations support the notion that posture-related blood pressure disturbances in animals lacking vestibular inputs are more related to altered peripheral resistance than to peripheral blood pooling and reduced cardiac output.
Perspectives and Significance
The present data reveal the existence of mechanisms to balance arterial inflow and venous outflow from the hindlimb. Because of the existence of these mechanisms, accumulation of additional blood in the hindlimb only persists for the first few seconds of a passive head-up tilt, then the reduction in venous return from the dependent limbs appears to be precisely equalized by vasoconstriction and a decrease in arterial perfusion. Although this vasoconstriction was attenuated by the removal of vestibular inputs, the balance in the movement of blood into and out of the hindlimb was not appreciably affected. These data therefore challenge the notion that vestibular lesions result in attenuated venous return and cardiac output during movements that promote orthostasis (32). Instead, alterations in total peripheral resistance following this dysfunction could affect the regulation of blood pressure.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC-00693. Core support was provided by National Institute on Deafness and Other Communication Disorders Grant P30-DC-05205. K. J. Yavorcik was supported by an American Physiological Society Undergraduate Summer Research Fellowship.
DISCLOSURES
No conflicts of interests are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Michael Bonadio, Vince Bottaro, Monica Erwin, and Kenneth Hobbs for assisting with data collection and Dr. Edwin Klein for performing spay surgeries on the animals.
REFERENCES
- 1.Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol 294: R681– R688, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Cotter LA, Arendt HE, Cass SP, Jian BJ, Mays DF, 2nd, Olsheski CJ, Wilkinson KA, Yates BJ. Effects of postural changes and vestibular lesions on genioglossal muscle activity in conscious cats. J Appl Physiol 96: 923– 930, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cotter LA, Arendt HE, Jasko JG, Sprando C, Cass SP, Yates BJ. Effects of postural changes and vestibular lesions on diaphragm and rectus abdominis activity in awake cats. J Appl Physiol 91: 137– 144, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Cui J, Iwase S, Mano T, Katayama N, Mori S. Muscle sympathetic outflow during horizontal linear acceleration in humans. Am J Physiol Regul Integr Comp Physiol 281: R625– R634, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Davenport PW, Thompson FJ. Mechanosensitive afferents of femoral-saphenous vein. Am J Physiol Regul Integr Comp Physiol 252: R367– R370, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Doba N, Reis DJ. Role of the cerebellum and vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res 34: 9– 18, 1974 [DOI] [PubMed] [Google Scholar]
- 7.Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110: 2931– 2937, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gotoh TM, Fujiki N, Matsuda T, Gao S, Morita H. Roles of baroreflex and vestibulosympathetic reflex in controlling arterial blood pressure during gravitational stress in conscious rats. Am J Physiol Regul Integr Comp Physiol 286: R25– R30, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Groothuis JT, Thijssen DH, Kooijman M, Paulus R, Hopman MT. Attenuated peripheral vasoconstriction during an orthostatic challenge in older men. Age Ageing 37: 680– 684, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Holmes MJ, Cotter LA, Arendt HE, Cass SP, Yates BJ. Effects of lesions of the caudal cerebellar vermis on cardiovascular regulation in awake cats. Brain Res 938: 62– 72, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol 86: 1552– 1560, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res 143: 463– 469, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Kerman IA, Emanuel BA, Yates BJ. Vestibular stimulation leads to distinct hemodynamic patterning. Am J Physiol Regul Integr Comp Physiol 279: R118– R125, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol 275: R824– R835, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kerman IA, Yates BJ, McAllen RM. Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol 279: R109– R117, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Macpherson JM, Everaert DG, Stapley PJ, Ting LH. Bilateral vestibular loss in cats leads to active destabilization of balance during pitch and roll rotations of the support surface. J Neurophysiol 97: 4357– 4367, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibres supplying the saphenous vein in the cat. J Physiol 474: 233– 243, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188: 175– 186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 563: 925– 943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monahan KD, Ray CA. Vestibulosympathetic reflex during orthostatic challenge in aging humans. Am J Physiol Regul Integr Comp Physiol 283: R1027– R1032, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Mori RL, Cotter LA, Arendt HE, Olsheski CJ, Yates BJ. Effects of bilateral vestibular nucleus lesions on cardiovascular regulation in conscious cats. J Appl Physiol 98: 526– 533, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y, Matsuo S, Hosogai M, Kawai Y. Vestibular control of arterial blood pressure during head-down postural change in anesthetized rabbits. Exp Brain Res 194: 563– 570, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ray CA. Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol 279: H2399– H2404, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation 105: 956– 961, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Rushmer RF. Cardiovascular Dynamics Philadelphia: Saunders, 1976 [Google Scholar]
- 26.Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol Heart Circ Physiol 272: H1780– H1784, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Starling EH. The Linacre Lecture on the Law of the Heart London: Longmans, Green, 1918 [Google Scholar]
- 28.Tanaka K, Abe C, Awazu C, Morita H. Vestibular system plays a significant role in arterial pressure control during head-up tilt in young subjects. Auton Neurosci 148: 90– 96, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Thompson FJ, Barnes CD, Wald JR. Interactions between femoral venous afferents and lumbar spinal reflex pathways. J Auton Nerv Syst 6: 113– 126, 1982 [DOI] [PubMed] [Google Scholar]
- 30.Voustianiouk A, Kaufmann H, Diedrich A, Raphan T, Biaggioni I, Macdougall H, Ogorodnikov D, Cohen B. Electrical activation of the human vestibulo-sympathetic reflex. Exp Brain Res 171: 251– 261, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol 100: 1475– 1482, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Vestibular inputs elicit patterned changes in limb blood flow in conscious cats. J Physiol 575: 671– 684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res 130: 151– 158, 2000 [DOI] [PubMed] [Google Scholar]