Abstract
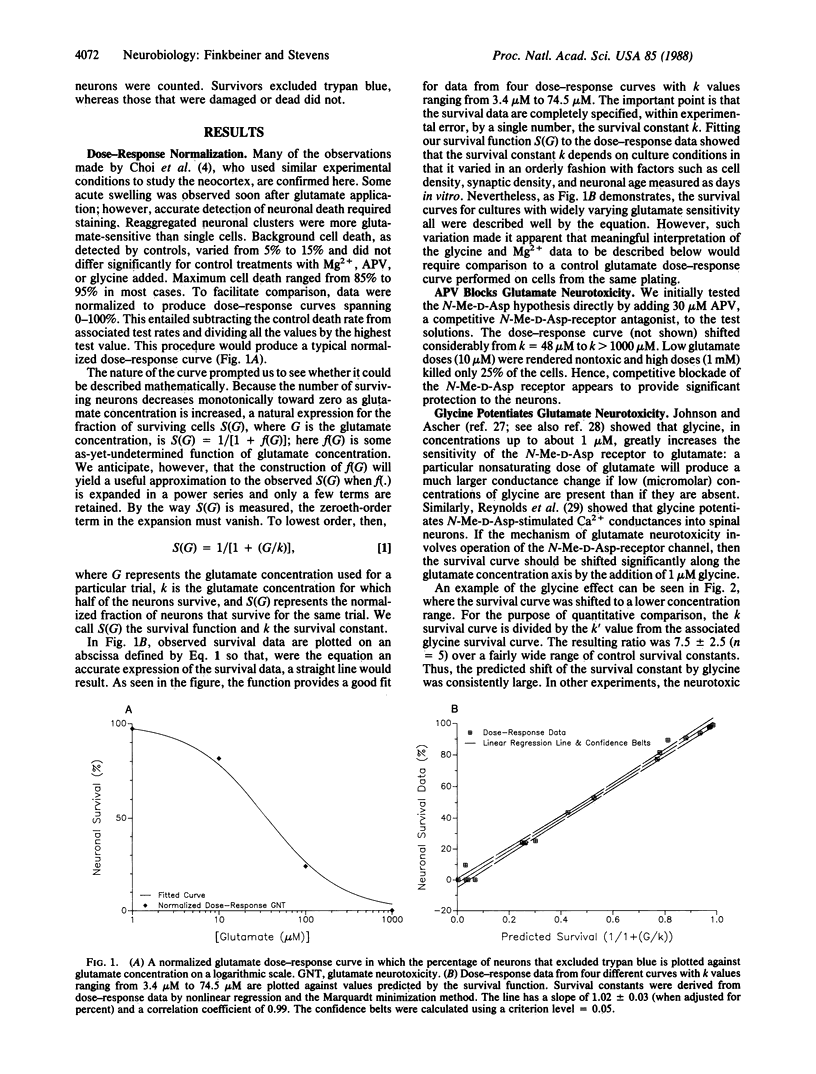
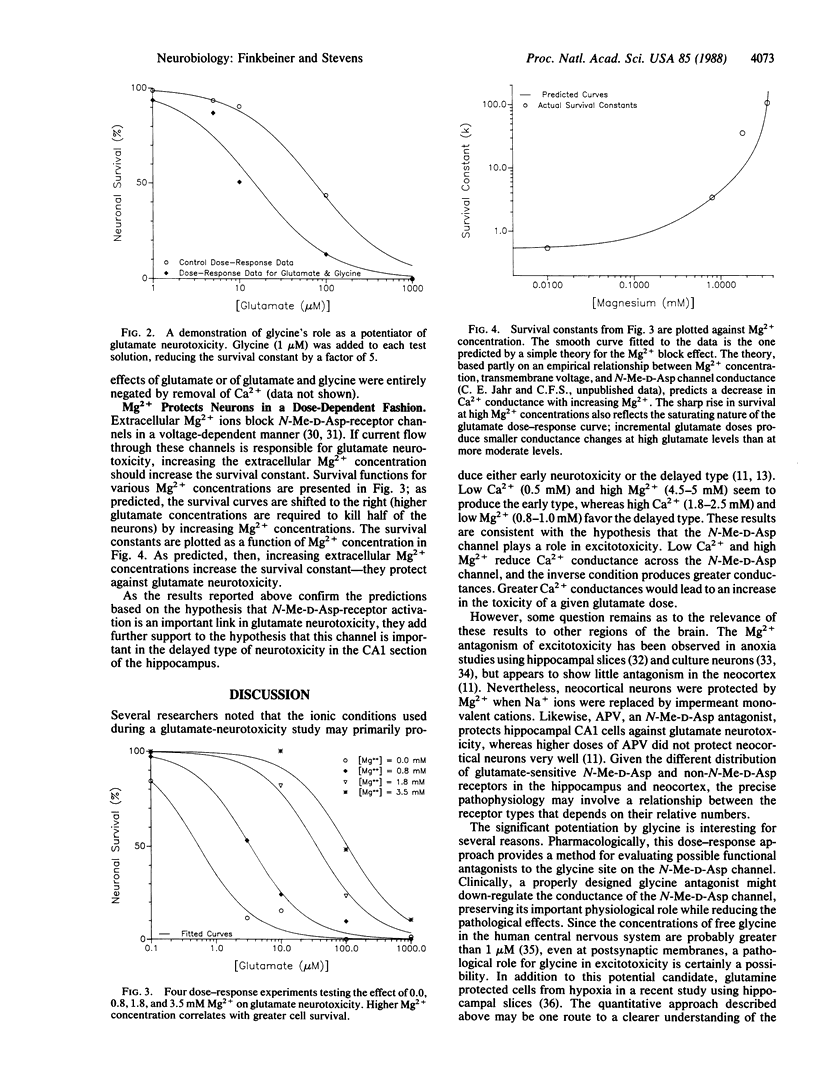
The role of the N-methyl-D-aspartate receptor channel in glutamate neurotoxicity was investigated in cultured hippocampal neurons of the CA1 region. An equation, the survival function, was developed to quantify the effects of putative modulators of neurotoxicity. 2-Amino-5-phosphonovaleric acid (30 microM) reduced the neuronal sensitivity to glutamate by a factor greater than 20, whereas glycine (1 microM) enhanced it by a factor of 7.5 +/- 2.5. Neurons were protected by increasing Mg2+ concentrations in a predictable way based on the ion's ability to block the N-methyl-D-aspartate channel. These findings provide a quantitative basis for the assessment of various neuroprotective agents and add further support to the hypothesis that the N-methyl-D-aspartate channel is central to glutamate neurotoxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi D. W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985 Aug 5;58(3):293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro T. N., Hare T. A. Free and conjugated amino acids in human CSF: influence of age and sex. Brain Res. 1985 Jul 8;338(1):53–60. doi: 10.1016/0006-8993(85)90247-1. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984 Jan;42(1):1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Neurotoxicity of excitatory amino acid receptor agonists in rat cerebellar slices: dependence on calcium concentration. Neurosci Lett. 1986 May 15;66(2):193–198. doi: 10.1016/0304-3940(86)90189-8. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Hajós F., Garthwaite J. Ionic requirements for neurotoxic effects of excitatory amino acid analogues in rat cerebellar slices. Neuroscience. 1986 Jun;18(2):437–447. doi: 10.1016/0306-4522(86)90164-8. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kass I. S., Lipton P. Mechanisms involved in irreversible anoxic damage to the in vitro rat hippocampal slice. J Physiol. 1982 Nov;332:459–472. doi: 10.1113/jphysiol.1982.sp014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Nairn A. C., Greengard P. Autophosphorylation reversibly regulates the Ca2+/calmodulin-dependence of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4253–4257. doi: 10.1073/pnas.83.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., McGeer P. L. Duplication of biochemical changes of Huntington's chorea by intrastriatal injections of glutamic and kainic acids. Nature. 1976 Oct 7;263(5577):517–519. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S. Cell damage in epilepsy and the role of calcium in cytotoxicity. Adv Neurol. 1986;44:849–855. [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Identification and properties of N-methyl-D-aspartate receptors in rat brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7532–7536. doi: 10.1073/pnas.83.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Holets V. R., Toy D. W., Cotman C. W. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Collins R. C., Sloviter R. S. Excitotoxic mechanisms of epileptic brain damage. Adv Neurol. 1986;44:857–877. [PubMed] [Google Scholar]
- Peinado J. M., Mora F. Glutamic acid as a putative transmitter of the interhemispheric corticocortical connections in the rat. J Neurochem. 1986 Nov;47(5):1598–1603. doi: 10.1111/j.1471-4159.1986.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Murphy S. N., Miller R. J. 3H-labeled MK-801 binding to the excitatory amino acid receptor complex from rat brain is enhanced by glycine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7744–7748. doi: 10.1073/pnas.84.21.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S. M., Olney J. W. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986 Feb;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. Synaptic activity mediates death of hypoxic neurons. Science. 1983 Apr 29;220(4596):536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- Rothman S. M., Thurston J. H., Hauhart R. E. Delayed neurotoxicity of excitatory amino acids in vitro. Neuroscience. 1987 Aug;22(2):471–480. doi: 10.1016/0306-4522(87)90347-2. [DOI] [PubMed] [Google Scholar]
- Schurr A., Changaris D. G., Rigor B. M. Glutamine protects neuronal function against cerebral hypoxia: a study using the in vitro hippocampal slice preparation. Brain Res. 1987 May 26;412(1):179–181. doi: 10.1016/0006-8993(87)91457-0. [DOI] [PubMed] [Google Scholar]
- Simon R. P., Swan J. H., Griffiths T., Meldrum B. S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984 Nov 16;226(4676):850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- Squire L. R. Mechanisms of memory. Science. 1986 Jun 27;232(4758):1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science. 1985 Nov 8;230(4726):681–683. doi: 10.1126/science.2996146. [DOI] [PubMed] [Google Scholar]



