Abstract
Hemorrhagic shock (HS) due to major trauma predisposes the host to the development of acute lung inflammation and injury. The lung vascular endothelium is an active organ that plays a central role in the development of acute lung injury through generating reactive oxygen species and synthesizing and releasing of a number of inflammatory mediators, including leukocyte adhesion molecules that regulate neutrophils emigration. Previous study from our laboratory has demonstrated that in a setting of sepsis, Toll-like receptor-4 (TLR4) signaling can induce TLR2 expression in endothelial cells (ECs), thereby increasing the cells' response to TLR2 ligands. The present study tested the hypothesis that TLR4 activation by HS and the resultant increased TLR2 surface expression in ECs might contribute to the mechanism underlying HS-augmented activation of lung ECs. The results show that high-mobility group box 1 (HMGB1) through TLR4 signaling mediates HS-induced surface expression of TLR2 in the lung and mouse lung vascular endothelial cells (MLVECs). Furthermore, the results demonstrate that HMGB1 induces activation of NAD(P)H oxidase and expression of ICAM-1 in the lung, and MLVECs sequentially depend on TLR4 in the early phase and on TLR2 in the late phase following HS. Finally, the data indicate an important role of the increased TLR2 surface expression in enhancing the activation of MLVECs and augmenting pulmonary neutrophil infiltration in response to TLR2 agonist peptidoglycan. Thus, induction of TLR2 surface expression in lung ECs, induced by HS and mediated by HMGB1/TLR4 signaling, is an important mechanism responsible for endothelial cell-mediated inflammation and organ injury following trauma and hemorrhage.
Keywords: acute lung injury, neutrophil, Toll-like receptors
global ischemia/reperfusion related to resuscitation from hemorrhagic shock (HS) is a major cause of multiorgan failure, in which acute lung injury (ALI) is an important component and often serves as a direct cause of death (18). Resuscitated HS is believed to promote the development of lung injury by priming the immune system for an exaggerated inflammatory response to a second, often trivial stimulus, the so-called “two-hit hypothesis” (41). Previous studies have demonstrated that a period of sustained shock followed by resuscitation leads to augmented lung neutrophil sequestration and lung injury in response to a small dose of intratracheal bacterial cell wall constituent, such as LPS (11, 16). This effect is due, in part, to the increased LPS-stimulated release of cytokines and chemokines from alveolar macrophages (11, 16). From another aspect, the lung vascular endothelium is a multifunctional cell monolayer that plays important roles in the regulation of vascular tone, coagulation, and fibrinolysis, as well as immune and inflammatory responses (6, 25, 37). Therefore, lung endothelial cell (EC) activation is critically involved in the development of ALI. However, the mechanism underlying HS initiating and enhancing lung EC activation presents a significant gap in our knowledge.
Reactive oxygen species (ROS) have been implicated as important in the pathogenesis of ALI through regulating the expression of a number of inflammatory mediators and activation of signaling pathways (19). The major source of ROS within ECs is the nonphagocytic NAD(P)H oxidase (3), which is composed of membrane-bound gp91phox and p22phox, as well as cytosolic subunits such as p47phox, p67phox, and small GTPase Rac. In addition to these components, ECs also express homologues of gp91phox (Nox2) including Nox1, Nox4, and Nox5. Endothelial NAD(P)H oxidase is activated by many factors including growth factors, cytokines, shear stress, hypoxia, and G protein-coupled receptor agonists (21). It has been reported that HS-induced P-selectin expression in vascular tissue depends on functional NAD(P)H oxidase (1), suggesting that HS is an initial factor for NAD(P)H oxidase, although direct activation of endothelial NAD(P)H oxidase by HS has not been reported.
The accumulation of polymorphonuclear neutrophils (PMN) in the lung vasculature, interstitium, and alveolar space is considered a critical event in ALI and has been the target of various preventative strategies. The lung EC-derived intercellular adhesion molecule-1 (ICAM-1), a counter receptor for the leukocyte β2-integrins LFA-1 and Mac-1 (CD11a/CD18 and CD11b/CD18) (8, 24), plays an important role in the regulation of PMN sequestration. The interaction of ICAM-1 with CD11/CD18 integrins enables PMN to adhere firmly to the vascular endothelium and thereby migrate across the microvascular barrier (53). Studies have shown that HS can activate ECs and induce ICAM-1 expression (20, 38, 54, 63). However, the mechanisms underlying this process have not been fully elucidated.
Toll-like receptors (TLRs), a family of pattern recognition receptors, are now defined as the receptors for recognizing pathogen-associated molecular pattern molecules as well as endogenous molecules released by damaged tissues (“danger signals”) (2, 39). TLR4 and TLR2 sit at the interface of microbial and sterile inflammation by selectively responding to both bacterial products and multiple other endogenous ligands, including hyaluronic acid (56), heparan sulfate (28), fibrinogen (52), heat shock proteins (62), and high-mobility group box 1 (HMGB1) (43, 60, 61). Both inflammation and injury responses in organs subjected to ischemia/reperfusion partially depend on TLR4 (46, 60, 61, 69). Previous studies from both our group and others have demonstrated that ECs express a low level of TLR2, which can be upregulated by TLR4 signaling (9, 26). These studies suggest a mechanism of inducible cellular sensitivity to both exogenous and endogenous stimuli.
HMGB1 was originally identified as a nuclear protein that functions to stabilize nucleosome formation and also acts as a transcription factor that regulates the expression of several genes (36). HMGB1 can be secreted by innate immune cells in response to microbial products or other inflammatory stimuli (64, 66), be released by injured cells, and is known as one of the main prototypes of the emerging damage-associated molecular pattern molecules (39, 50, 68). HMGB1 was initially identified as an inflammatory cytokine that is a late mediator of lethality in sepsis (64, 66). However, recent studies suggest that HMGB1 also acts as an early mediator of inflammation contributing to the development of ALI after trauma/hemorrhage (30, 44, 67) and hepatic injury after liver ischemia-reperfusion (60).
The present study aimed to test the hypothesis that TLR4 activation by HS and the resultant increased TLR2 expression in ECs might contribute to the mechanism underlying the HS-augmented activation of lung ECs. The role of HMGB1-TLR4-TLR2 signaling in HS/resuscitation (HS/R)-augmented activation of lung ECs was addressed. The study shows that HMGB1/TLR4 signaling mediates the HS-induced increase in TLR2 surface expression and decrease in TLR4 surface expression in the lung as well as in mouse lung vascular endothelial cells (MLVECs). These alterations in TLR4 and TLR2 surface expression result in HMGB1-mediated activation of NAD(P)H oxidase and expression of ICAM-1 in MLVECs that is TLR4-dependent in the early phase and switches to being TLR2-dependent in the late phase following HS. More importantly, the HS-induced surface expression of TLR2 contributes to an enhanced activation of MLVECs and augmented pulmonary PMN infiltration in response to the TLR2 agonist peptidoglycan (PGN). Thus, the present study demonstrates a novel mechanism underlying HS-augmented lung inflammation, namely that induction of increased TLR2 surface expression in lung ECs, which is induced by HS/R and mediated by HMGB1 activation of TLR4 signaling, is an important mechanism responsible for EC-mediated inflammation and organ injury following HS/R.
MATERIALS AND METHODS
Materials.
Recombinant HMGB1 was purchased from R&D Systems (Minneapolis, MN). Stimulating activity of the recombinant HMGB1 was confirmed in mouse macrophages by assay of TNF release, with an ED50 of 3–12 μg/ml. Polyclonal neutralizing antibody against HMGB1 prepared as described previously (66) was provided by Dr. K. J. Tracey (Feinstein Institute for Medical Research, North Shore-Long Island Jewish Health System, Manhasset, NY). Polyclonal anti-HMGB1 antibody for Western blot analysis, and kinase assay kits for IRAK4 were purchased from Cell Signaling Technology (Danvers, MA). Polyclonal rabbit anti-IRAK4 antibody and MyD88 homodimerization inhibitory peptide set were purchased from Imgenex (San Diego, CA). Nonimmune rabbit IgG (cat. no. I5006), diphenyleneiodonium, and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO), except where noted.
Hemorrhagic shock and resuscitation.
Male C3H/HeJ mice, which are not responsive to LPS because of a point mutation of tlr4 affecting the TIR domain(45, 47) and control wild-type (WT) C3H/HeOuJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). TLR2 −/− mice were obtained from Dr. Shizuo Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) (55), and C57BL/6 mice that are WT control for TLR2−/− mice were purchased from the Jackson Laboratory. All experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of Veterans Affairs Pittsburgh Healthcare System. Mice were 12–14 wk of age at the time of experiments and were maintained on standard rodent chow and water ad libitum. The mice were not fasted before experiments. Animals were anesthetized with 50 mg/kg of ketamine and 5 mg/kg of xylazine via intraperitoneal administration. Femoral arteries were cannulated for monitoring of mean arterial pressure, blood withdrawal and resuscitation. HS was initiated by blood withdrawal and reduction of the mean arterial pressure to 30 mmHg within 20 min. Blood was collected into a 1-ml syringe and heparinized to prevent clotting. To exclude the effect of heparin on immune processes, equal amounts of heparin (10 units) were injected into sham animals through the cannulated femoral artery during the sham operation. After a hypotensive period of 2 h, animals were resuscitated by transfusion of the shed blood and Ringer's lactate in a volume equal to that of shed blood over a period of 60 min. The catheters were then removed, the femoral artery was ligated, and the incisions were closed. Sham animals underwent the same surgical procedures without hemorrhage and resuscitation. In some experiments, neutralizing antibodies against HMGB1 (600 μg per mouse) or nonimmune control IgG was injected intraperitoneally into the mice 10 min before hemorrhage, respectively. The animals were kept anesthetized during the whole experiment period by xylazine and ketamine. At various time points after resuscitation (0 to 8 h) whole lung tissue was harvested for Western blot and RT-PCR analysis.
MLVEC isolation and characterization.
MLVECs were isolated by using a previously described method (23, 57) but modified in our laboratory. Briefly, mice were anesthetized with 50 mg/kg of ketamine and 5 mg/kg of xylazine ip. The chest cavity was opened, and the right ventricle was cannulated. PBS was infused to remove blood from lungs. Peripheral lung tissue diced into a size about 1 mm3 were prepared and cultured in a 60-mm culture dish in growth medium (MEM D-Val medium containing 2 mM glutamine, 10% FBS, 5% human serum, 50 μg/ml penicillin/streptomycin, 5 μg/ml heparin, 1 μg/ml hydrocortisone, 80 μg/ml endothelial cell growth supplement from bovine brain, 5 μg/ml amphotericin, and 5 μg/ml mycoplasma removal agent) at 37°C with 5% CO2 for 60 h. The adherent cells were continued to culture for 3 days after removal of the tissue dices, followed by a purification procedure with biotin-conjugated rat anti-mouse CD31 (PECAM-1) monoclonal antibody and BD IMag streptavidin particles plus-DM, and the immunomagnetic separation system (BD Biosciences Pharmingen, San Diego, CA), following the manufacturer's instruction. The cells were allowed to grow for 3 to 4 days after purification. The cells were characterized by their cobblestone morphology, uptake of Dil-Ac-LDL (Biomedical Technologies, Stoughton, MA), and staining for factor VIII-related antigen (Sigma, St. Louis, MO). The MLVEC passaged between 3 and 5 times were used in experiments in which cells were treated with HMGB1 (0.5 μg/ml) for 30 min, and washed with HBSS three times. At various time points (0 to 8 h) after HMGB1 treatment, the cells were harvested for further analysis. In experiments carried out to confirm the role of MyD88 and NF-κB in the mechanism of HMGB1-induced TLR2 upregulation, NAD(P)H oxidase activation, and ICAM-1 expression, the MLVECs were preincubated with either MyD88 inhibitory peptide (100 μM; Imgenex, San Diego, CA) (35) or NF-κB inhibitor IKK-NBD (100 μM; Biomol, Plymouth Meeting, PA) (40) for 2 h before HMGB1 treatment.
Immunoprecipitation and detection of phosphorylated p47phox.
Mouse lung tissue or MLVECs were homogenized or lysed (∼1 × 106 cells/ml) in lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM NaF, 1 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 20 mM PMSF). The supernatants were then immunoprecipitated with anti-p47phox antibody as described (42). The immunoprecipitated proteins were separated on a 10% SDS-PAGE gel and were then electroblotted onto PVDF membrane and blocked for 1 h at room temperature with Tris-buffered saline containing 3% nonfat dried milk. The membranes were probed with anti-phosphoserine antibody (Invitrogen, South San Francisco, CA) (17, 29) at 1:500 dilution and detected with an horseradish peroxidase-conjugated second antibody at a dilution of 1:3,000 using the enhanced chemiluminesence Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, NJ). The specific binding of anti-phosphoserine antibody was tested using anti-phosphoserine inhibitor (O-phospho-l-serine) (Invitrogen) added in the membrane incubation solution with primary anti-phosphoserine antibody in a concentration of 20 mM (32). Blots were then stripped and reprobed with anti-p47phox antibody to detect total amount of p47phox.
Western blot analysis.
Lung tissue homogenate samples or aliquots of MLVEC lysates were separated on a 10% SDS-PAGE under a nonreducing condition. Equivalent loading of the gel was determined by quantification of protein as well as by reprobing membranes for actin detection. Separated proteins were electroblotted onto PVDF membrane and blocked for 1 h at room temperature with Tris-buffered saline containing 5% nonfat dry milk. The membranes were then probed with a 1:500 dilution of either a purified antibody against mouse TLR2, TLR4 (R&D Systems), or ICAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA) at room temperature for 1 h. After being washed, primary antibodies associated with the membranes were detected on autoradiographic film by horseradish peroxidase-conjugated secondary antibodies and the Enhanced Chemiluminesence Plus System (Amersham, Arlington Heights, IL), according to the manufacturer's instructions. Blots were quantified using Scion (Frederick, MD) image software and normalized by actin signal.
Measurement of superoxide generation in live MLVECs.
Live MLVECs that were cultured in 12-well cell culture plate were stained with the cell-permeable ROS detection reagent H2DFFDA (Invitrogen Molecular Probes, Carlsbad, CA) in the concentration of 10 μM for 10 min. The cells were then washed with HBSS for three times followed by incubation in the growth medium in the presence or absence of HMGB1 (0.5 μg/ml) for 8 h. The ROS production was then detected by fluorescence microscopy at different time points.
RT-PCR.
Total RNA from the lung tissue and MLVECs was isolated using TRI-reagent (Molecular Research Center, Cincinnati, OH) following the manufacturer's instruction. Total RNA was then reverse-transcribed using a SuperScript Preamplification kit (Invitrogen, Carlsbad, CA). Primer pairs for mouse TLR2, TLR4. and GAPDH amplification were purchased from R&D Systems. The product of reverse transcription was amplified following the kit instruction. The amplified product size for TLR2 and TLR4 is 252 bp and 225 bp, respectively, and the PCR products were separated using 1.2% agarose gel and identified by ethidium bromide staining. Expression of mRNA was quantified using Scion Image software and normalized by the GAPDH signal.
Nuclear protein extraction and NF-κb DNA binding assay.
Nuclear protein extracts were prepared from lung tissue or MLVEC by the method of Deryckere and Gannon (7). Aliquots of frozen tissue were ground to powder with a mortar in liquid nitrogen. The thawed powder or 1× 107 cells were homogenized in a Dounce tissue homogenizer with 4 ml of solution A (0.6% Nonidet P-40, 150 mM NaCl, 10 mM HEPES, pH 7.9, 1 mM EDTA, and 0.5 mM PMSF). The cells were lysed with five strokes of the pestle followed by briefly centrifuging at 2,000 rpm for 30 s to pellet debris. The supernatant was incubated on ice for 5 min, and centrifuged for 10 min at 5,000 rpm. Nuclear pellets were then resuspended in 300 μl of solution B (25% glycerol, 20 mM HEPES, pH 7.9, 420 mM NaCl, 1.2 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 2 mM benzamidine, 5 μg/ml pepstatin, 5 μg/ml leupeptin, and 5 μg/ml aprotinin) and incubated on ice for 20 min. The mixture was centrifuged at 14,000 rpm for 1 min. Supernatants containing nuclear proteins were quantified using the protein assay dye reagent (Bio-Rad, Hercules, CA). Nuclear NF-κB binding capacity was measured with the nonradioactive NF-κB-p65-DNA binding assay kit (Chicago West Group, Lisle, IL) following the manufacturer's instruction.
IRAK4 kinase assay.
Equal amounts of lung tissue homogenate supernatant were incubated with polyclonal rabbit anti-IRAK4 antibody for 2 h at 4°C on a rotor, after which 50 μl of 50% protein G plus agarose was added to each sample and incubated for an additional 2 h at 4°C. The samples were precipitated in a microcentrifuge, and the beads were washed twice with lysis buffer and twice with kinase buffer following the kit instruction. The beads were incubated at 25°C for 30 min in a final volume of 37.5 μl of kinase buffer in the presence of Biotinylated ezrin/radixin/moesin peptide as a substrate (1.5 μM/sample) and 200 μM ATP, both of which were provided in the HTScan IRAK4 kinase assay kit (Cell Signaling Technologies). After adding 50 μl/sample stop buffer (50 mM EDTA, pH 8), 25 μl of each reaction and 75 μl dH2O were transferred to 96-well streptavidin-coated plate (PerkinElmer Life Sciences, Waltham, MA) and incubated at room temperature for 60 min. IRAK4 activity was then measured following the manufacturer's instructions for the kit by using primary anti-phospho-ezrin/radixin/moesin antibody (Cell Signaling Technologies) and secondary Europium labeled anti-rabbit antibody with Delfia enhancement solution (PerkinElmer Life Sciences). Fluorescence emission at 615 nm was detected with SpectraMax M2 Multidetection reader (Molecular Devices, Sunnyvale, CA).
Statistics.
The data are presented as means ± SE of the indicated number of experiments. Statistical significance among group means was assessed by ANOVA. Student Neuman-Keuls post hoc test was performed. Differences were considered significant at P < 0.01.
RESULTS
HS/R modulates TLR2 and TLR4 expression in the lung and MLVECs through HMGB1-TLR4 signaling.
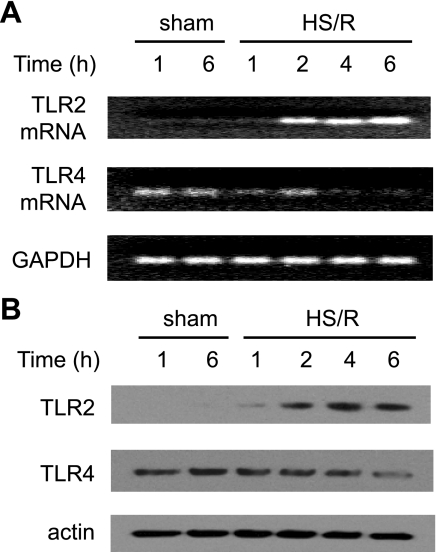
HS/R caused an increase in TLR2 mRNA and protein expression in the lung at 2 h and a more marked increase at 4 and 6 h, compared with the sham group (Fig. 1). By contrast, HS/R induced a decrease in TLR4 mRNA and protein expression starting at 4 h following HS/R (Fig. 1).
Fig. 1.
Heat shock resusitation (HS/R) regulation of expression of Toll-like receptor-2 (TLR2) and TLR4 in the lung. Wild-type (WT; C3H/HeOuJ) mice were subjected to HS/R or sham operation. Lung tissue was harvested 1 to 6 h after resuscitation or sham operation, and TLR2 and TLR4 mRNA (A) and protein (B) expression in the lung were detected by RT-PCR and Western blot analysis, respectively. The blots are representative of 3 independent studies; n = 3 mice/group.
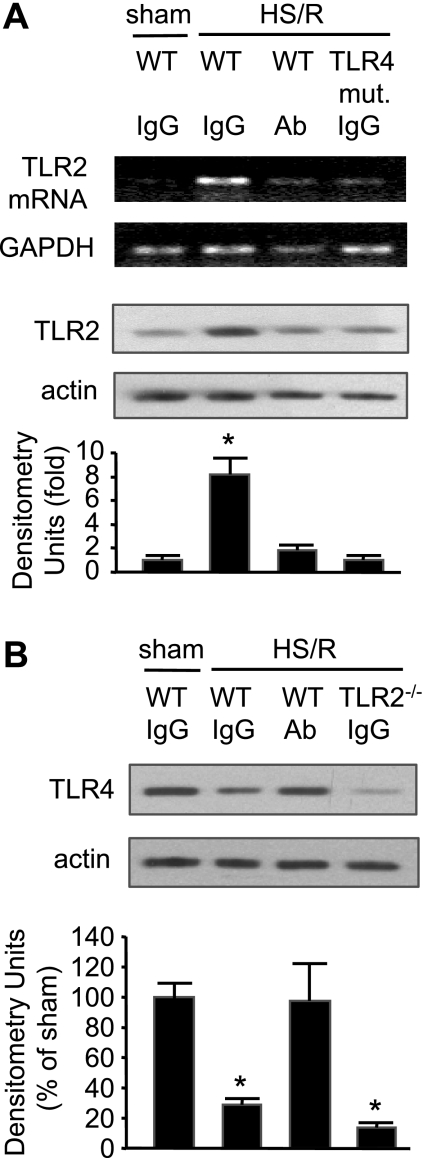
Our previous studies have shown that TLR4 signaling plays an important role in mediating HS/R-induced activation of innate immunity (10, 14, 46). To address the role of TLR4 in HS/R-induced TLR2 expression, TLR4-mutant C3H/HeJ mice were subjected to HS/R, and TLR2 expression in the lung was detected. As shown in Fig. 2, HS/R failed to induce the TLR2 upregulation in TLR4-mutant mice at 4 h after resuscitation, indicating an essential role for functional TLR4 in HS/R-induced TLR2 upregulation.
Fig. 2.
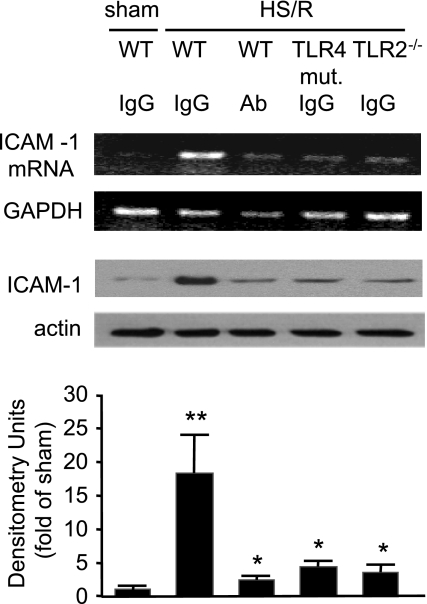
Role of high-mobility group box 1 (HMGB1) in mediating HS/R regulation of TLR2 and TLR4 expression in the lung. A: pretreatment with neutralizing antibody to HMGB1 or TLR4 mutation (mut.) prevents HS/R-induced TLR2 upregulation in the lung. Anti-HMGB1 antibody (Ab; 600 μg per mouse) or nonspecific control IgG was given intraperitoneally to WT (C3H/HeOuJ) or TLR4-mutant mice 10 min before hemorrhage or sham operation. Lung tissue was then collected from the mice at 4 h after HS/R or sham operation for detection of TLR2 mRNA and protein expression using RT-PCR and Western blot analysis, respectively. Images are representative of 3 independent studies. The graph depicts the means ± SE of the fold changes in TLR2 protein expression. *P < 0.01 compared with other groups. B: pretreatment with neutralizing antibody to HMGB1 prevents HS/R-induced TLR4 downregulation in the lung. Anti-HMGB1 antibody or nonspecific control IgG was given to WT (C57BL/6) or TLR2−/− mice 10 min before HS or sham operation, as described in A. Lung tissue was then collected from the mice at 4 h after HS/R or sham operation for detection of TLR4 expression using Western blot analysis. The blots are representative of 3 independent studies. The graph depicts the means ± SE of the %changes in TLR4 expression from 3 mice. *P < 0.01 compared with sham group.
TLR4 recognizes a variety of endogenous ligands including HMGB1 (36, 51). A previous report has shown that HS/R causes a significant increase of HMGB1 in the serum, lung, and liver at 2 h after HS/R (13). To determine whether endogenous HMGB1 contributes to HS/R-induced TLR2 expression in the lung, neutralizing antibody to HMGB1 was administered to mice 10 min before HS/R. Treatment with anti-HMGB1 antibody prevented the HS/R-induced increase in TLR2 mRNA and protein and decrease in TLR4 protein in the lung compared with nonspecific IgG-treated animals (Fig. 2). Furthermore, while TLR4 mutation attenuated the HS/R-induced TLR2 expression, TLR2 deficiency could not prevent HS/R-induced TLR4 downregulation (Fig. 2). Compared with the WT sham/IgG group, no changes in the TLR2 and TLR4 expression were found in the WT sham/HMGB1 Ab, TLR4-mutant sham/IgG, and TLR2−/− sham/IgG groups (data not shown). These results suggest that HMGB1 regulates TLR2 and TLR4 expression through TLR4 but not TLR2.
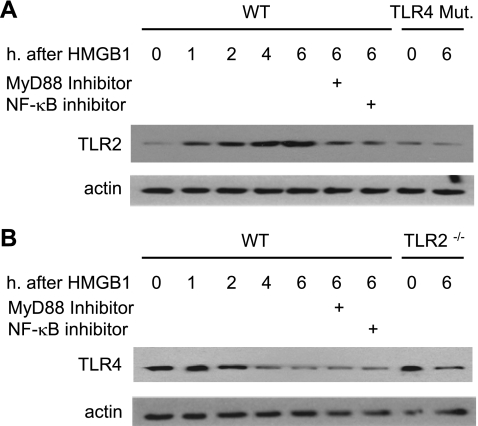
Lung vascular ECs are one of the major pulmonary cell populations and the most important source of lung ICAM-1. Since in vivo experiments have shown a role of HMGB1 in mediating HS/R-induced increase in TLR2 expression in the lung, we next isolated the MLVECs to directly address the role of HMGB1 activation of TLR4 signaling in inducing TLR2 surface expression in lung ECs. MLVECs were isolated from WT (C3H/HeOuJ) and TLR4-mutant mice, treated with HMGB1 for 0 to 6 h, and assessed for TLR2 surface expression. HMGB1 increased TLR2 surface expression in WT MLVECs at as early as 1 h, and reached a peak at 4 h (Fig. 3A). However, HMGB1 failed to induce an increase in TLR2 surface expression in TLR4-mutant MLVECs.
Fig. 3.
Dynamic changes in TLR2 and TLR4 in mouse lung vascular endothelial cells (MLVECs) in response to HMGB1. MLVECs were isolated from TLR4-mutant, TLR4 WT (C3H/HeOuJ), TLR2−/−, and TLR2 WT (C57BL/6) mice and incubated with recombinant HMGB1 (0.5 μg/ml) for up to 6 h; TLR2 and TLR4 expression in the MLVECs were then assessed using Western blot analysis. In some experiments, MyD88 inhibitory peptide (100 μM) or NF-κB inhibitor IKK-NBD (100 μM) was added to WT MLVECs 2 h prior to HMGB1. The images are representative of 3 independent studies.
Previous studies have shown that activation of TLR4 can signal through both MyD88-dependent and MyD88-independent pathways (22). To determine whether TLR2 expression induced by HMGB1 activation of TLR4 in the MLVECs is a MyD88-dependent event, the MyD88 inhibitor homodimerization inhibitory peptide (35) was applied. MLVECs isolated from TLR4 WT mice were preincubated with MyD88 inhibitory peptide (100 μM) for 2 h and subsequently treated with HMGB1 for 6 h. As shown in Fig. 3A MyD88 inhibitor significantly attenuated the effect of HMGB1 on TLR2 induction in the MLVECs, suggesting that HMGB1/TLR4 induces TLR2 expression through a MyD88-dependent pathway.
To address the role of NF-κB in mediating HMGB1-induced upregulation of TLR2, the effects of IKK-NBD, an NF-κB inhibitor (5), were assessed on the TLR2 expression in MLVECs. The NF-κB inhibitor (100 μM) partially, but notably, attenuated the HMGB1-induced increase in TLR2 expression in WT MLVECs at 6 h after stimulation with HMGB1 (Fig. 3A). This result indicates an important role for NF-κB signaling in mediating TLR4-TLR2 interaction.
TLR4 surface expression in the MLVECs that were isolated from TLR2−/− and WT (C57BL/6) mice was also evaluated as shown in Fig. 3B. HMGB1 decreased TLR4 surface expression in WT MLVECs, as well as in TLR2−/− MLVECs, excluding a role for TLR2 in mediating the HMGB1-induced decrease in TLR4 surface expression. Inhibitors for MyD88 and NF-κB also did not alter the decreased expression of TLR4 in response to HMGB1 stimulation as well (Fig. 3B).
TLR4 and TLR2 sequentially mediate HS/R-induced activation of lung ECs.
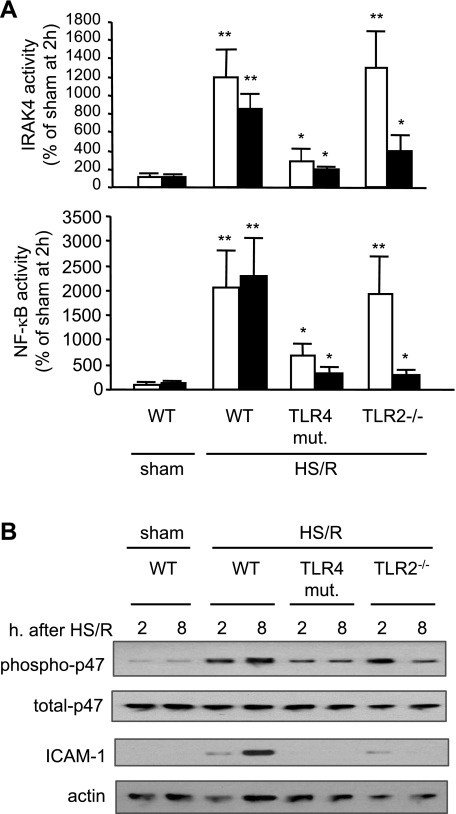
The role of TLR2 and TLR4 in HS/R-induced lung EC activation was addressed. The TLR2- and TLR4-dependency in HS/R-activated IRAK4, NF-κB, NAD(P)H oxidase, and ICAM-1 expression in the lung was measured at 2 h (representing an early phase) and 8 h (representing a late phase). As shown in Fig. 4A, HS/R induced a ∼12-fold increase in IRAK4 activity and a ∼20-fold increase in NF-κB nuclear translocation in the lung at 2 h after HS/R, respectively, and sustained these increases for at least 8 h in WT mice. However, TLR4 mutation significantly attenuated the inductions at both early and late time points. In TLR2−/− mice, a marked increase in HS/R-induced activities of IRAK4, and NF-κB was observed in the early phase, but not in the late phase. The changes in NAD(P)H oxidase activation, in the form of p47phox phosphorylation, and ICAM-1 expression in the lung were consistent with the changes in IRAK4 and NF-κB (Fig. 4B). These results indicate that TLR4 is required for early response of the cells and induction of TLR2 surface expression following HS/R; whereas, increased TLR2 surface expression is critical for a sustained and enhanced response to a HS/R insult.
Fig. 4.
Time-dependent alteration in TLRs dependency of lung activation following HS/R. WT (C3H/HeOuJ), TLR4-mutant, and TLR2−/− mice were subjected to HS/R or sham operation. Lung tissue was harvested at 2 and 8 h after resuscitation or sham operation, and activities of IRAK4 and nuclear NF-κB (A) as well as phosphorylation of p-47phox and expression of ICAM-1 (B) in the lung were detected. The graph depicts the means ± SE of the %changes, and the white bars and the black bars represent 2 h and 8 h time points, respectively. The graphs and images are representative of 3 independent studies; n = 3 mice/group. *P < 0.01 compared with sham group; **P < 0.01 compared with other groups.
To confirm that the HS/R-induced ICAM-1 expression is mediated by HMGB1, WT (C3H/HeOuJ) mice were pretreated with neutralizing antibody against HMGB1 before HS/R. As shown in Fig. 5, neutralizing antibody against HMGB1 markedly attenuated HS/R-induced ICAM-1 expression in the lung at an 8-h time point compared with that in WT shock mice treated with nonspecific IgG. In the experiments shown in Figs. 4 and 5, only C3H/HeOuJ mice were used to reduce the usage of WT control mice based on our finding that there was no statistical significance in the changes of multiple parameters, including those shown in Fig. 4, between C3H/HeOuJ and C57BL/6 mice in response to HS/R (data not shown).
Fig. 5.
HS/R-induced ICAM-1 expression in the lung is mediated by HMGB1 through TLR4 and TLR2. TLR4 mutation, TLR2 deficiency, or pretreatment with neutralizing antibody to HMGB1 prevents HS/R-induced ICAM-1 expression in the lung. Anti-HMGB1 antibody (600 μg/mouse ip) or nonspecific control IgG was given intraperitoneally to WT (C3H/HeOuJ), TLR4-mutant, and TLR2−/− mice 10 min before HS/R or sham operation. Lung tissue was then collected from the mice at 8 h after HS/R or sham operation for detection of ICAM-1 expression using RT-PCR and Western blot analysis, respectively. The graph depicts the means ± SE of the %changes in ICAM-1 expression from 3 mice. The images and graphs are representative of at least 3 independent studies. *P < 0.01 compared with sham group; **P < 0.01 compared with other groups.
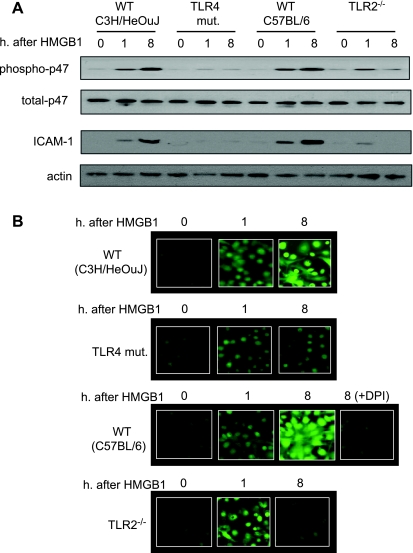
The role of TLR2 and TLR4 in the activation of MLVECs by HMGB1 was also studied in vitro. Figure 6A shows that WT MLVECs treated with HMGB1 for 8 h exhibited a sustained activation of NAD(P)H oxidase and ICAM-1 expression. By contrast, HMGB1 failed to induce a high-level activation of NAD(P)H oxidase and ICAM-1 expression in TLR4-mutant MLVECs. However, in TLR2−/− MLVECs, the HMGB1-induced activation of NAD(P)H oxidase and increased ICAM-1 expression was exhibited in the early phase (1 h) but not in the late phase (8 h) (Fig. 6A).
Fig. 6.
TLR4 and TLR2 sequentially mediate HS/R-induced activation of lung ECs. A: MLVECs were isolated from TLR4-mutant, TLR4 WT (C3H/HeOuJ), TLR2−/−, and TLR2 WT (C57BL/6) mice and incubated with recombinant HMGB1 (0.5 μg/ml) for 0 to 8 h; p47phox phosphorylation and ICAM-1 expression in the MLVECs were then assessed. Treatment with HMGB1 for 8 h exhibited a sustained activation of NAD(P)H oxidase and ICAM-1 expression in the WT MLVECs, but not in TLR4-mutant and TLR2−/− MLVECs. B: reactive oxygen species (ROS) production in live MLVECs. MLVECs that were cultured in 12-well cell culture plate were stained with the cell-permeable ROS detection reagent H2DFFDA in the concentration of 10 μM for 10 min. Cells were then washed with HBSS 3 times followed by incubation in the growth medium in the presence or absence of HMGB1 (0.5 μg/ml) for 8 h. The ROS production was then detected by fluorescence microscopy at different time points as indicated. In some experiments, the NAD(P)H oxidase-specific inhibitor diphenyleneiodonium (DPI) (100 μM) was added to the WT (C57BL/6) MLVECs immediately before the treatment of HMGB1 to specify the source of ROS. Blots are representative of at least 3 independent studies.
To address whether the NAD(P)H oxidase activation is functionally associated with an increase in intracellular ROS production, the ROS in live MLVECs was directly detected using fluorescence microscopy. As shown in Fig. 6B, the changes in ROS production in the MLVEC were consistent with the alterations in NAD(P)H oxidase activation as shown in Fig. 6A. To specify that the ROS is derived from NAD(P)H oxidase, the NAD(P)H oxidase-specific inhibitor diphenyleneiodonium (100 μM) was added to the MLVECs that were isolated from WT C57BL/6 mice immediately before the treatment of HMGB1. As shown in Fig. 6B, diphenyleneiodonium markedly diminished the ROS production in the MLVEC in response to HMGB1, indicating that the observed ROS production is mainly derived from NAD(P)H oxidase.
Increased surface expression of TLR2 mediates HS/R-augmented activation of lung ECs.
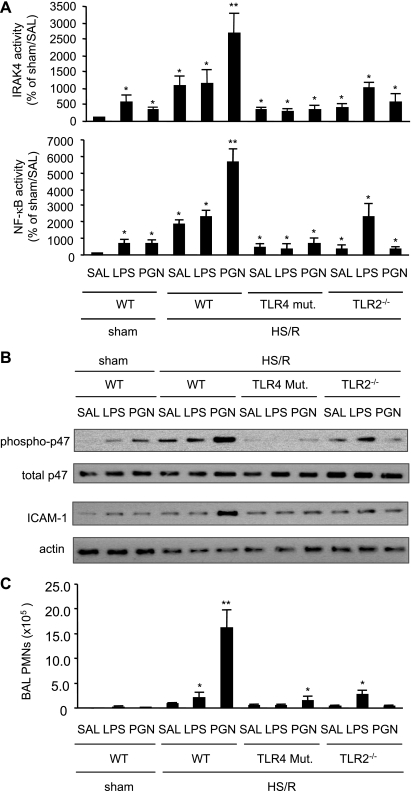
An inducible surface expression of TLR2 may imply inducible cell sensitivity to TLR2 agonist. To elucidate the role of increased surface expression of TLR2 in the HS/R-augmented activation of lung ECs, the effect of a small dose of intratracheal PGN on the lung EC activation was tested. Mice were subjected to HS/R followed by intratracheal PGN (3 μg/kg body wt), LPS (3 μg/kg body wt), or saline at 6 h after resuscitation. Activities of IRAK, NF-κB, and NAD(P)H oxidase as well as ICAM-1 expression in the lung were measured 2 h thereafter. The small dose of either LPS or PGN alone induced a slight increase in the activities of IRAK4 and NF-κB in the sham groups, as shown in Fig. 7A. While HS/R alone caused ∼ 11-fold and ∼19-fold increases in the activities of IRAK4 and NF-κB, respectively, the antecedent HS/R markedly augmented the PGN-induced activation of IRAK4 and NF-κB to a level of ∼27-fold and ∼56-fold, respectively, compared with sham control (Fig. 7A). By contrast, HS/R did not further increase the activities of IRAK4 and NF-κB in response to LPS in a significant manner. Either TLR4 mutation or TLR2 deficiency diminished the HS/R-augmented activation of IRAK4 and NF-κB in response to PGN (Fig. 7A). HS/R also enhanced p47phox phosphorylation and ICAM-1 expression in the lung in response to PGN in WT mice, but not in TLR4-mutant or TLR2−/− mice, as shown in Fig. 7B.
Fig. 7.
Induced TLR2 mediates HS/R-augmented lung activation and inflammation in response to peptidoglycan (PGN). Mice were subjected to HS/R followed by intratracheal PGN (3 μg/kg body wt), LPS (3 μg/kg body wt), or saline (SAL) at 6 h after resuscitation. Activities of IRAK and NF-κB (A), as well as p47phox phosphorylation and ICAM-1 expression (B) in the lung were measured 2 h thereafter. To address the physiological relevance of the altered p47phox phosphorylation and ICAM-1 expression in the lung, PMN in bronchoalveolar lavage fluid were counted 2 h after intratracheal administration of saline, PGN, or LPS (C). The blots and graphs are representative of at least 3 independent studies; n = 3 mice/group. *P < 0.01 compared with control group. **P < 0.01 compared with other groups.
To address the physiological relevance of the altered p47phox phosphorylation and ICAM-1 expression in the lung, PMN in bronchoalveolar lavage (BAL) fluid were counted 2 h after intratracheal administration of saline, PGN, or LPS. As shown in Fig. 7C, HS/R alone induced a small increase in BAL PMN counts. However, while LPS caused a further slight increase in the BAL PMN in WT and TLR2−/− mice, PGN induced an augmented elevation of BAL PMN following antecedent HS/R in WT mice, but not in TLR4-mutant and TLR2−/− mice.
DISCUSSION
The mechanism underlying HS/R-primed ALI is yet unclear. The development of ALI is a cascading event in which macrophages, PMN, ECs, and epithelial cells work in concert. Most previous studies on HS/R-induced ALI focused on the role of macrophages and PMN. For example, our and other reports have demonstrated that the TLR4 upregulation of TLR2 in alveolar macrophages is critical in the pathogenesis of HS/R-induced ALI (14, 15), and HMGB1 is an important mediator causing PMN activation (13) and organ inflammation following trauma and HS (33, 34). However, the role of HMGB1-TLR4 signaling in HS/R initiation and priming of EC activation and subsequent lung inflammation was not reported. The present study showed that HMGB1-TLR4 signaling mediated HS/R-induced expression of TLR2 in the lung and lung ECs, and therefore resulted in prolonged cell activation in response to HS/R as well as augmented cell sensitivity to TLR2 agonist, and consequently, led to exaggerated ROS production, adhesion molecules expression, and pulmonary PMN infiltration. Thus, the present study explores a novel EC-dependent mechanism underlying HS-augmented lung inflammation.
The present study demonstrated a temporal change in TLRs-dependency of lung EC activation following HS/R. The constitutive level of TLR2 in lung ECs is low, but can be induced to a high expression by HS/R. The inducible expression of TLR2 suggests an important physiological significance of TLR-TLR cooperativity, namely that as ligand activation of TLR4 signaling wanes, the signaling functions can be transferred to other TLRs, and thus the TLR-mediated cellular response can be maintained over a prolonged period of time (9, 31). More importantly, the induction of TLR2 surface expression suggests a mechanism by which cell sensitivity to a TLR2 ligand can be amplified in a setting of HS/R, as demonstrated in the results where antecedent HS/R dramatically exaggerated activation of pulmonary cells in response to PGN. Taken together, these observations reveal a new mechanism of HS/R-primed lung inflammation, which would be important when proposing therapeutic strategies for posttrauma ALI.
The role of activation of TLR4 in the induction of TLR2 expression was demonstrated as HS/R caused the increase in TLR2 expression in WT mice but not in TLR4-mutant mice. The results also demonstrate that the TLR4-dependent induction of TLR2 expression is a MyD88- and NF-κB-dependent event as shown in Fig. 3. The role of endogenous TLR4 ligand HMGB1 was defined in the study as well. HMGB1 is increasingly recognized as the prototypic alarmin (65). Evidence indicates that HMGB1 acts as an early inflammatory mediator in ischemia (60, 61), trauma/hemorrhagic shock (13, 44, 67), and noninfectious hepatitis (48). A previous study has shown that direct stimulation of human umbilical vein ECs with HMGB1 could cause ICAM-1 expression (59). However, no role had been ascribed to HMGB1 in HS/R-enhanced ICAM-1 expression and NAD(P)H oxidase activation. The present study shows an important role of HMGB1 in inducing TLR2 and subsequent augmented activation of endothelial NAD(P)H oxidase and expression of ICAM-1 in lung ECs in a setting of HS/R through a TLR4 signaling-dependent mechanism. This mechanism is supported by the findings that a neutralizing antibody to HMGB1 blocks HS/R-induced TLR2 surface expression and ICAM-1 in vivo (Fig. 2, 4, and 5), and that HMGB1 directly induces TLR2 surface expression in WT MLVECs, but not in TLR4-mutant MLVECs (Fig. 3). In addition, it has already been reported that HMGB1 levels in serum, lungs, and liver were significantly increased within 2 h after HS/R in mice (13). However, the mechanism by which HS induces HMGB1 release has not been fully addressed. In our previous study, it has been demonstrated that macrophage β-adrenergic receptor activation by catecholamines serves as an important mechanism for HS/R-induced HMGB1 secretion (34). That study showed that a β-adrenergic receptor antagonist could prevent HS/R-induced increase in serum HMGB1, and epinephrine could directly increase serum HMGB1 in vivo. The study also demonstrated that in vitro direct treatment of alveolar macrophages with epinephrine caused HMGB1 release from the cells, and this effect was suppressed by a β-adrenergic receptor antagonist (34).
HMGB1 endothelial NAD(P)H oxidase is a major source of intracellular ROS, which have an important signaling function (19). ROS in ECs contribute to transcriptional regulation of a number of inflammatory mediators through NF-κB (49), AP-1 (c-Jun and c-Fos) (58), and hypoxia-inducible factor-1α (4), etc. Importantly, ROS mediate stable ICAM-1 expression-dependent endothelial adhesivity, resulting in the arrest of PMN (27). Amplified activation of EC NAD(P)H oxidase and expression of ICAM-1 in response to HS/R and PGN were found to be dependent on increased surface expression of TLR2 that is, in turn, induced by HMGB1-TLR4 signaling (Fig. 7). Thus, the present study explores an important role of shock-induced activation of TLR4 in activating TLR2 signal, which in turn leads to exaggerated lung inflammation.
TLR4 expression in the lung and MLVECs was decreased by HS/R as shown in the results. How HS/R downregulates TLR4 is not clear, but it appears that the downregulation of TLR4 is dependent on HMGB1 but is not mediated by a pathway that involves MyD88, NF-κB, or TLR2 signaling, since suppression of MyD88 and NF-κB activation, as well as TLR2 deficiency, did not prevent the TLR4 decrease in HMGB1-stimulated MLVECs. A previous study from our laboratory has shown that in alveolar macrophages LPS downregulates TLR4 and that HS/R attenuated the effect of LPS (12). This modulation may serve as a mechanism by which the role of TLR4 signaling is prolonged, and the resultant inflammatory response induced by TLR4 signaling is enhanced in HS/R (12). The present study, however, revealed a different mechanism of HS/R regulation of TLR4. Nevertheless, HS either acting as a preconditioning factor to prevent TLR4 decrease in response to LPS or working as a coordinator to transfer the signaling momentum from TLR4 to TLR2 through inducing TLR2 expression in a setting without LPS stimulation is believed to be important in promoting the development of inflammation and organ injury after HS/R.
Perspectives and Significance
The burden of disease related to trauma is considerable. The epidemiology of trauma mortality suggests two major phases. In the early phase, death most commonly results from neurologic injury and/or exsanguination. During the later phase, more than 7 days posttrauma, > 60% of patients die of causes related to the development of systemic inflammatory response syndrome (SIRS). Because of the time lag in the onset of organ dysfunction and subsequent failure, this patient population is well suited to prophylactic interventions aimed at preventing these occurrences. Since ECs play important and broad roles in HS-induced inflammation, an insight of the mechanisms of HS initiation of EC activation will provide us novel targets for preventive and therapeutic interventions of post-HS SIRS and ALI. In a broader sense, the studies in this field will contribute to a greater understanding of the pathogenesis of a number of human inflammatory diseases in which endothelium is involved in the inflammatory process.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-079669 (to J. Fan and M. A. Wilson), National Institute of General Medical Sciences Grant P50-GM-53789 (T. R. Billiar and Y. Vodovotz), a Veterans Affairs Merit Award (to J. Fan), National Key Basic Research Program Grant 2010CB529704 (to Y. Jiang, China), Grant PCSIRT-IRT0731 (to Y. Jiang), National Natural Science Foundation of China (NSFC) Grant 30670828 (to Y. Jiang), and Joint Fund of NSFC with the Guangdong Province Grant U0632004 (to Y. Jiang).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Akgur FM, Brown MF, Zibari GB, McDonald JC, Epstein CJ, Ross CR, Granger DN. Role of superoxide in hemorrhagic shock-induced P-selectin expression. Am J Physiol Heart Circ Physiol 279: H791– H797, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499– 511, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life 50: 267– 269, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1α promoter via a functional NFκB site. Arterioscler Thromb Vasc Biol 27: 755– 761, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bowie A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol 67: 508– 514, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Daniel TO, Abrahamson D. Endothelial signal integration in vascular assembly. Annu Rev Physiol 62: 649– 671, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. Biotechniques 16: 405, 1994 [PubMed] [Google Scholar]
- 8.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 65: 961– 971, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Frey RS, Malik AB. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest 112: 1234– 1243, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J, Frey RS, Rahman A, Malik AB. Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-α-induced NF-κB activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 277: 3404– 3411, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Kapus A, Li YH, Rizoli S, Marshall JC, Rotstein OD. Priming for enhanced alveolar fibrin deposition after hemorrhagic shock: role of tumor necrosis factor. Am J Respir Cell Mol Biol 22: 412– 421, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Kapus A, Marsden PA, Li YH, Oreopoulos G, Marshall JC, Frantz S, Kelly RA, Medzhitov R, Rotstein OD. Regulation of Toll-like receptor 4 expression in the lung following hemorrhagic shock and lipopolysaccharide. J Immunol 168: 5252– 5259, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol 178: 6573– 6580, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am J Physiol Lung Cell Mol Physiol 290: L738– L746, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fan J, Li Y, Vodovotz Y, Billiar TR, Wilson MA. Neutrophil NAD(P)H oxidase is required for hemorrhagic shock-enhanced TLR2 up-regulation in alveolar macrophages in response to LPS. Shock 28: 213– 218, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol 161: 440– 447, 1998 [PubMed] [Google Scholar]
- 17.Fleming IN, Elliott CM, Collard JG, Exton JH. Lysophosphatidic acid induces threonine phosphorylation of Tiam1 in Swiss 3T3 fibroblasts via activation of protein kinase C. J Biol Chem 272: 33105– 33110, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, Petty TL, Hyers TM. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med 98: 593– 597, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Frey RS, Ushio-Fukai M, Malik A. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal 11: 791– 810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez RJ, Moore EE, Ciesla DJ, Nieto JR, Johnson JL, Silliman CC. Post-hemorrhagic shock mesenteric lymph activates human pulmonary microvascular endothelium for in vitro neutrophil-mediated injury: the role of intercellular adhesion molecule-1. J Trauma 54: 219– 223, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494– 501, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Henneke P, Golenbock DT. TIRAP: how Toll receptors fraternize. Nat Immunol 2: 828– 830, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hohler B, Holzapfel B, Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochem Cell Biol 114: 29– 37, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci USA 91: 11641– 11645, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iivanainen E, Kahari VM, Heino J, Elenius K. Endothelial cell-matrix interactions. Microsc Res Tech 60: 13– 22, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol 11: 304– 311, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Issekutz AC, Rowter D, Springer TA. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol 65: 117– 126, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233– 5239, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J 17: 4056– 4065, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JY, Park JS, Strassheim D, Douglas I, Diaz Del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 288: L958– L965, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Latz E, Golenbock DT. Receptor “cross talk” in innate immunity. J Clin Invest 112: 1136– 1137, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine L, Gjika HB, Van Vunakis H. Antibodies and radioimmunoassays for phosphoserine, phosphothreonine and phosphotyrosine. Serologic specificities and levels of the phosphoamino acids in cytoplasmic fractions of rat tissues. J Immunol Methods 124: 239– 249, 1989 [DOI] [PubMed] [Google Scholar]
- 33.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol 293: R1538– R1544, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Yuan Y, Li Y, Zhang J, Xiao G, Vodovotz Y, Billiar TR, Wilson MA, Fan J. Interacting neuroendocrine and innate and acquired immune pathways regulate neutrophil mobilization from bone marrow following hemorrhagic shock. J Immunol 182: 572– 580, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loiarro M, Sette C, Gallo G, Ciacci A, Fanto N, Mastroianni D, Carminati P, Ruggiero V. Peptide-mediated interference of TIR domain dimerization in MyD88 inhibits interleukin-1-dependent activation of NF-κB. J Biol Chem 280: 15809– 15814, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331– 342, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J 6: 2591– 2599, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Matsutani T, Kang SC, Miyashita M, Sasajima K, Choudhry MA, Bland KI, Chaudry IH. Liver cytokine production and ICAM-1 expression following bone fracture, tissue trauma, and hemorrhage in middle-aged mice. Am J Physiol Gastrointest Liver Physiol 292: G268– G274, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Matzinger P. The danger model: a renewed sense of self. Science 296: 301– 305, 2002 [DOI] [PubMed] [Google Scholar]
- 40.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289: 1550– 1554, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am 75: 257– 277, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Okuyama M, Sakon M, Kambayashi J, Kawasaki T, Monden M. Involvement of protein phosphatase 2A in PKC-independent pathway of neutrophil superoxide generation by fMLP. J Cell Biochem 60: 279– 288, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370– 7377, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. Hmgb1 is markedly elevated within six hours of mechanical trauma in humans. Shock 32: 17– 22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085– 2088, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg 202: 407– 417, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189: 615– 625, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191– 195, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J 10: 2247– 2258, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4: 469– 478, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med 10: 251– 257, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887– 2894, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Smith CW, Rothlein R, Hughes BJ, Mariscalco MM, Rudloff HE, Schmalstieg FC, Anderson DC. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest 82: 1746– 1756, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun LL, Ruff P, Austin B, Deb S, Martin B, Burris D, Rhee P. Early up-regulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression in rats with hemorrhagic shock and resuscitation. Shock 11: 416– 422, 1999 [PubMed] [Google Scholar]
- 55.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165: 5392– 5396, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99– 111, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91: 70– 76, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 22: 1141– 1149, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med 254: 375– 385, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 175: 7661– 7668, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201: 1135– 1143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107– 15112, 2002 [DOI] [PubMed] [Google Scholar]
- 63.van Meurs M, Wulfert FM, Knol AJ, De Haes A, Houwertjes M, Aarts LP, Molema G. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock 29: 291– 299, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248– 251, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol 81: 59– 66, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101: 296– 301, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, Harbrecht BG, Billiar TR, Fink MP. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med 12: 105– 114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeh HJ, 3rd, Lotze MT. Addicted to death: invasive cancer and the immune response to unscheduled cell death. J Immunother 28: 1– 9, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol 173: 7115– 7119, 2004 [DOI] [PubMed] [Google Scholar]