Abstract
The risk for cardiovascular disease (CVD) increases with advancing age; however, the age at which CVD risk increases significantly is delayed by more than a decade in women compared with men. This cardioprotection, which women experience until menopause, is presumably due to the presence of ovarian hormones, in particular, estrogen. The purpose of this study was to determine how age and ovarian hormones affect flow-induced vasodilation in the coronary resistance vasculature. Coronary arterioles were isolated from young (6 mo), middle-aged (14 mo), and old (24 mo) intact, ovariectomized (OVX), and ovariectomized + estrogen replaced (OVE) female Fischer-344 rats to assess flow-induced vasodilation. Advancing age impaired flow-induced dilation of coronary arterioles (young: 50 ± 4 vs. old: 34 ± 6; % relaxation). Ovariectomy reduced flow-induced dilation in arterioles from young females, and estrogen replacement restored vasodilation to flow. In aged females, flow-induced vasodilation of arterioles was unaltered by OVX; however, estrogen replacement improved flow-induced dilation by ∼160%. The contribution of nitric oxide (NO) to flow-induced dilation, assessed by nitric oxide synthase (NOS) inhibition with NG-nitro-l-arginine methyl ester (l-NAME), declined with age. l-NAME did not alter flow-induced vasodilation in arterioles from OVX rats, regardless of age. In contrast, l-NAME reduced flow-induced vasodilation of arterioles from estrogen-replaced rats at all ages. These findings indicate that the age-induced decline of flow-induced, NO-mediated dilation in coronary arterioles of female rats is related, in part, to a loss of ovarian estrogen, and estrogen supplementation can improve flow-induced dilation, even at an advanced age.
Keywords: endothelial nitric oxide synthase, Akt, nitric oxide, ovariectomy
the risk for cardiovascular disease (CVD) and heart failure increase with advancing age; however, sexual dimorphism exists in the chronological development of these risks (22, 47). Although the chronological rate of aging is independent of sex, mechanisms that regulate the cardiovascular system across the lifespan may differ dramatically between men and women. The risk for CVD in men begins to increase at approximately the same age that flow-mediated vasodilation begins to decline (5). Women also exhibit this age-related impairment of flow-mediated vasodilation; however, significant reduction of flow-mediated dilation becomes apparent at the age of menopause, more than a decade later than in men (5). The cardioprotection that women experience until menopause is presumably due to the presence of ovarian estrogen and results in a sex-related delay of the expression of CVD (49).
Chronic estrogen treatment has been shown to enhance endothelial function in a number of vascular beds (27, 31, 39), in part, through a pathway involving activation of Akt/PKB and subsequent phosphorylation of endothelial nitric oxide synthase (eNOS) (3, 10, 15, 43, 44). Endothelium-dependent vasodilation to acetylcholine in the peripheral vasculature is preserved or potentiated with chronic estrogen treatment in male, ovariectomized (OVX) and sham-operated rats (26, 27, 34, 52). A number of studies have shown that chronic estrogen treatment enhances endothelium-dependent vasodilation in large peripheral arteries of postmenopausal women (7, 24, 38).
In contrast to these reports of the cardioprotective effects of estrogen, the Women's Health Initiative (37) and Heart and Estrogen/Progestin Replacement Study (19) found an increased risk for coronary heart disease and stroke in postmenopausal women taking hormone-replacement therapy (HRT) compared with nonusers of HRT. This negative effect of HRT in postmenopausal women in the randomized clinical trials prompted investigations into the disparate results of animal studies, which have found a beneficial effect of chronic estrogen treatment (2, 26, 52). Even in humans, postmenopausal women have shown improved endothelium-dependent function in both small and large coronary arteries after short-term administration of estrogen (11, 13), raising questions as to why long-term HRT increases the risk for coronary heart disease in women after menopause. Discrepancies in the timing of hormone-replacement initiation, type and dose of estrogen/progesterone, and the age and health of women in clinical studies (32) warrants an optimized, pertinent animal model that more closely resembles reproductive aging in women. The present study utilizes a unique model, which allows identification of age-related changes in the coronary resistance vasculature while incorporating interventions such as the loss of ovarian hormones and/or estrogen replacement at appropriate timepoints in the reproductive lifespan.
The purpose of this study was to determine how advancing age, ovariectomy, and estrogen-replacement affect endothelium-dependent, flow-induced vasodilation in the coronary resistance vasculature. We hypothesized that increasing age and lack of ovarian hormones would decrease flow-induced dilation in coronary arterioles. This study specifically investigated whether alterations in nitric oxide (NO) signaling underlie age- and estrogen-mediated changes in endothelium-dependent vasodilation.
METHODS
Animals.
Young (4 mo), middle-aged (12 mo), and old (22 mo) female Fischer-344 rats were obtained from Harlan (Indianapolis, IN). At the time of arrival, rats were maintained with ovaries intact (Intact), ovariectomized (OVX), or ovariectomized + estrogen-replaced (OVE) and housed for 6 to 8 wk postoperatively. Ages at the time of death were 6, 14, and 24 mo for young, middle-aged, and old rats, respectively. All procedures were approved by the Institutional Animal Care and Use Committee at West Virginia University and the University of Florida and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington D.C., rev. 1996). Rats were housed individually at 23°C and were maintained on a 12:12-h light-dark cycle. All rats were fed a phytoestrogen-free rat chow and water ad libitum. Prior to death, at least two complete estrous cycles were monitored in all female rats by daily vaginal smears. At the time of death, 3 ml of blood were collected into chilled tubes containing dipotassium EDTA. Blood samples were centrifuged at 10,000 rpm at 4°C for 5 min, and the plasma was collected and stored at −80°C for later analysis. The ovaries (in intact females), uterus, and cervix were dissected and trimmed of connective tissue and fat to obtain uterine weight (UW). Plasma estradiol was determined with ELISA immunoassay (Estradiol EIA kit, Oxford Biomedical; Oxford, MI).
Surgical procedures.
OVX surgeries were performed as described previously (35). Briefly, rats were anesthetized with isoflurane (3%/oxygen balance). Bilateral dorsolateral incisions were made through the top layer of skin and the underlying muscles were bluntly dissected to locate the ovary and fallopian tube. The fallopian tube was ligated with absorbable suture, and the ovary was removed. The surrounding fat pad was returned to the abdominal cavity, and the musculature and outer layer of skin were sutured. Nonabsorbable suture used to suture the skin was removed 7–10 days following surgery. OVE was performed simultaneously with the OVX procedure. Two 0.05 mg 17β-estradiol 60-day slow-release pellets (Innovative Research, Novi, MI) were implanted subcutaneously near the scapulas.
Microvessel preparation.
Six to eight weeks after surgery, rats were anesthetized (isoflurane 5%/O2 balance) and euthanized by removal of the heart. Coronary arterioles from the left anterior descending artery (LAD) distribution were isolated and cannulated as described previously (42). Arterioles were cannulated on pipettes matched (within 1%) for size and resistance and pressurized at 60 cm H2O (∼45 mmHg). Arterioles unable to hold pressure due to leaks were discarded. Those without leaks were warmed to 37°C and allowed to develop spontaneous tone.
Evaluation of vasodilator responses to intraluminal flow.
Responses to flow were used to determine endothelial responsiveness to intraluminal shear stress. Once steady tone was achieved, arterioles were exposed to graded increases in intraluminal flow at constant pressure by adjusting the height of the fluid reservoirs in equal but opposite directions, thereby creating a pressure difference across the arterioles without altering pressure within the arterioles (21). Diameter measurements were determined in response to pressure differences of 2, 4, 10, 20, 40, and 60 cm H2O, corresponding to physiologically significant flow rates from 5 to 50 nl/s (28).
Responses to Dea-NONOate.
Concentration-response relations to cumulative addition of the nitric oxide donor Dea-NONOate [2-(N,N-diethyl-amino)-diazenolate-2-oxide, sodium salt] (3 × 10−9 M − 1 × 10−4 M) were determined to determine responsiveness of the vascular smooth muscle to NO.
Maximal diameter.
At the conclusion of the experiment, the vessels were washed with Ca2+-free PSS every 15 min for 1 h to obtain maximal passive diameter at 60 cm H2O.
Blockade of nitric oxide synthase and cyclooxygenase.
In a second set of experiments, the contribution of nitric oxide to flow-induced vasodilation was reevaluated in the presence of NG-nitro-l-arginine methyl ester (l-NAME; 1 × 10−5 M), a nonspecific blocker of nitric oxide synthase (NOS). To determine the role of cyclooxygenase (COX) signaling, indomethacin (INDO; 1 × 10−5 M) or a combination of both inhibitors (l-NAME+INDO) was applied to vessels during exposure to flow. Inhibitor incubation was performed for a minimum of 30 min prior to beginning experiments.
mRNA levels.
Arterioles were snap frozen and stored at −80°C. Arterioles were later pulverized in lysate buffer and total RNA was extracted using an aqueous and ethanol filter isolation method (RNAqueous Isolation Kit, Ambion, Austin, TX). Real-time PCR was performed with TaqMan probes (Applied Biosystems, Foster City, CA) specific for rat Akt-1 (Applied Biosystems). Custom TaqMan probes were designed from the published sequences for rat eNOS (eNOS primers at exon 8–9 junction: forward, GTG ACC CTC ACC GAT ACA ACA TAC; reverse, TGT CCG GGT GTC TAG ATC CAT). The fluorescent signal from the probe (FAM-labeled reporter dye; NFQ labeled-quencher dye) was measured by the ABI Prism 7900HT Fast Real-Time PCR system, as described previously (45). Levels of the target sequence and levels of coamplified 18S ribosomal RNA were quantified relative to the cycle number (cycle threshold, CT), at which the target and 18S reach a fixed threshold as described previously (45).
Protein expression.
Segments were dissected off of the LAD (≤150 μm DM; ∼1,000 μm in length) in cold PSS solution (-Albumin) (4°C), snap frozen (four per tube), and stored at −80°C. After addition of 20 μl Price-Laemmli lysis buffer, arterioles were solubilized and sonicated. Protein determination was assessed using NanoOrange (Molecular Probes, Carlsbad, CA). Equal amounts of whole vessel homogenates were electrophoresed on 8% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Following blocking, membranes were incubated with primary antibodies for eNOS (1:1,000) (BD Transduction Laboratories, Lexington, KY), p-Akt serine 473 (1:500), p-eNOS serine 1177 (1:500), Akt (1:1,000), or β-actin (1:1,500) (Cell Signaling Technology, Beverly, MA) overnight (4°C). Antibody binding was assessed by enhanced chemiluminescence (Super Signal; Pierce, Rockford, IL) following incubation with secondary anti-rabbit or anti-mouse antibodies as appropriate (1 h). Exposure was the same for each specific protein across all blots. Densitometric analysis of immunoblot films was performed using National Institutes of Health ImageJ 1.38 × Analysis Software (National Institutes of Health, Bethesda, MD). Data were normalized by expressing p-eNOS, p-AKT, eNOS, and Akt values relative to the β-actin loading control. p-eNOS and p-AKT were expressed relative to the β-actin loading control to distinguish between absolute differences in protein levels in the absence of possible age-related changes to either total eNOS or AKT.
Solutions and chemicals.
Albumin was purchased from USB Chemicals (Cleveland, OH, USA). All other chemicals were purchased from Sigma Chemical (St. Louis, MO).
Data analysis.
Data are expressed as means ± SE. Spontaneous tone was calculated as a percent constriction in relation to maximal diameter as determined by the following equation: Spontaneous tone (%) = [(DM − DT)/DM] × 100, where DM is the maximal diameter recorded at 60 cm H2O and DT is the steady-state baseline diameter recorded at the same pressure. The vasodilator responses to flow and Dea-NONOate are expressed as percent relaxation as calculated by the formula: Relaxation % = [(DS − DB)/(DM − DB)] × 100, where DS is the arteriolar diameter at the respective stage, DB is the diameter recorded immediately prior to initiation of the flow- or concentration-diameter curves, and DM is the maximal diameter for the arteriole. The vasodilator concentration that produced 50% of the maximal relaxation to the Dea-NONOate was designated as the IC50.
Flow-diameter and concentration-diameter curves were evaluated by three-way ANOVA with repeated measures on one factor to detect differences within (flow rate or concentration) and between (experimental groups) factors. Pairwise comparisons were made by post hoc analysis (Bonferroni's) when a significant main effect was found. Two-way ANOVA was used to determine group differences in body weight, heart weight, heart weight/body weight ratio, uterine weight, uterine weight/body weight ratio, spontaneous tone, and maximal diameter. One-way ANOVA was used to compare numeric plasma estradiol concentrations between treatments (Intact, OVX, OVE) within each age group. In some samples, estradiol concentrations were low enough to fall below the limit of detection of the assay (∼10 pg/ml); therefore, uterine weight/body weight ratios and estradiol concentrations were transformed to rank order, and a nonparametric correlation coefficient using Spearman's rho test was calculated to determine whether there was a significant predictive relationship between uterine weight/body weight ratio and plasma estradiol concentration. In all statistical analyses, n indicates the number of animals in each group. Significance was defined as P ≤ 0.05.
RESULTS
Animal characteristics.
Middle-age and old intact rats exhibited a greater body weight and heart weight than young intact rats (Table 1). The ratio of heart weight (HW) to body weight (BW) was lower in middle-aged and old intact rats compared with young intact rats (Table 1). OVX induced a significant increase in BW in young females and decreased HW/BW ratio in young and middle-aged females (Table 1). Estrogen replacement caused a decrease in BW and an increase in HW/BW ratio compared with OVX in all age groups. In middle-aged and old females, BW decreased and HW/BW increased in OVE compared with age-matched intact (Table 1). Serum estradiol measurements were positively correlated with UW/BW ratios (slope = 0.450, P = 0.001, Fig. 1A). Vaginal smears performed for eight consecutive days prior to death revealed that the majority of old intact females are in constant diestrous at the time of death compared with regularly cycling young intact females (Fig. 1B). Subsequent analysis of the estrous cycle at time of death revealed no significant effect of cycle on vascular reactivity (Fig. 1C), which is consistent with findings reported in rat aorta (46). Uterine weight was reduced by OVX in rats of all age groups, whereas uterine weight was increased in OVE rats compared with OVX rats of all age groups (Fig. 1D). Young intact females had greater plasma estradiol compared with middle-aged and old intact females (Fig. 1D). OVX decreased plasma estradiol in young intact, and estrogen-replacement restored estradiol to levels to those of young intact females (Fig. 1D). OVX did not alter plasma estradiol in middle-aged and old females compared with the age-matched intact rats (Fig. 1D). Estrogen replacement increased estradiol in old females compared with old intact rats (Fig. 1D).
Table 1.
Animal characteristics of young, middle-aged, and old intact, OVX, and OVE rats
| Young Intact | Middle-Age Intact | Old Intact | Young OVX | Middle-Age OVX | Old OVX | Young OVE | Middle-Age OVE | Old OVE | |
|---|---|---|---|---|---|---|---|---|---|
| BW, g | 211±3 | 266±4* | 303±4* | 242±5† | 278±6 | 307±6 | 219±6§ | 249±4‡§ | 272±8‡§ |
| n | (30) | (20) | (22) | (19) | (21) | (16) | (15) | (14) | (12) |
| HW, mg | 587±10 | 707±13* | 745±15* | 617±11 | 700±16 | 767±18 | 617±17 | 744±15 | 824±36‡ |
| HW/BW, mg/g | 2.78±0.04 | 2.65±0.03* | 2.47±0.05* | 2.56±0.05† | 2.52±0.04† | 2.50±0.06 | 2.87±0.07§ | 2.99±0.04‡§ | 3.04±0.11‡§ |
Values are expressed as means ± SE; n indicates number of rats. BW, body weight; HW, heart weight.
Indicates significant age-related difference vs. young intact.
Indicates significant OVX effect vs. age-matched intact;
Indicates significant OVE effect vs. age-matched intact.
Indicates significant OVE effect vs. age-matched OVX (P ≤ 0.05).
Fig. 1.
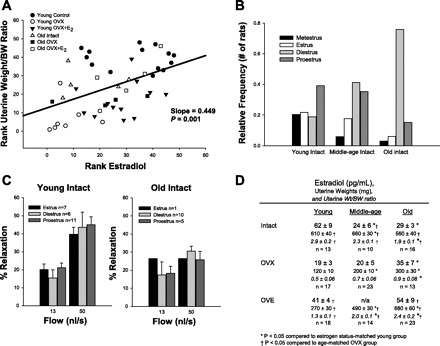
Effects of age, ovariectomy, and estrogen supplementation on estradiol, uterine weight/body weight, and estrous cycle. A: plasma estradiol measurements are positively correlated with uterine weight/body weight ratios (slope = 0.449; P ≤ 0.05). B: relative frequencies of estrous cycle status at time of death in young, middle age, and old intact rats. C: estrous cycle status at time of death does not affect flow vasodilation measurements in young or old intact rats. D: plasma estradiol, uterine weights, and uterine weight/body weight [uterine wt/body weight (BW)] for intact, OVX, and OVE groups. OVX decreased uterine weight in all groups compared with age-matched intact females, whereas uterine weight was increased with estrogen replacement after OVX in all groups. Plasma estradiol was greater in young intact compared with middle-aged and old intact females. OVX decreased estradiol in young rats only. Estrogen replacement after OVX increased estradiol in old females compared with old intact, whereas OVE in young rats brought estradiol levels back to those seen in young intact. Values are expressed as means ± SE. *Significant compared with estrogen status-matched young group, †Significant compared with age-matched OVX group (P ≤ 0.05).
Vessel characteristics.
Maximal diameter was similar among arterioles from all female groups except young OVE rats, in which maximal diameter was reduced (Table 2). Spontaneous tone was not altered by age or changes in estrogen status. Treatment with l-NAME increased tone to a similar degree in arterioles from all groups (Table 2). Indomethacin did not significantly alter tone in arterioles from any group compared with spontaneous tone prior to treatment (Table 2). l-NAME + INDO increased spontaneous tone to a similar degree in coronary arterioles from all groups except for young intact and middle-aged OVX rats (Table 2).
Table 2.
Vessel characteristics of isolated coronary arterioles in young, middle-aged, and old intact, OVX, and OVE rats
| Young Intact | Middle-Age Intact | Old Intact | Young OVX | Middle-Age OVX | Old OVX | Young OVE | Middle-Age OVE | Old OVE | |
|---|---|---|---|---|---|---|---|---|---|
| Maximum diameter, μm | 136±4 | 138±5 | 143±5 | 131±5 | 142±5 | 144±5 | 123±5* | 141±4 | 136±5 |
| n | (31) | (23) | (33) | (28) | (31) | (31) | (23) | (29) | (29) |
| Spontaneous tone, % | 43±3 | 49±3 | 46±3 | 49±4 | 51±3 | 52±3 | 45±5 | 52±3 | 45±3 |
| (31) | (23) | (33) | (28) | (31) | (31) | (23) | (29) | (29) | |
| Pre-l-NAME | 42±3 | 49±4 | 46±4 | 49±4 | 45±3 | 50±4 | 40±6 | 50±3 | 42±4 |
| (14) | (14) | (14) | (16) | (15) | (13) | (14) | (16) | (15) | |
| Post-l-NAME | 54±5† | 59±3† | 67±5† | 62±7† | 70±4† | 68±3† | 60±8† | 63±5† | 63±6† |
| (14) | (14) | (14) | (16) | (15) | (13) | (14) | (16) | (15) | |
| Pre-INDO | 39±5 | 37±3 | 34±6 | 30±5 | 42±4 | 34±7 | 30±3 | 46±5 | 37±4 |
| (5) | (5) | (8) | (8) | (8) | (5) | (6) | (7) | (9) | |
| Post-INDO | 36±4 | 35±3 | 39±5 | 39±5 | 48±5 | 43±4 | 33±4 | 53±4 | 46±6 |
| (5) | (5) | (8) | (8) | (8) | (5) | (6) | (7) | (9) | |
| Pre-l-NAME + INDO | 39±5 | 39±3 | 34±7 | 30±5 | 41±5 | 35±4 | 31±3 | 46±6 | 36±4 |
| (5) | (6) | (7) | (8) | (7) | (7) | (7) | (6) | (11) | |
| Post-l-NAME + INDO | 50±5 | 52±5† | 60±6† | 62±9† | 53±9 | 68±3† | 53±5† | 74±4† | 65±6† |
| (5) | (6) | (7) | (8) | (7) | (7) | (7) | (6) | (11) |
Values are expressed as means ± SE.; n indicates number of vessels.
Indicates significant OVE effect vs. age-matched intact.
Indicates significant inhibitor effect vs. preinhibitor (control) (P ≤ 0.05).
Vasodilator responses to flow.
Flow-induced dilation in coronary arterioles from old females was impaired compared with those from young females (Fig. 2). Flow-induced dilation in arterioles from middle-aged females was intermediate between young and old females but not significantly different from the dilation in arterioles from either young or old rats.
Fig. 2.
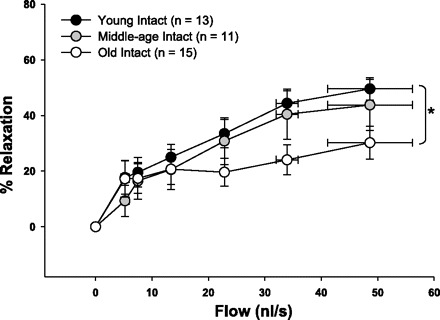
Flow-induced dilation in young, middle-aged, and old intact females. Coronary arterioles from old females dilated less to flow than arterioles from young females. Values are expressed as means ± SE. *Significant age-related difference vs. young intact (P ≤ 0.05).
OVX effect on flow-induced dilation.
Flow-induced dilation of coronary arterioles from young females declined following OVX (Fig. 3A), whereas dilation of coronary arterioles to flow in middle-age OVX females was slightly, but not significantly, decreased compared with intact females of the same age (Fig. 3B). Flow-induced dilation of coronary arterioles was unchanged in old females after OVX (Fig. 3C).
Fig. 3.
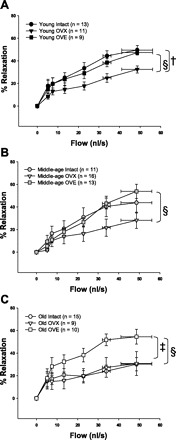
Effects of ovariectomy (OVX) and ovariectomy with estrogen replacement (OVE) on flow-induced vasodilation of coronary arterioles. A: flow-induced dilation is decreased after OVX in young females but restored to intact levels after OVE. B: OVX does not decrease flow-induced dilation in middle-age females, but OVE increases flow-induced dilation compared with OVX. C: OVE increases flow-induced dilation in old females. Values are expressed as means ± SE. †Significant OVX effect vs. intact. ‡Significant OVE effect vs. age-matched intact. §Significant OVE effect vs. OVX (P ≤ 0.05).
OVE effect on flow-induced dilation.
OVE significantly improved dilation of coronary arterioles to flow in all age groups compared with OVX (Fig. 3). In old females, estrogen replacement augmented flow-induced dilation to a level significantly greater than that of arterioles from either intact or OVX rats (Old OVE: 54.5% ± 6.5, Old OVX: 23.2% ± 8.2, Old Intact: 30.2% ± 5.8) (Fig. 3C).
NOS inhibition.
To determine whether NO contributed to flow-induced dilation in coronary arterioles, dilation to flow was assessed in the presence of a nonspecific inhibitor of NOS (l-NAME). In intact females, only arterioles from young rats exhibited a decrease in flow-induced dilation after l-NAME treatment (Fig. 4A), indicating a loss of NO contribution to flow with advancing age. Flow-induced dilation in OVX females of all ages was impervious to prior incubation with l-NAME (Fig. 5A). Conversely, blockade with l-NAME abolished dilation to flow in all OVE females, indicating a reliance on NO-dependent vasodilation after estrogen replacement, regardless of age (Fig. 5B).
Fig. 4.
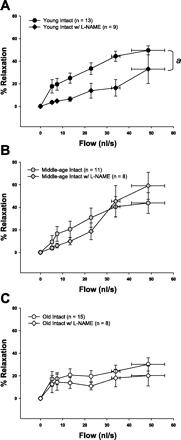
Flow-induced dilation in the presence and absence of l-NAME (1×10−5 M), a nitric oxide sysnthase (NOS) inhibitor, in young, middle-age, and old intact females. Flow-induced dilation of arterioles from young females was reduced by pretreatment with l-NAME (A). l-NAME did not alter flow-induced dilation of arterioles from middle-age (B) or old (C) females. Values are expressed as means ± SE. aSignificant effect of l-NAME vs. control (P ≤ 0.05).
Fig. 5.
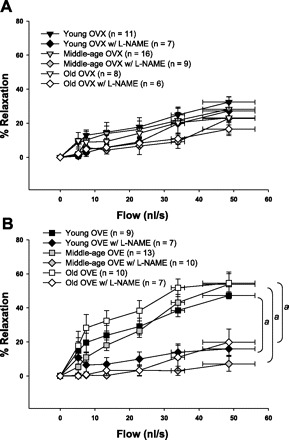
Flow-induced dilation in the presence and absence of l-NAME (1×10−5 M), a NOS inhibitor, in young, middle-age, and old OVX and OVE females. A: l-NAME did not alter flow-induced dilation in OVX females at any age. B: Flow-induced dilation was decreased by l-NAME treatment in coronary arterioles from OVE females in all age groups. Values are expressed as means ± SE. aSignificant effect of l-NAME vs. control (P ≤ 0.05).
COX inhibition.
In arterioles from young and middle-aged rats, indomethacin treatment had no effect on flow-induced dilation, regardless of estrogen status (data not shown). In arterioles from old intact females, indomethacin treatment increased maximal flow-induced vasodilation (Fig. 6), suggesting that a COX-dependent constrictor pathway limits flow-induced dilation at an advanced age (Fig. 6). In contrast, indomethacin reduced flow-induced dilation in arterioles from old OVE females (Fig. 6), suggesting that estrogen replacement increases the contribution of COX-mediated signaling to flow-induced dilation in arterioles from old rats.
Fig. 6.
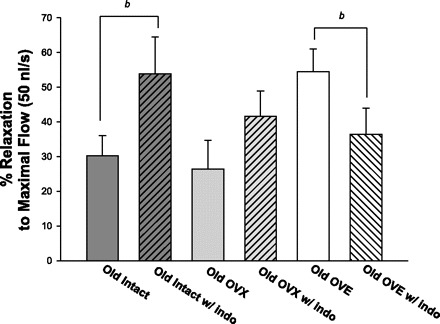
Maximal dilation to flow (occurring at 50 nl/s) in coronary arterioles from old females before and after incubation with indomethacin. Indomethacin treatment increased vasodilation to flow in arterioles from old intact females. In contrast, arterioles from old OVE females exhibited decreased flow-induced dilation after treatment with indomethacin. Values are expressed as means ± SE. bSignificant effect of indomethacin vs. control for maximal vasodilation to flow at 50 nl/s, and for the entire flow-response curve (not shown) (P ≤ 0.05).
Combined NOS and COX inhibition.
In all groups, combined NOS and COX inhibition on flow-induced dilation in coronary arterioles was similar to NOS inhibition alone (data not shown).
Vasodilator responses to DEA-NONOate.
To determine whether the age-related impairment of vasodilation in coronary arterioles was due to a decrease in smooth muscle responsiveness to NO, vasodilation to DEA-NONOate was measured. DEA-NONOate elicited similar dilation in coronary arterioles from young, middle-aged, and old intact females (Fig. 7A). In young and old rats, neither OVX or OVE altered dilation of coronary arterioles to DEA-NONOate (Fig. 7, B and D). Coronary arterioles from middle-aged OVE rats exhibited increased sensitivity to DEA-NONOate compared with those from middle-aged OVX rats (Fig 7C; IC50: Middle-age OVX = 2.31 × 10−6 M, Middle-age OVE = 7.01 × 10−7 M).
Fig. 7.
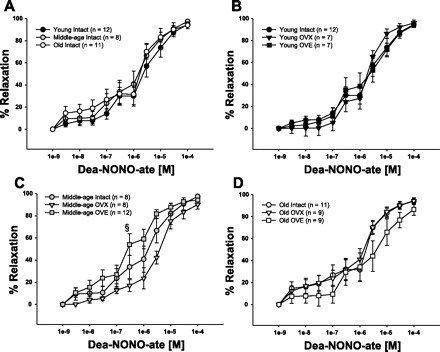
Concentration-response curves to Dea-NONOate, an exogenous NO donor. Vasodilation to Dea-NONOate did not change with advancing age (A). Ovariectomy and ovariectomy with estrogen replacement did not alter vasodilator responses to Dea-NONOate in arterioles from young (B) or old (D) rats. Sensitivity (IC50) in coronary arterioles from middle-aged OVE was greater than those from middle-aged OVX (middle-age OVX: 2.31×10−6 M, Middle-age OVE 7.01×10−7 M) (C). Values are expressed as means ± SE. §Significant OVE effect vs. age-matched OVX (P ≤ 0.05).
AKT and eNOS mRNA levels.
In coronary arterioles from intact females, Akt mRNA expression declined with age (Fig. 8A). OVX decreased (vs. intact), whereas OVE increased (vs. OVX) AKT mRNA in coronary arterioles from both young and middle-aged females (Fig. 8A). In coronary arterioles from middle-aged and old OVX rats, estrogen-replacement increased Akt mRNA expression to levels greater than those of arterioles from age-matched intact rats (Fig. 8A).
Fig. 8.
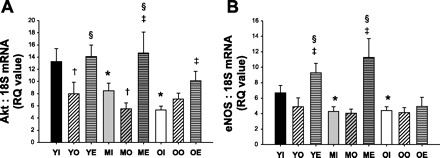
mRNA RQ values for Akt (A) and endothelial NOS (eNOS) (B) for all female groups (n ≥ 8 per group). Young (Y), Middle-age (M), or Old (O), and either Intact (I), OVX (O), or OVE (E). Values are expressed as means ± SE. *Significant age-related difference vs. young intact. †Significant OVX effect vs. age-matched intact. ‡Significant OVE effect vs. age-matched intact. §Significant OVE effect vs. age-matched OVX (P ≤ 0.05).
Similar to Akt mRNA, advancing age also caused a decrease in eNOS mRNA in coronary arterioles from middle-aged and old intact females compared with arterioles from young females; however, OVX did not alter eNOS mRNA in any age group (Fig. 8B). OVE upregulated eNOS mRNA in both young and middle-aged females compared with intact and OVX (Fig. 8B). Surprisingly, OVE did not alter eNOS mRNA in coronary arterioles from old rats (Fig. 8B).
p-eNOS, p-Akt, eNOS, and Akt protein levels.
Basal p-Akt was undetectable in coronary arterioles from all groups. Total Akt and eNOS protein levels of coronary arterioles were not altered by age or changes in ovarian hormones (Fig. 9). Coronary arterioles from young females exhibited no changes in total eNOS protein or p-eNOS after OVX or OVE (Fig. 9B). In middle-aged females, neither OVX nor OVE altered total eNOS protein levels (Fig. 9C); however, there was a 93% increase in p-eNOS after OVE treatment in middle-aged females (Fig. 9C). Similarly, total eNOS did not change with OVX or OVE in coronary arterioles from old females (Fig. 9D), but estrogen replacement increased p-eNOS levels by 45% in arterioles from old OVE females compared with arterioles from old OVX rats.
Fig. 9.
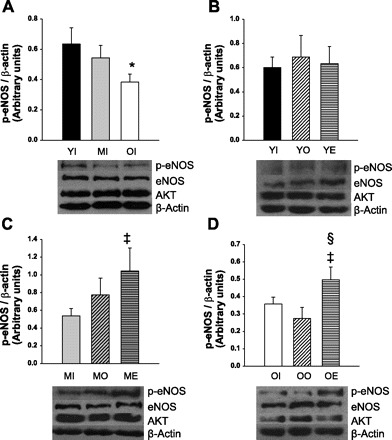
Densitometric analysis of p-eNOS with respective representative blots for total eNOS, Akt, and β-actin below. Phospho-Akt was undetectable in all groups. Levels of total Akt and total eNOS were similar in all age and estrogen treatment groups. There was an age-dependent decrease in phosphorylated-eNOS (p-eNOS) protein (A) (YI: n = 8; MI: n = 4; OI: n = 7). There was no OVX or OVE effect on p-eNOS in arterioles from young females (B) (YI: n = 14; YO: n = 6; YE: n = 6). Middle-aged females exhibited more p-eNOS protein after OVE compared with intact (C) (MI: n = 6; MO: n = 7; ME: n = 5). p-eNOS protein was increased after OVE compared with intact and OVX rats (D) (OI: n = 10, OO: n = 6, OE: n = 6). Young (Y), middle-age (M), or old (O), and either intact (I), OVX (O), or OVE (E). Values are expressed as means ± SE. *Significant age-related difference vs. young intact. ‡Significant OVE effect vs. age-matched intact. §Significant OVE effect vs. age-matched OVX (P ≤ 0.05).
DISCUSSION
Although there are numerous reports in the literature detailing the beneficial influence of estrogen replacement on the vasculature in young animals (4, 10, 14, 41), to our knowledge this is the first study to examine the effects of estrogen supplementation on the coronary vasculature in female rats of an advanced age. The foremost finding of this study is that age reduces flow-induced, NO-mediated vasodilation of coronary arterioles of female rats, and estrogen replacement improves flow-induced dilation in coronary arterioles of old female rats, indicating a favorable coronary endothelial response to estrogen replacement in this aged population. This improvement in endothelium-dependent, NO-mediated dilation is accompanied by an increase in phosphorylation of eNOS protein after estrogen replacement. Congruent with the finding that estrogen replacement improved flow-induced dilation in rats with low levels of circulating estradiol, ovariectomy reduced flow-induced, NO-mediated vasodilation in coronary arterioles from young females, and estrogen replacement restored NO-mediated dilation to flow in coronary arterioles from young and middle-age ovariectomized rats. Together, these findings indicate that the age-related loss of endothelium-dependent dilation to flow in coronary arterioles occurs, in part, as a result of declining levels of ovarian estrogen, and that estrogen supplementation can improve flow-induced dilation, even at an advanced age.
Endothelium-dependent vasodilation has been shown to correlate inversely with age in large epicardial coronary arteries (8). Vasodilation of coronary arteries in response to noradrenaline (29), adenosine (16), and testosterone (9) declines with age. Additionally, impaired endothelium-dependent vasodilation of septal arteries (∼200 μm) in middle-aged male rats has been reported (6); however, much of the current literature regarding aging effects in the coronary vasculature is confined to studies of larger resistance arteries from males. The novelty of this study is the examination of the coronary resistance vasculature at three critical stages in the reproductive lifespan of female rats: young adulthood, reproductive senescence, and senescence. The present study illustrates an age-related impairment in endothelium-dependent dilation in coronary arterioles from female rats, which is associated with a loss of NO-dependent dilation (Fig. 4) and a decrease in phosphorylation of eNOS protein (Fig. 9A). These findings extend previous studies showing that flow-induced dilation in females decreases with advancing age in brachial arteries (5, 33) and mesenteric arteries (1) by demonstrating that age-related endothelial impairment also occurs in resistance arterioles where endothelial responsiveness is critical to flow distribution.
In the present study, young females were the only age group in which OVX decreased flow-induced dilation of coronary arterioles. l-NAME treatment reduced flow-induced dilation of arterioles from young females before OVX, but l-NAME had no effect on the response to flow after OVX. In contrast, l-NAME did not alter flow-induced dilation of arterioles from either intact or OVX middle-age and old females, suggesting that a loss of ovarian hormones, whether induced by ovariectomy or aging, leads to a decline in flow-induced NOS signaling. Estrogen replacement after OVX enhanced flow-induced, NO-mediated dilation of coronary arterioles from all age groups (Fig. 3). Restoration of NO-dependent dilation by estrogen replacement has been observed previously in cerebral arteries (34) and in the coronary microcirculation of guinea pigs (50) at a young age. Additionally, several human studies have shown that HRT in postmenopausal women improves flow-induced dilation in peripheral conduit arteries (7, 24, 38). The current data indicate that estrogen supplementation also improves flow-induced vasodilation in the coronary resistance vasculature at all ages, primarily through enhancement of NOS-mediated signaling.
Vasodilation to flow was unaltered by l-NAME treatment in coronary arterioles from OVX rats. In addition, substantial dilation to flow remained in arterioles from all groups of rats, even during simultaneous inhibition of NOS and COX signaling (data not shown). In the cerebral microcirculation, OVX abolished endothelium-dependent dilation through up-regulation of caveolin-1, a negative regulator of eNOS (34). Xu et al. (51) found that NO-mediated signaling decreased in cerebral arterioles from ovariectomized rats, whereas signaling through KCa2+ channels increased following ovariectomy, suggesting a conversion to dependency on hyperpolarizing factor in the absence of estrogen. Golding and Kepler have shown that in cerebral arteries of control female rats, endothelium-derived hyperpolarizing factor (EDHF)-mediated dilations are negligible but can be enhanced after OVX (12). Additionally, Huang et al. (17) showed that when eNOS was inhibited in murine gracilis arterioles of WT mice, EDHF substitutes for NO-dependent dilation in response to flow (17). We did not directly assess the EDHF contribution to flow-induced dilation of coronary arterioles; however, it seems plausible that a conversion from NO dependency to EDHF dependency of flow-induced dilation may occur when ovarian hormones decline. Future studies are necessary to assess the effects of age and ovarian hormones on the contribution of EDHF to flow-induced vasodilation in coronary arterioles.
Flow-induced dilation was augmented in coronary arterioles from old intact females following pretreatment with indomethacin (Fig. 6), suggesting that COX-dependent vasoconstriction increases with age. Stewart and colleagues have established that a decline in circulating estrogen (2) and advancing age (48) enhances prostaglandin H synthase (PGHS)-2-dependent vasoconstriction in mesenteric arteries while simultaneously decreasing NO-dependent vasodilation. In mesenteric arteries from aged rats, an increase in circulating estrogen improved vasodilation by decreasing PGHS-dependent constriction in mesenteric arteries (2). Similarly, our results indicate that a decrease in COX-dependent vasoconstriction and an increase in COX-mediated vasodilation contributed to the enhancement of flow-induced vasodilation in coronary arterioles that occurred with estrogen supplementation in old rats.
Improvement of NO-mediated vasodilation by estrogen treatment may involve changes in expression and/or regulation of eNOS (4, 10, 15). Numerous reports have documented both the presence (25, 34, 36, 40) and absence (3, 20, 30) of estrogenic modulation of eNOS expression in various vascular beds. The present data show that the removal of ovarian estrogen does not alter eNOS mRNA expression in coronary arterioles at any age; however, eNOS mRNA is upregulated by estrogen replacement in coronary arterioles from young and middle-aged females (Fig. 8B). Surprisingly, but in keeping with findings reported in cerebral microvessels (20), mesenteric arteries (30), and left ventricular tissue (3), neither age nor estrogen status altered total eNOS protein in coronary arterioles. Although total eNOS was not affected by estrogen status, phosphorylation of eNOS protein (serine 1177) was increased in arterioles from middle-aged and old females after estrogen replacement. In coronary arterioles from male rats, we have previously reported that advancing age impairs flow-induced vasodilation through reductions in Flk-1 activation and PI3-kinase/Akt signaling (23). Similar to our findings in coronary arterioles from old male rats, our current results indicate that basal NO remained constant (Table 2) despite changes in NO-mediated dilation that occurred with advancing age and manipulation of circulating estrogen. Thus, our data suggest that changes in NO-mediated signaling that occur with age and alterations of estrogen status in female rats are more likely related to modifications in regulation of eNOS activity rather than adaptations in eNOS expression. Future studies in which phosphorylation of eNOS at other regulatory sites and feedback of NO and reactive oxygen species on eNOS transcription and translation will be needed to provide a more comprehensive understanding of the effects of age and estrogen status on regulation of eNOS expression and function in the coronary endothelium of female rats.
Although molecular evidence shows that Akt is responsive to the presence of estrogen (10, 18, 44), previous studies focused on the acute effects of estrogen exposure rather than the chronic estrogen supplementation. In endothelial cells, acute administration of estrogen causes phosphorylation of Akt within 1 min (10); however, we found that phosphorylated AKT was undetectable in coronary arteriolar samples under basal conditions. Bhuiyan et al. (3) found no changes in phosphorylated Akt or total Akt protein after OVX in left ventricular tissue from female Wistar rats. Although it would seem reasonable that circulating estrogen affects both Akt mRNA and Akt protein similarly, our data indicate that chronic changes in estrogen altered Akt mRNA expression, but not Akt protein levels. The results suggest that estrogen exerts differential effects on transcription, translation, and activation of Akt. Our results also suggest that modifications of Akt mRNA expression induced by alterations of estrogen status are unlikely to be a critical factor in the changes in NO signaling that occur with advancing age or estrogen supplementation.
Perspectives and Significance
The major finding from this study is that estrogen replacement following ovariectomy restores or enhances flow-induced dilation in coronary arterioles from all ages of female Fischer-344 rats. Estrogen replacement reversed the age-related impairment of flow-induced dilation in coronary arterioles from aged females by increasing the contribution of NO to flow-induced dilation. This estrogen-induced increase in NO signaling in arterioles from aged female rats was paralleled by an increase in phosphorylation of eNOS protein. These observations indicate the endothelium of coronary resistance vasculature retains its ability to increase NO signaling in response to elevation of circulating estrogen, even at an advanced age. Thus, estrogen supplementation could remain beneficial to the coronary resistance vasculature of postmenopausal women, depending on the timing of administration and the overall health of the endothelium upon initiation of replacement.
GRANTS
This work was supported by National Institutes of Health Grant R01HL077224.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Charles Wood for performance of plasma estradiol assays. The authors gratefully acknowledge the technical contributions of Yanduan Hu.
REFERENCES
- 1. Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-α antagonism. Am J Physiol Heart Circ Physiol 290: H1259– H1263, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong SJ, Zhang Y, Stewart KG, Davidge ST. Estrogen replacement reduces PGHS-2-dependent vasoconstriction in the aged rat. Am J Physiol Heart Circ Physiol 283: H893– H898, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bhuiyan MS, Shioda N, Fukunaga K. Ovariectomy augments pressure overload-induced hypertrophy associated with changes in Akt and nitric oxide synthase signaling pathways in female rats. Am J Physiol Endocrinol Metab 293: E1606– E1614, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bucci M, Roviezzo F, Cicala C, Pinto A, Cirino G. 17-β-oestradiol-induced vasorelaxation in vitro is mediated by eNOS through hsp90 and akt/pkb dependent mechanism. Br J Pharmacol 135: 1695– 1700, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471– 476, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159– 1166, 2002 [DOI] [PubMed] [Google Scholar]
- 7. de Kleijn MJ, Wilmink HW, Bots ML, Bak AA, van der Schouw YT, Planellas J, Engelen S, Banga JD, Grobbee DE. Hormone replacement therapy and endothelial function. Results of a randomized controlled trial in healthy postmenopausal women. Atherosclerosis 159: 357– 365, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77– 81, 1993 [DOI] [PubMed] [Google Scholar]
- 9. English KM, Jones RD, Jones TH, Morice AH, Channer KS. Aging reduces the responsiveness of coronary arteries from male Wistar rats to the vasodilatory action of testosterone. Clin Sci (Lond) 99: 77– 82, 2000 [PubMed] [Google Scholar]
- 10. Florian M, Lu Y, Angle M, Magder S. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids 69: 637– 645, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gilligan DM, Quyyumi AA, Cannon RO., 3rd Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation 89: 2545– 2551, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Golding EM, Kepler TE. Role of estrogen in modulating EDHF-mediated dilations in the female rat middle cerebral artery. Am J Physiol Heart Circ Physiol 280: H2417– H2423, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Guetta V, Quyyumi AA, Prasad A, Panza JA, Waclawiw M, Cannon RO., 3rd The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation 96: 2795– 2801, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem 280: 19704– 19710, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87: 677– 682, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Headrick JP. Impact of aging on adenosine levels, A1/A2 responses, arrhythmogenesis, and energy metabolism in rat heart. Am J Physiol Heart Circ Physiol 270: H897– H906, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462– H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450–mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res 94: 245– 252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 280: 605– 613, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Koyuncu FM, Ozbilgin K, Kuscu NK, Inan S, Vatansever S, Ceylan E. The effect of oestradiol and neta on immunohistochemical staining of iNOS and eNOS in coronary arteries of ovariectomized rats. Histol Histopathol 21: 367– 371, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kuo L, Davis M, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063– H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 7: 29– 49, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Leblanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280– H2288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med 121: 936– 941, 1994 [DOI] [PubMed] [Google Scholar]
- 25. McNeill AM, Kim N, Duckles SP, Krause DN, Kontos HA. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke 30: 2186– 2190, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Moien-Afshari F, Kenyon E, Choy JC, Battistini B, McManus BM, Laher I. Long-term effects of ovariectomy and estrogen replacement treatment on endothelial function in mature rats. Maturitas 45: 213– 223, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Momoi H, Ikomi F, Ohhashi T. Estrogen-induced augmentation of endothelium-dependent nitric oxide-mediated vasodilation in isolated rat cerebral small arteries. Jpn J Physiol 53: 193– 203, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662– H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Murohara T, Yasue H, Ohgushi M, Sakaino N, Jougasaki M. Age related attenuation of the endothelium dependent relaxation to noradrenaline in isolated pig coronary arteries. Cardiovasc Res 25: 1002– 1009, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, Miwa S, Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol 144: 178– 189, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. New G, Duffy SJ, Harper RW, Meredith IT. Long-term oestrogen therapy is associated with improved endothelium-dependent vasodilation in the forearm resistance circulation of biological males. Clin Exp Pharmacol Physiol 27: 25– 33, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol 47: 1741– 1753, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol 291: H3043– H3049, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Pelligrino DA, Ye S, Tan F, Santizo RA, Feinstein DL, Wang Q. Nitric-oxide-dependent pial arteriolar dilation in the female rat: effects of chronic estrogen depletion and repletion. Biochem Biophys Res Commun 269: 165– 171, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Prisby RD, Muller-Delp J, Delp MD, Nurkiewicz TR. Age, gender, and hormonal status modulate the vascular toxicity of the diesel exhaust extract phenanthraquinone. J Toxicol Environ Health 71: 464– 470, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Rahimian R, Dube GP, Toma W, Dos Santos N, McManus BM, van Breemen C. Raloxifene enhances nitric oxide release in rat aorta via increasing endothelial nitric oxide mRNA expression. Eur J Pharmacol 434: 141– 149, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288: 321– 333, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, D'Anna R, Lasco A, Squadrito G, Gaudio A, Cancellieri F, Arcoraci V, Squadrito F. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol 21: 1512– 1519, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Sakuma I, Liu MY, Sato A, Hayashi T, Iguchi A, Kitabatake A, Hattori Y. Endothelium-dependent hyperpolarization and relaxation in mesenteric arteries of middle-aged rats: influence of oestrogen. Br J Pharmacol 135: 48– 54, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation 111: 3473– 3480, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Shaw L, Taggart MJ, Austin C. Mechanisms of 17β-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br J Pharmacol 129: 555– 565, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374– 383, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538– 541, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simoncini T, Rabkin E, Liao JK. Molecular basis of cell membrane estrogen receptor interaction with phosphatidylinositol 3-kinase in endothelial cells. Arterioscler Thromb Vasc Biol 23: 198– 203, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947– 958, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stallone JN, Crofton JT, Share L. Sexual dimorphism in vasopressin-induced contraction of rat aorta. Am J Physiol Heart Circ Physiol 260: H453– H458, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses' health study. N Engl J Med 325: 756– 762, 1991 [DOI] [PubMed] [Google Scholar]
- 48. Stewart KG, Zhang Y, Davidge ST. Aging increases PGHS-2-dependent vasoconstriction in rat mesenteric arteries. Hypertension 35: 1242– 1247, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576– 582, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Thompson LP, Pinkas G, Weiner CP. Chronic 17β-estradiol replacement increases nitric oxide-mediated vasodilation of guinea pig coronary microcirculation. Circulation 102: 445– 451, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Xu HL, Santizo RA, Baughman VL, Pelligrino DA. Nascent EDHF-mediated cerebral vasodilation in ovariectomized rats is not induced by eNOS dysfunction. Am J Physiol Heart Circ Physiol 285: H2045– H2053, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Yamaguchi K, Honda H, Wakisaka C, Tohei A, Kogo H. Effects of phytoestrogens on acetylcholine- and isoprenaline-induced vasodilation in rat aorta. Jpn J Pharmacol 87: 67– 73, 2001 [DOI] [PubMed] [Google Scholar]


