Abstract
Knockout (KO) of IL-6 has been shown to attenuate ANG II hypertension, and mineralocorticoid receptors (MR) have been reported to contribute to the increase in IL-6 during acute ANG II infusion. This study determined whether that MR action is sustained with chronic ANG II infusion and whether it plays a role in mediating ANG II hypertension. ANG II infusion (90 ng/min) increased plasma IL-6 from 1.6 ± 0.6 to 22.7 ± 2.2 and 19.9 ± 3.2 pg/ml on days 7 and 14, respectively, and chronic MR blockade with spironolactone attenuated that only at day 7 (7.2 ± 2.2 pg/ml). ANG II increased MAP (19 h/day with telemetry) ∼40 mmHg, but in ANG II+spironolactone mice (25 or 50 mg·kg−1·day−1), mean arterial pressure (MAP) was not significantly different despite a tendency for lower pressure the first 6 days. To isolate further the mineralocorticoid link to IL-6 and blood pressure, DOCA-salt hypertension was induced in IL-6 KO and wild-type (WT) mice. Plasma IL-6 increased from 4.1 ± 1.7 to 34.5 ± 7.0 pg/ml by day 7 of DOCA treatment in the WT mice but was back to control levels by day 14. An IL-6 bioassay using the murine B9, B-cell hybridoma cell line demonstrated that plasma IL-6 measurements reflected actual IL-6 bioactivity. The hypertension was not different and virtually superimposable in WT vs. IL-6 KO mice, averaging 145 ± 2 and 144 ± 3 mmHg, respectively. Both experiments confirm chronic stimulation of IL-6 by mineralocorticoids but show that it is transient. In addition, IL-6 was not required for mineralocorticoid hypertension. This suggests that aldosterone contributes to the increase in plasma IL-6 in the early stage of ANG II hypertension but that the blood pressure actions of IL-6 in that model are linked most likely to ANG II rather than aldosterone.
Keywords: angiotensin II, bioactivity
inflammatory cytokines have been implicated in the etiology of cardiovascular disease at many levels, ranging from the initiation of the atherosclerotic process (6, 10) to the progression of end-organ injury (25, 26, 39). Cytokines and markers of inflammation also have shown a strong correlation with hypertension in human studies (2, 4, 7, 16, 23, 33, 38, 40). Guzik et al. (13) recently have shown that generalized loss of T cell-mediated inflammatory processes significantly attenuates angiotensin II (ANG II) hypertension, and our laboratory (22) and Coles et al. (9) have reported that ANG II hypertension in mice is dependent significantly on IL-6. The mechanism for that effect of IL-6 is not known, but Luther et al. (24) reported that the mineralocorticoid receptor antagonist, spironolactone, prevented the increase in plasma IL-6 concentration caused by acute ANG II infusion, suggesting a potential role for aldosterone in linking IL-6 to chronic ANG II hypertension.
We tested this potential role of aldosterone in this study by inducing chronic ANG II hypertension in wild-type (WT) mice, with or without chronic treatment with spironolactone. However, if plasma IL-6 concentration and blood pressure both decreased in the ANG II+spironolactone mice, we would not be able to tell whether the drop in blood pressure was due to an IL-6-dependent blood pressure action of ANG II at the AT1 receptor or to an IL-6-dependent blood pressure action of aldosterone at the mineralocorticoid receptor, or both. Therefore, to isolate further the mineralocorticoid link to IL-6 and blood pressure, DOCA-salt hypertension was induced in IL-6 knockout (KO) and WT mice. The results of both experiments together are needed to determine how a mineralocorticoid-IL-6 link may or may not contribute to the dependence of ANG II hypertension on IL-6.
In addition to testing those relationships, this study determined the link between plasma IL-6 and bioactivity in hypertension. This is a critical issue for IL-6, because the IL-6 receptor can be tissue bound or soluble in the plasma (14, 15, 19, 32, 34). Moreover, certain stimuli, such as IL-1β and TNF-α, can increase the plasma levels of the soluble receptor (11). The ramifications of that effect are that plasma IL-6 bioactivity can change significantly independent of a change in plasma IL-6 concentration, and changes in plasma IL-6 concentration may not accurately reflect changes in IL-6 bioactivity (34, 37). However, the relationship between plasma IL-6 concentration and actual plasma IL-6 bioactivity has not been tested in any model of hypertension. We addressed this by coupling an IL-6 bioactivity assay with our IL-6 enzyme immunoassay to determine the link between circulating IL-6 levels and IL-6 bioactivity.
METHODS
Role of Aldosterone in the IL-6 and Blood Pressure Responses in ANG II Hypertension
Procedures involving animals were approved by the Animal Care and Use Committee of the Medical College of Georgia. The experiments were conducted in 12- to 14-wk-old (27–30 g) male mice (C57BL/6J, Jackson Laboratories, Bar Harbor, ME). The animals were anesthetized using isoflurane, and biotelemetry transmitter devices (PA-C10, Data Sciences, St. Paul, MN) were implanted using aseptic technique. The catheter was implanted in the left carotid artery through an incision in the vessel wall made with a custom-shaped 25-gauge needle. The transmitter body was tunneled subcutaneously above the right shoulder and secured above the scapula. All incisions were infiltrated with Marcaine, and mice were given buprenorphine subcutaneously and placed in warmed plastic cages lined with sterile paper to recover following surgery. Mice were transferred to a light- and temperature-controlled room in the animal facilities and were housed individually in standard mouse cages with tap water and standard rodent chow available ad libitum. They were given 5–7 days to recover from surgery before control measurements were made.
Experimental protocol.
During the control period, all groups received 100 mg of peanut butter per day along with their rodent chow (4% NaCl), because it served as the vehicle for spironolactone administration. By the 3rd control day, the mice were eating the entire 100 mg every day. After 4 days of control measurements, mice were assigned randomly to vehicle, ANG II, or ANG II+spironolactone treatment (low and high dose) groups, and the ANG II groups had a 14-day osmotic minipump (Alzet, Durect, Cupertino, CA) implanted subcutaneously to infuse ANG II at a dose of 90 ng/min (22). The spironolactone mice also began receiving spironolactone (low: 25 mg·kg−1·day−1, high: 50 mg·kg−1·day−1) in their peanut butter at that time. Blood samples for cytokine assays were drawn from subgroups of isoflurane-anesthetized ANG II and ANG II+Spir (low dose only) mice on day 7 of the treatment period using tapered PE-50 catheters placed in the left carotid artery. The remainder of those mice along with the vehicle mice had blood samples taken at day 14.
Role of IL-6 in Mineralocorticoid Hypertension: DOCA Treatment in IL-6 Knockout Mice
These experiments were conducted in 12- to 14-wk-old (27–30 g) male IL-6 KO mice (B6.129S6-Il6 tm1Kopf, Jackson Laboratories) and their wild-type (WT) controls. Transmitters were implanted as described above, and the left kidney was removed from all mice through a flank incision during the same surgical procedure. The same housing and recovery period methods as noted above were followed.
Experimental protocol.
Animals were divided randomly into four groups: WT, IL-6 KO, WT+DOCA, and IL-6 KO+DOCA. After 4 days of control hemodynamic measurements, the DOCA animals were anesthetized briefly with isoflurane for subcutaneous implantation of a silicone rubber sheet containing DOCA using the method of Ormsbee and Ryan (29), at a dose established for mice [1 mg per 1 gram body wt (31)] using a silicone to DOCA ratio of 2:1. Although more than the 0.2 mg/g (200 mg/kg) dose typically used with this method in rats (3), it is lower than the ∼8 mg/g (200 mg/mouse) dose recently employed to study vascular inflammation in mice (20). All mice were started on a drinking solution of 1% NaCl and 0.2% KCl made with tap water for the 14-day treatment period. Blood samples for cytokine assays were drawn from subgroups of WT mice on day 7 of the treatment period as described above, and the remainder of the mice had blood samples taken at day 14. Blood samples also were drawn from randomly selected IL-6 KO mice to verify knockout of IL-6 and to provide another control for the IL-6 assays.
Analytical Methods
Plasma IL-6.
Plasma IL-6 concentrations were measured by enzyme immunoassay (R&D Systems, Minneapolis, MN) from terminal carotid artery blood samples, obtained from separate groups, on days 7 and 14 of DOCA and ANG II treatment. For each study, animals from every group were included on each assay plate to control for interassay variability.
IL-6 bioactivity assay.
Cells from the murine B9, B-cell hybridoma, cell line (Dr. L. Aarden, Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands) require IL-6 for survival and proliferation in vitro and have been shown to respond to murine and rat IL-6 (1, 30). The cells were grown in RPMI 1640 supplemented with 10% heat-inactivated FBS, 50 μM 2-mercaptoethanol (2-ME), 2 mM glutamine, antibiotics, and 100 pg/ml recombinant mouse IL-6 (R&D systems) (1, 27, 35). The saturated cultures were split 1:3 every 2–3 days at a density of 5 × 105 cells/ml.
For assay of plasma IL-6 bioactivity, plasma aliquots were thawed, heated at 57°C for 30 min to inactivate nonspecific cytotoxic factors in serum, and centrifuged for 15 min. Seven dilutions of each plasma sample were made to make sure our samples would fall on the linear portion of the standard curve, and 50 μl aliquots were added in triplicate on a 96-well plate. The standard curve was constructed using serial twofold dilutions of recombinant mouse IL-6 (R&D Systems) from 500 to 0.015 pg/ml, with 50-μl aliquots added to the plate in triplicate. All dilutions were made using RPMI 1640 without phenol red supplemented with 10% FBS, 50 μM 2-ME, 2 mM glutamine, and antibiotics (assay medium). The plate-containing samples and standards was equilibrated at 37°C in a humidified, 5% CO2 atmosphere, while the cells were harvested for the assay.
The cells were harvested and washed three times in assay medium by centrifugation at 300 g for 5 min. The cell number and trypan blue viability were determined, and the cells were resuspended at a final concentration of 1 × 105 cells/ml in assay medium. Fifty microliters of the cell suspension (5,000 cells) were dispensed into each well of the plate bringing the total fluid volume to 100 μl per well. The plate then was incubated for 72 h at 37°C in a humidified, 5% CO2 atmosphere (27). After 66 h, 20 μl of Cell Titer 96 Aqueous One Solution Reagent (Promega-MTS, Madison, WI) was added to each well. The plate then was incubated for 6 h at 37°C in a humidified, 5% CO2 atmosphere, and the absorbance at 490 nM was measured.
Blood pressure measurement.
Mouse cages were placed individually on Data Sciences receivers, and pulsatile arterial pressure was recorded from 1500 to 1000 (i.e., 19 h) each day. Analog signals from the transmitters were sampled for 5 s every 1–2 min at 500 Hz, and the average of those measurements was recorded as the daily mean arterial pressure for each animal.
Statistical analysis.
Data were analyzed with a two-factor, repeated-measures ANOVA. Significant F-test from the ANOVA at P < 0.05 was followed by post hoc comparisons using the Newman-Keuls multiple range test.
RESULTS
Spironolactone Treatment in ANG II Hypertension
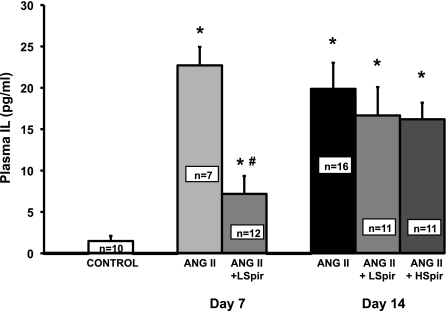
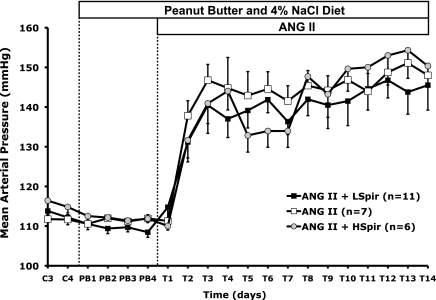
Figure 1 shows that plasma IL-6 concentration increased significantly during ANG II infusion, consistent with our previous report (22), averaging 22.7 ± 2.2 and 19.9 ± 3.2 pg/ml on days 7 and 14 of ANG II infusion, respectively. Moreover, spironolactone decreased that response significantly at day 7 in the low-dose group, even though plasma IL-6 still was significantly greater than control mice. By day 14, however, plasma IL-6 in neither spironolactone group was different from the 14-day ANG II group, and both were significantly different from the 7-day ANG II+spironolactone group. This shows that spironolactone caused a significant, but transient, attenuation of the increase in plasma IL-6 in ANG II hypertension. Figure 2 shows that mean arterial pressure increased significantly during 14 days of ANG II treatment in all groups. Although there appeared to be a tendency for MAP to be lower in the two ANG II+spironolactone groups during the 1st wk, there were no statistically significant between-group differences.
Fig. 1.
Plasma IL-6 concentrations for control mice with saline minipumps (control), ANG II, and ANG II+Spironolactone (ANG II+LSpir or HSpir for 25 and 50 mg·kg−1·day−1 Spir doses, respectively) mice on days 7 and 14 of ANG II treatment. *P < 0.05 compared with control. #P < 0.05 between ANG II and ANG II+Spir on that day.
Fig. 2.
Mean arterial pressure in WT mice with ANG II treatment (□, n = 7) and WT mice with ANG II+Spironolactone (LSpir, 25 mg·kg−1·day−1, closed squares, n = 11; HSpir, 50 mg·kg−1·day−1, shaded circles, n = 6) during the last 2 days of a run-in control period (C), 4 days of peanut butter vehicle feeding (PB), and 14 days of treatment (T) with spironolactone.
DOCA-Salt Hypertension in WT and IL-6 KO Mice
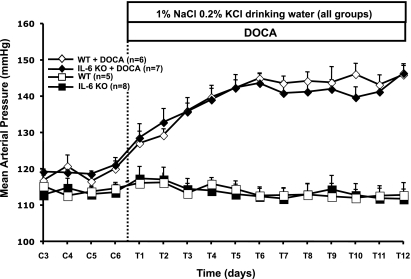
Mean arterial pressure was not different between groups during the control period, averaging 117 ± 2 and 119 ± 2 mmHg in the WT and IL-6 KO mice, respectively (Fig. 3). The salt drinking solution of 1% NaCl and 0.2% KCl alone did not change blood pressure in the control WT or IL-6 KO mice, averaging 116 ± 2 and 117 ± 3 mmHg, respectively. DOCA-salt treatment, however, had a significant hypertensive effect. Blood pressure increased steadily for the first 6 days, and it began to plateau on day 6 at an average of 145 ± 2 and 144 ± 3 mmHg in WT and IL-6 KO mice, respectively. There was no difference in mean arterial pressure between the two groups at any time during DOCA-salt treatment.
Fig. 3.
Mean arterial pressure in control IL-6 KO mice (IL-6 KO; ▪), control WT mice (WT; □), DOCA-treated IL-6 KO mice (IL-6 KO+DOCA; ⧫), and DOCA-treated WT mice (WT+DOCA; ◊) during the last 4 days of the control period (C) and 12 days of DOCA treatment (T).
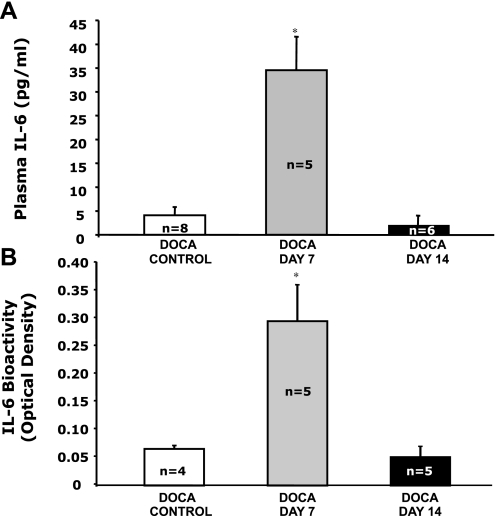
Plasma IL-6 concentration averaged 4.1 ± 1.7 pg/ml in WT mice during the control period and increased to an average of 34.5 ± 7.0 pg/ml after 1 wk of DOCA treatment (Fig. 4A). However, that response was transient, because after 2 wk of DOCA treatment, the plasma IL-6 concentration was back down to control levels at an average of 1.8 ± 1.0 pg/ml. Duplicate samples from those mice and mice from the first experiment (Fig. 1) were reassayed on the same plates to verify the sustained IL-6 increase in ANG II-treated mice compared with the transient increase in DOCA-salt-treated mice. In addition, our bioactivity assay revealed that plasma IL-6 bioactivity paralleled plasma IL-6 concentration. Reported as optical density (OD), the plasma IL-6 bioactivity for DOCA control mice averaged 0.061 ± 0.005, increased significantly to an average of 0.291 ± 0.066 on day 7 of DOCA treatment, and returned to levels not different from control on day 14 of DOCA treatment (Fig. 4B).
Fig. 4.
A: plasma IL-6 concentrations for WT mice without DOCA treatment (DOCA control) and DOCA-treated mice on days 7 and 14 of treatment. B: plasma IL-6 bioactivity for WT mice without DOCA treatment (DOCA control) and DOCA-treated mice on days 7 and 14 of treatment. *P < 0.05 compared with DOCA Control.
DISCUSSION
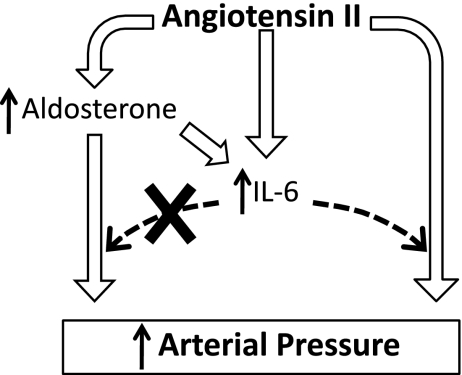
These results extend the observation of Luther et al. (24) by showing that the effect of spironolactone to attenuate the increase in plasma IL-6 concentration during ANG II hypertension was maintained for ∼1 wk before waning. We also showed that DOCA treatment had a transient (∼1 wk) effect to increase plasma IL-6 concentration, so together, these data suggest that aldosterone contributes significantly to the increase in plasma IL-6 that occurs during the early stage of ANG II hypertension (9, 22). The results from DOCA treatment in IL-6 KO mice also show that mineralocorticoid hypertension does not require IL-6, which suggests that any increase in aldosterone caused by chronic ANG II infusion would not have its own IL-6-dependent blood pressure action. Therefore, on the basis of these and previous findings, we hypothesize that aldosterone could play a transient role in the dependence of ANG II hypertension on IL-6 through an effect to increase plasma IL-6 concentration (Fig. 5).
Fig. 5.
Hypothesis linking previous results, showing stimulation of IL-6 by ANG II and IL-6-dependent ANG II hypertension, with current results regarding the actions of aldosterone.
Our laboratory reported that ANG II-salt hypertension was attenuated significantly in IL-6 KO mice, and it was particularly important that the different blood pressure response to ANG II in KO vs. WT mice was evident early and was independent of renal injury (22). Those findings suggested that IL-6 played a significant role in the physiological mechanism(s) that underlie the hypertensive actions of ANG II. The ANG II dose (90 ng/min), the same as in the present study, was high, however, calculating out to roughly 3.5 times the dose of 1,000 ng·kg−1·min−1 that the laboratory of Welch and colleagues (18) showed took 3 days to increase blood pressure, but the rapid elevation at day 1 likely isolates vascular actions of ANG II. Nonetheless, it is important that Coles et al. (9) recently confirmed our findings at a lower dose (∼22 ng/min or ∼ 800 ng·kg−1·min−1) with an elegant study that showed significant attenuation of ANG II hypertension either by IL-6 KO or by blocking IL-6 binding to target tissues with injection of soluble gp130 (IL-6β-receptor subunit), which prevents normal coupling of the IL-6-α-receptor subunit complex to the membrane-bound gp130 subunits required for signaling. In addition, those authors measured this effect of IL-6 in mice that were on normal, rather than 4%, salt intake. With this evidence that ANG II hypertension depends significantly on IL-6, the next task was to determine which components of the physiological response to ANG II are responsible for that relationship.
Plasma IL-6
We focused on aldosterone because of its undeniable importance in the renin-angiotensin-aldosterone system for chronic blood pressure control and because Luther et al. (24) recently used spironolactone to demonstrate that the increase in plasma IL-6 measured during a 3-h ANG II infusion in human subjects required aldosterone. That suggested that aldosterone could contribute to ANG II hypertension not only through classic mineralocorticoid receptor mechanisms, but also by contributing to the increase in plasma IL-6. Our data provide additional support for that, by demonstrating that the increase in plasma IL-6 concentration during chronic ANG II hypertension was attenuated significantly by spironolactone treatment. It should be noted that Luther et al. reported complete blockade of the increase in IL-6 with spironolactone, whereas we did not (Fig. 1). It is possible that the acute (3 h) vs. chronic (14 days) nature of the respective experiments, and the much greater increase in plasma IL-6 caused by chronic ANG II infusion in the present study, may explain the different effects of spironolactone on IL-6. In addition, ANG II has been shown to stimulate IL-6 release directly from several tissues (5, 12, 21, 24, 36), so it is unlikely that the more than eight-fold increase in IL-6 that we measured during chronic ANG II infusion would have been blocked completely even by a higher dose of spironolactone. Therefore, regardless of the magnitude, these results provide evidence that the increase in plasma IL-6 in chronic ANG II hypertension is dependent significantly on aldosterone.
Another important finding, however, is that the effect of spironolactone proved to be transient, waning in week 2, even in mice with a twofold higher spironolactone dose. The mechanism for that effect is not known, but it was corroborated by the DOCA-treated WT mice, in which plasma IL-6 increased during week one of DOCA before returning to control levels during week 2. Thus, the effect of aldosterone during chronic ANG II infusion and the effect of chronic DOCA treatment both were shown to cause transient, i.e., ∼1-wk, increases in plasma IL-6 concentration.
It is important to note the implications of our IL-6 bioactivity data in this regard, because plasma levels of soluble IL-6 receptor (sIL-6R) or gp130 can increase or decrease IL-6 bioactivity independent of a change in plasma IL-6 concentration. The effect of sIL-6R on IL-6 bioactivity is a process called trans-signaling, which involves binding of a circulating IL-6/IL-6R complex with gp130 (also known as IL-6 receptor β subunit) that is ubiquitously expressed (34, 37). This means that IL-6 can act more readily in tissues that do not express IL-6R, and Coles et al. (9) reported that the chronic hypertensive action of ANG II is dependent on this mechanism. In fact, they did not measure an increase in plasma IL-6 at their lower dose of ANG II infusion in WT mice, yet knockout of IL-6 and blockade of sIL-6R each were effective in attenuating ANG II hypertension. Thus, if sIL-6R had increased by day 14 in the DOCA-salt WT mice, IL-6 bioactivity could have been maintained at an elevated level even though plasma IL-6 concentration decreased. However, our data in the DOCA-treated mice showed that plasma IL-6 concentration reflected IL-6 bioactivity, indicating that, not only did plasma IL-6 concentration decrease to control levels during week 2, but, indeed, IL-6 bioactivity in the 2nd wk of DOCA was not different from control levels.
Arterial Pressure
Luther et al. (24) did not measure an effect of spironolactone on blood pressure, which we initially believed may have been because their study was only 3 h in duration, but we also did not measure a significant blood pressure-lowering effect of spironolactone despite 14 days of ANG II treatment. This is consistent with previous chronic studies in mice (8, 17, 28). However, on the basis of our hypothesis that IL-6 plays a significant role in chronic ANG II hypertension, it could appear problematic that the significant decrease in plasma IL-6 during week 1 in the ANG II+spironolactone mice did not cause a statistically significant decrease in MAP. On the other hand, even complete knockout of IL-6 did not completely block ANG II hypertension (9, 22), and, at most, could be shown to account for roughly 50% of the hypertension. A threshold effect of IL-6 on ANG II hypertension has not been established, and it is possible that the statistically significant increase in plasma IL-6 at week 1 in the ANG II+spironolactone mice, although significantly lower than the ANG II mice without spironolactone, was sufficient to contribute to ANG II hypertension. It also is worth noting that even though there was not a statistically significant blood pressure effect of spironolactone, there appeared to be a tendency for lower blood pressure during the 1st wk of ANG II+spironolactone in both the low- and high-dose spironolactone groups (Fig. 2), and it is interesting that it coincided with the period in which plasma IL-6 concentration was most mineralocorticoid dependent, whether looking at the effect of spironolactone (Fig. 1) or DOCA (Fig. 4). Thus, we might attribute the apparent trend for decreased MAP in week 1 to the transient decrease in plasma IL-6.
Nevertheless, MAP between the ANG II and ANG II+spironolactone groups was not significantly different, but although those data suggest that aldosterone is not a major contributor to overall ANG II hypertension in this model, it remains possible that aldosterone needs IL-6 for full development of its own hypertensive action. In fact, Guzik et al. (13) showed that loss of T cells also ameliorates mineralocorticoid hypertension. However, that possibility cannot be evaluated in the ANG II+spironolactone experiment because IL-6 remained significantly increased from control levels even on day 7. This is why we used IL-6 knockout mice to test the role of IL-6 in mineralocorticoid hypertension, using the DOCA-salt model as did Guzik et al. (13). Figure 3 shows very clearly that there is no requirement for IL-6 in mineralocorticoid hypertension. Thus, although the DOCA-salt model is not exactly the same as aldosterone elevations during ANG II infusion, these data strongly suggest that in ANG II hypertension, any contribution that aldosterone itself might, or could, have on blood pressure is not IL-6 dependent. However, the absence of an IL-6 effect on blood pressure in DOCA-salt hypertension despite the large stimulation of IL-6 by DOCA may raise a question of whether any model of hypertension actually is IL-6 dependent. We interpret the significant effect of IL-6 KO (9, 21) and blockade of IL-6 trans-signaling (9) to attenuate ANG II hypertension, plus the failure of IL-6 KO to attenuate DOCA-salt hypertension, to mean that chronic IL-6 blood pressure actions require interaction with ANG II at its target sites.
These results, therefore, confirm and extend the findings from Luther et al. (24) by suggesting that mineralocorticoids contribute to the increased IL-6 in ANG II-dependent hypertension, but also suggest that blood pressure response in ANG II hypertension is due to interaction between IL-6 and ANG II rather than to mineralocorticoid receptor-mediated effects. This is important, because it increases the focus and rationale for studying the specific AT1-receptor and cell signaling pathways that link IL-6 to the blood pressure actions of ANG II. In the overall scheme of the renin-angiotensin-aldosterone system, these findings suggest that if aldosterone contributes to the IL-6 dependence of ANG II hypertension, it most likely would be through an effect to help stimulate an early rise in plasma IL-6 concentration.
Perspectives and Significance
The report that loss of T cells ameliorates both ANG II and DOCA hypertension (13), whereas these data show that IL-6 knockout ameliorates only ANG II hypertension, reinforces the need to understand the multitude of potential mechanisms that can be lumped together under the umbrella of “inflammatory mechanisms for hypertension.” Moreover, it is critical to appreciate that “inflammatory mechanisms” should not uniformly invoke overt tissue injury as a factor underlying chronic blood pressure effects. In fact, the effect of IL-6 knockout or blockade to ameliorate ANG II hypertension is evident within days (9, 22) yet has no effect on proteinuria or the return to normal blood pressure after a 14-day ANG II infusion is stopped (22). Therefore, the present results focus future studies on mechanisms linking IL-6 to AT1-mediated increases in blood pressure, but we believe that focus should be narrowed further to studying specifically how ANG II chronically affects renal and vascular function in an IL-6-dependent manner.
GRANTS
This work was supported by National Heart, Lung and Blood Institute Grants HL74167, HL56259, and HL75625.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Ashlyn Allen for her technical assistance.
REFERENCES
- 1.Aarden LA, De Groot ER, Schaap OL, Lansdorp PM. Production of hybridoma growth factor by human monocytes. Eur J Immunol 17: 1411– 1416, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Abramson JL, Weintraub WS, Vaccarino V. Association between pulse pressure and C-reactive protein among apparently healthy U.S. adults. Hypertension 39: 197– 202, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Banes AK, Watts SW. Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension. BMC Pharmacol 3: 12– 26, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbieri M, Ferrucci L, Corsi AM, Macchi C, Lauretani F, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Paolisso G. Is chronic inflammation a determinant of blood pressure in the elderly? Am J Hypertens 16: 537– 543, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257– 1266, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Call JT, Deliargyris EN, Newby LK. Focusing on inflammation in the treatment of atherosclerosis. Cardiol Rev 12: 194– 200, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Carretero OA, Scicli AG. Local hormonal factors (intracrine, autocrine, and paracrine) in hypertension. Hypertension 18Suppl. I: I-58– I-69, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate Angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Br J Pharmacol 144: 443– 448, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control Angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol 171: 315– 325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 98: 121– 128, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Franchimont N, Lambert C, Huynen P, Ribbens C, Relic B, Chariot A, Bours V, Piette J, Merville MP, Malaise M. Interleukin-6 receptor shedding is enhanced by interleukin-1β and tumor necrosis factor α and is partially mediated by tumor necrosis factor α-converting enzyme in osteoblast-like cells. Arthritis Rheum 52: 84– 93, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Funakoshi Y, Ichiki T, Ito K, Takeshita A. Induction of interleukin-6 expression by angiotensin II in rat vascular smooth muscle cells. Hypertension 34: 118– 125, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 204: 2449– 2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 374: 1– 20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 334: 297– 314, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeper MM, Welte T. Systemic inflammation, COPD, and pulmonary hypertension. Chest 131: 634– 635; author reply 635, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates ANG iotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J 20: 1546– 1548, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol 13: 2860– 2868, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci 1: 340– 357, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 292: H1789– H1795, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kranzhofer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kubler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1623– 1629, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935– H940, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Li JJ, Fang CH, Hui RT. Is hypertension an inflammatory disease? Med Hypotheses 64: 236– 240, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Luther JM, Gainer JV, Murphey LJ, Yu C, Vaughan DE, Morrow JD, Brown NJ. Angiotensin II induces interleukin-6 in humans through a mineralocorticoid receptor-dependent mechanism. Hypertension 48: 1050– 1057, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Manhiani MM, Quigley JE, Socha MJ, Motamed K, Imig JD. IL6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney Blood Press Res 30: 195– 202, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Muller DN, Mervaala EM, Schmidt F, Park JK, Dechend R, Genersch E, Breu V, Loffler BM, Ganten D, Schneider W, Haller H, Luft FC. Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension 36: 282– 290, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Nakatani M, Seki T, Shinohara Y, Taki C, Nishimura S, Takaki A, Shioda S. Pituitary adenylate cyclase-activating peptide (PACAP) stimulates production of interleukin-6 in rat Muller cells. Peptides 27: 1871– 1876, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka-Yoshida K. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol 49: 261– 268, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Ormsbee HS, 3rd, Ryan CF. Production of hypertension with desoxycorticorticosterone acetate-impregnated silicone rubber implants. J Pharm Sci 62: 255– 257, 1973 [DOI] [PubMed] [Google Scholar]
- 30.Osawa H, Yamabe H, Inuma H, Miyata M, Sasaki T, Kaizuka M, Tamura N, Tsunoda S, Fujita Y, Kanazawa T, Nomura K, Onodera K. TGF-β upregulates interleukin 6 production by rat glomerular epithelial cells in vitro. Nephrol Dial Transplant 10: 1592– 1597, 1995 [PubMed] [Google Scholar]
- 31.Rhaleb NE, Peng H, Alfie ME, Shesely EG, Carretero OA. Effect of ACE inhibitor on DOCA-salt- and aortic coarctation-induced hypertension in mice: do kinin B2 receptors play a role? Hypertension 33: 329– 334, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol 80: 227– 236, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens 15: 152– 158, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 63: 321– 329, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Schwabe M, Cox GW, Bosco MC, Prohaska R, Kung HF. Multiple cytokines inhibit interleukin-6-dependent murine hybridoma/plasmacytoma proliferation. Cell Immunol 168: 117– 121, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Skurk T, van Harmelen V, Hauner H. Angiotensin II stimulates the release of interleukin-6 and interleukin-8 from cultured human adipocytes by activation of NF-κB. Arterioscler Thromb Vasc Biol 24: 1199– 1203, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Tenhumberg S, Waetzig GH, Chalaris A, Rabe B, Seegert D, Scheller J, Rose-John S, Grotzinger J. Structure guided optimization of the interleukin-6 transsignaling antagonist sgp130. J Biol Chem 283: 27200– 27207, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens 12: 181– 187, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Chen H, Xie HH, Shu H, Yuan WJ, Su DF. Inflammation is involved in the organ damage induced by sinoaortic denervation in rats. J Hypertens 21: 2141– 2148, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Zoccali C, Maio R, Tripepi G, Mallamaci F, Perticone F. Inflammation as a mediator of the link between mild to moderate renal insufficiency and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 17: S64– S68, 2006 [DOI] [PubMed] [Google Scholar]