Abstract
• Background and Aims Gesneriaceae is a pantropical plant family with over 3000 species. A great variety of pollination mechanisms have been reported for the neotropical members of the family, but the details of buzz-pollination and enantiostyly for the family have not been described. We investigated the floral biology and pollination ecology of Paraboea rufescens in Xishuangbanna, south-west China, considering three aspects: (1) the type of enantiostyly exhibited; (2) whether the species is self-compatible; and (3) whether pollinator behaviour could enhance the precision of pollen transfer between flowers of contrasting stylar orientation.
• Methods Flowering phenology was monitored once a month during vegetative growth, and once a week during flowering both in the field and under cultivation. Pollination manipulations and pollinator observation in the field were conducted.
• Key Results Anthesis occurred early during the morning, and flowers remained open for 1–5 d, depending on weather conditions. Controlled pollinations revealed that P. rufescens is self-compatible, and exhibited inbreeding depression in seed set. Plants were pollinator limited in natural populations. The similar stylar deflection among flowers within a plant limits autonomous self-pollination as well as pollination between flowers. Two species of bumble bees (Bombus spp.), Amegila malaccensis and Nomia sp. effectively pollinated P. rufescens. These pollinators visited flowers in search of pollen with almost the same frequency. None of the pollinators appeared to discriminate between left- or right-handed flowers.
• Conclusions Paraboea rufescens exhibits monomorphic enantiostylous flowers and a buzz-pollination syndrome. Floral morphology in P. rufescens and pollinator foraging behaviour seems likely to reduce self-pollination and pollinations between flowers of the same stylar deflection.
Keywords: Buzz-pollination, enantiostyly, Gesneriaceae, mirror-image flowers, Paraboea rufescens, reproductive biology, Xishuangbanna
INTRODUCTION
Enantiostyly (mirror-image flowers) is a sexual polymorphism in which left-styled flowers have anthers deflected to the right, and right-styled flowers have the opposite arrangement (Todd, 1882; Webb and Lloyd, 1986; Jesson and Barrett, 2003). This form of reciprocal herkogamy occurs in at least a dozen unrelated families of flowering plants (Jesson and Barrett, 2003). Enantiostyly occurs in two distinct forms (Barrett et al., 2000). In monomorphic enantiostyly, which is most common, left- and right-styled flowers occur on the same plant. In contrast, in dimorphic enantiostyly which has been reported for only seven species, the flowers of individual plants are entirely either left- or right-styled (Barrett et al., 2000; Jesson and Barrett, 2003). Enantiostyly is generally considered to promote insect-mediated cross-pollination by reducing sexual interference between female and male function (Wilson, 1887; Iyengar, 1923; Ornduff and Dulberger, 1978; Dulberger and Ornduff, 1980; Dulberger, 1981; Webb and Lloyd, 1986; Fenster, 1995; Barrett et al., 2000; Barrett, 2002a). Moreover, recent work has detailed the inheritance, developmental basis, evolution and adaptive significance of enantiostyly (Barrett et al., 2000; Barrett, 2002a, b; Jesson and Barrett, 2002a–c; Jesson and Barrett, 2003; Jesson et al., 2003). Nevertheless, the basic reproductive biology of most enantiostylous species remains unstudied.
Enantiostyly is often associated with buzz-pollination (Buchmann, 1983), a pollination mechanism in which bees must buzz flowers by rapidly vibrating their indirect flight muscles to release pollen from the anthers, which dehisce only at the tip so that their pollen remains hidden (Buchmann, 1983). Floral traits commonly associated with buzz-pollination include a bowl-shaped perianth of suitable size for pollinator activity, reflexed petals, lack of nectar, and brightly coloured anthers (Buchmann, 1983). These traits combined with specific pollinator behaviour may lead to precise pollen transfer (Harder, 1990; Harder and Barclay, 1994; King and Buchmann, 1996). Several species in unrelated enantiostylous families, for example, Solanum rostratum in the Solanaceae (Bowers, 1975) and Chamaecrista fasciculata in the Leguminosae (Fægri and van der Piji, 1979), have independently evolved the buzz pollination syndrome. Because the details of pollen dispersal by pollinators affects the mating patterns of animal-pollinated plants (Harder and Barrett, 1996), it is not unexpected that buzz pollination would reduce the levels of geitonogamy (pollination between different flowers of the same individual), especially in species with enantiostyly.
The Gesneriaceae is a large tropical family with about 3000 animal-pollinated species in 133 genera (Wiehler, 1983). This family exhibits extensive diversity in floral structure, even within genera, which appears to have resulted from adaptive changes in pollination mode (Burtt, 1970; Wiehler, 1983; Endress, 1994). In the 1500 neotropical species of Gesneriaceae, 60 % are pollinated by hummingbirds, 30 % are pollinated by nectar-feeding euglossine bees and 10 % are pollinated by bats, butterflies, hawkmoths, flies or male euglossine bees in search of nectar (Wiehler, 1983). In contrast, little is known about the pollination ecology of Gesneriaceae in China.
During work on the pollination biology of tropical Chinese plants casual observations suggested that Paraboea rufescens is enantiostylous and buzz pollinated. In this paper are reported the results of subsequent studies concerning three aspects of the pollination ecology of P. rufescens: (1) the type of enantiostyly exhibited; (2) whether this species is self-compatible; and (3) whether pollinator behaviour could enhance the precision of pollen transfer between flowers of contrasting stylar orientation.
MATERIALS AND METHODS
Research site and plant material
Paraboea rufescens (Franchet) B. L. Burtt is a small herb distributed in Guangdong, south-western Guangxi, southern Guizhou, eastern and southern Yunnan provinces in China, Thailand and northern Vietnam (Wang et al., 1998). It usually grows in sunny cracks and crevices of calcareous rocks with little soil. This species was studied on the peak of a limestone mountain in the monsoon rain forest in Xishuangbanna National Nature Reserve, south Yunnan Province, China (21°41′N, 101°25′E; 580 m a.s.l.), 5 km (south-east) from Xishuangbanna Tropical Botanical Garden (XTBG). At this site, P. rufescens is the main species in crevices and is restricted to very sunny mountain tops with sparse trees and shrubs, including Ficus pisocarpa, F. orthoneura, Pistacia weinmaniifolia and Agapetes burmanica (Zhu et al., 2003). Some observations of flowering phenology were also made on potted plants at XTBG.
Floral phenology and morphology
Flowering phenology was monitored once a month from May 2002 to September 2003, and once a week during the flowering season. The time of flower anthesis and withering, the region of stigmatic receptivity, and the position of the stamens and the style were observed and recorded. Also the longevity of pollinated and non-pollinated flowers was compared, and the number of left- and right-handed flowers produced each day on 20 marked plants recorded on 1–3 Jul. 2003.
Pollination experiments
To examine the effect of self- and cross-pollination of Paraboea rufescens, the duration of style receptivity, and the contribution of insect visitors to effective pollination, five pollination treatments were carried out on 115 randomly selected plants: (1) bagging—14 plants were bagged without pollination; (2) self-pollination—first-day flowers of 24 plants were hand-pollinated with pollen from flowers of the opposite stylar orientation from the same plant; (3) cross-pollination of first-day flowers—first-day flowers of 21 plants were hand-pollinated with pollen of the opposite stylar orientation from another individual; (4) cross-pollination to second-day flowers—second-day flowers of 16 plants were hand-pollinated with pollen of the opposite stylar orientation from another individual: and (5) open-pollinaton—40 plants were left for unmanipulated natural pollination. Inflorescences subjected to hand-pollination were bagged with nylon mesh before anthesis and bagged again after hand-pollination. The number of inflorescence and open flowers was recorded for all treatments. These experiments were conducted during 5–17 Jul. 2003, and fruit set of each treatment was counted 5 weeks later (on 25 Aug.). The seeds produced per fruit were counted, but undeveloped ovules were not counted because each ovary contains >1500 tiny ovules, which are stuck together by a glue-like substance. The fruit and seed set data for the open-pollinated and bagged flowers were analysed using a one-way ANOVA (SPSS ver. 9·0; Norusis, 1999) with pollination treatment as fixed effects. Fruit set data were arcsine transformed and seed set data were square root transformed prior to analysis.
Pollinator visitation and behaviour
Pollinators were identified and their behaviour observed between 0700 and 1900 h on 29–30 Jun. and 4 Jul. 2003. During a bee's visit, its position was observed while entering and exiting a flower, and whether it buzzed the anthers and collected pollen grains to determine if pollinator visits could result in self- or cross-pollination. Also pollinator movements were recorded among flowers on individual plants to assess whether they discriminated between left- and right-handed flowers. Observations were made synchronously by several observers posted at three sites in the population. After observations were completed, at least three individuals of each pollinator morphospecies were captured and identified. All species are deposited in the insect collections of XTBG.
RESULTS
Floral traits and flowering phenology
Paraboea rufescens is a deciduous herb, which is dormant from late October to the end of April during the dry season of the Xishuangbanna area. Flowering begins in June, after a month of vegetative growth, and continues for 3 months.
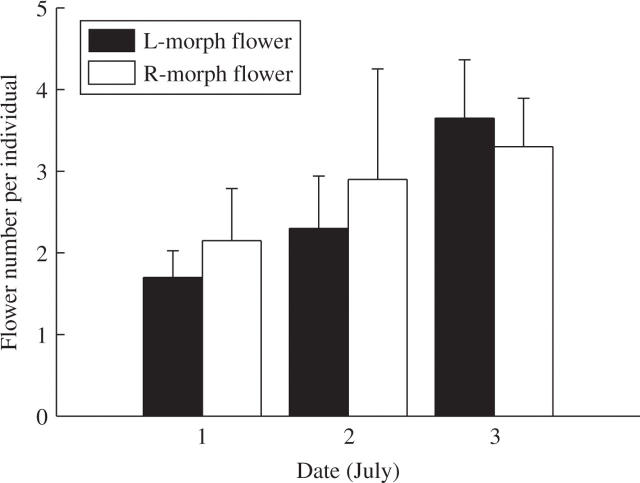
Paraboea rufescens produces flowers in cymes. Five united blue petals form a cup-shaped blossom containing one style, two stamens and two staminodes. The style, which is deflected either to the left or right of the main floral axis, places the stigma just beyond the stamens and parallel to the edge of corolla. Two white fertile anthers and two yellow staminodes are coherent and deflected in the opposite direction from the style (Fig. 1). The flower produces no nectar and the anther dehisces by an apical pore. Paraboea rufescens inflorescences bear both left- and right-styled flowers (Fig. 1), so this species exhibits monomorphic enantiostyly. On average (± standard error), plants produced 8·45 ± 1·1 inflorescences (range 2–20, n = 20), with one to three left- and right-handed flowers open per inflorescence per day (mean 0·46 and 0·48, respectively; n = 169 inflorescences of 20 individuals) from 1 to 3 Jul. 2003 (Fig. 2).
Fig. 1.
Left- and right-styled flowers, inflorescence and visitors of Paraboea rufescens: (A) an inflorescence bearing a left- and a right-styled flower; (B) Amegila malaccensis visiting a flower; (C) Amegila malaccensis entering or exiting a flower; (D) Trigona pagdeni visiting a flower.
Fig. 2.
The mean (± standard error) number of left- and right-styled flowers produced by Paraboea rufescens on 1, 2 and 3 Jul. 2003. Each plant produces the same frequencies of left- and right-handed flowers each day. Sample size = 20 plants with a total of 169 inflorescences.
Flowers opened during very early morning (0400–0500 h) and remained open for 1–5 d depending on environmental conditions. Once a flower was pollinated, the corolla and attached anthers fell off during the morning after pollination, if touched by a visitor, or during the following late afternoon. In contrast, unpollinated flowers at the study site remained open for 2–3 consecutive days and for up to 5 d in shade at XTBG, before withering gradually during over hours.
Experimental pollinations
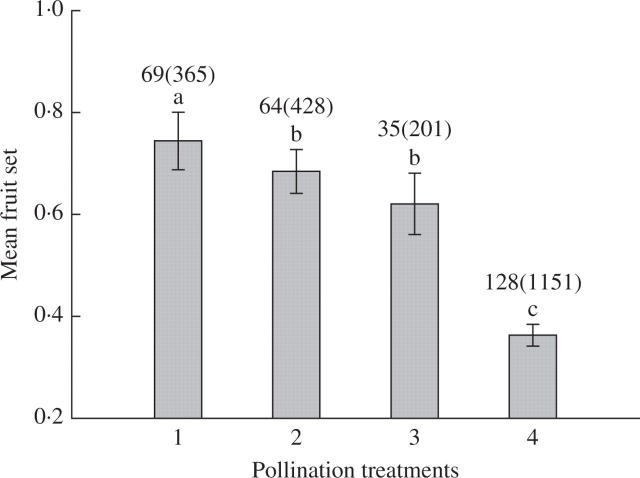
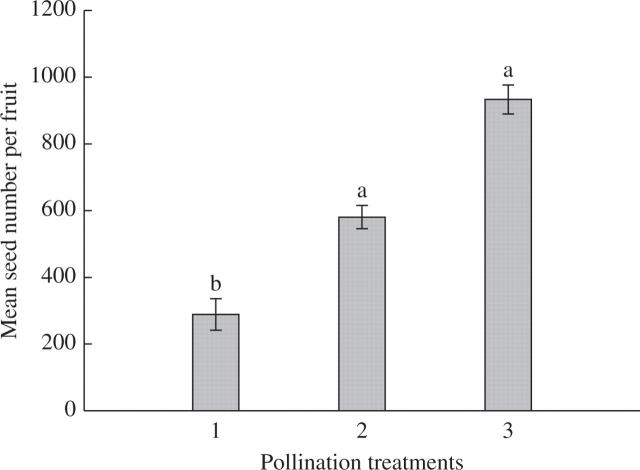
The pollination treatments revealed that P. rufescens is self-compatible, but does not self-pollinate autonomously (Figs 3 and 4). Bagged inflorescences that were not hand-pollinated set no fruit. The three main treatments (selfed, outcrossed and open) had significant fruit sets (Fig. 3; one-ANOVA on arcsine-transformed fruit set, F3,97 = 24·45, P = 7·21 × 10−12 < 0·001), in which, self-pollinated inflorescences had the highest fruit set. In addition, cross-pollination during a flower's first or second day resulted in equivalent fruit set, and hand cross-pollination flowers had significantly higher fruit set than open-pollinated flowers. In contrast, open-pollinated flowers produced significantly more seeds than hand cross-pollinated flowers, which in turn produced more seeds than self-pollinated flowers (Fig. 4, one-way ANOVA on square root-transformed seed set, F2,87 = 57·14, P = 1·42 × 10−16 < 0·001).
Fig. 3.
Mean (± standard error) fruit set of Paraboea rufescens inflorescences subjected to four pollination treatments: 1, self-pollination; 2, cross-pollination of first-day flowers; 3, cross-pollination of second-day flowers; and 4, open-pollination. Sample sizes are given below the bars [inflorescence number (flower number)], and statistically homogeneous groupings based on a one-way analysis of variance are indicated by the same letter (a, b or c) above the bars.
Fig. 4.
Mean (± standard error) seed number per Paraboea rufescens fruit resulting from one of three pollination treatments: 1, self-pollination; 2, cross-pollination of first-day flowers; and 3, open-pollination. Statistically homogeneous groupings based on a one-way analysis of variance are indicated by the same letter (a or b) above the bars.
Pollinator visitation and behaviour
Six bee species (two species of Bombus, Amegilla malaccensis, Nomia sp., Apis florea and Trigona pagdeni) visited Paraboea rufescens flowers during the observations. Of the bees that visited P. rufescens flowers regularly, bumble bees, Amegila malaccensis, and Nomia sp. are considered the primary pollinators, because their bodies contacted the stigma and they were observed to buzz flowers, which caused pollen grains to stream out of the anthers and collect on one side of their body. (A video recording is available from the senior author on request.) Apis florae and Trigona pagdeni are poor pollinators because their small bodies rarely touch the stigma (Fig. 1D). Instead, these species generally gleaned displaced pollen grains from the corolla.
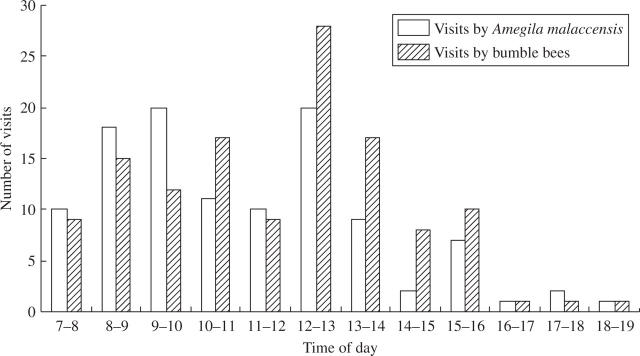
During a total of 108 h of observations, bumble bees (Bombus spp.) made 128 visits (53·6 %, n = 239) and Amegilla malaccensis and Nomia sp. made 111 visis (46·4 %). All of these major pollinators visited primarily during the morning, reaching a peak at noon, and declining during the afternoon (Fig. 5). They entered P. rufescens flowers with their thorax upwards, curled their body and grasped the anthers and staminodes (Fig. 1B), and then buzzed. Because the stamens bend in the opposite direction from the style and the pollinator's body occupied almost the entire flower, pollen was dispatched on one side of a pollinator's body while the stigma touched the corresponding position on the other side (Fig. 1C). Pollinators did not discriminate between right-styled and left-styled flowers. They visited flowers randomly and sometimes visited all flowers of an individual one by one. During 3 d of observation, in which 239 visits to 717 flowers were observed, 376 were to right-styled flowers, and 341 were to left-styled flowers (χ2 = 1·612, P > 0·1, n = 1).
Fig. 5.
Frequency of visits by bumble bees and Amegila malaccensis to flowers of Paraboea rufescens during 108 h of observation of 20 plants on 29 and 30 Jun. and 4 Jul. 2003.
DISCUSSION
A prominent feature in the Gesneriaceae is the four post-genitally united anthers either in pairs or combined together (Lamond and Vieth, 1972; Endress, 1994). Many gesneriad species also show marked protandry combined with herkogamy, and many are pollinated by birds (Wiehler, 1983). As far as is known, the present work on Paraboea rufescens represents the best-documented case in the Gesneriaceae of enantiostyly combined with buzz-pollination. In this family, some species of Streptocarpus and all species of Saintpaulia are enantiostylous and usually buzz-pollinated by bees (Harrison et al., 1999; Jesson et al., 2003), but no detailed studies on the basic reproductive biology have been reported. Although enantiostyly has evolved independently in at least 12 unrelated angiosperm families (Jesson and Barrett, 2003), convergence in floral morphology occurs among enantiostylous species. For example, enantiostyly is often associated with heteranthery, the specialization of anthers into brightly coloured feeding anthers and a cryptically coloured pollinating anther (Graham and Barrett, 1995). In P. rufescens, two yellow staminodes appear to serve as an attractant to insect visitors, whereas the two white anthers produce pollen.
Our pollination experiments provided insights into the floral biology of P. rufescens. The lack of fruit production in the bagging treatment indicates that P. rufescens is dependent upon insects for pollination; spontaneous self-pollination in this species does not occur. Fruit set was significantly higher in hand pollinations than in flowers visited by natural pollinators, suggesting that fruit production in natural populations is pollen limited. As in other enantiostylous species (Bowers, 1975; Dulberger, 1981; Fenster, 1995), floral morphology and pollinator foraging behaviour in P. rufescens seems likely to reduce self-pollination and pollinations between flowers of the same stylar deflection. However, pollen can be transferred between the two flower types within the same individual, resulting in geitonogamy. The relative degree of geitonogamy and xenogamy (pollination between different individuals) depends on the flight pattern of pollinators, the number of open flowers per plant and population size (Bowers, 1975). However, in buzz-pollinated Solanum rostratum, Jesson and Barrett (2002b) demonstrated that monomorphic enantiostyly can significantly reduce levels of geitonogamy compared with levels of geitonogamy characteristic of straight-styled flowers. It seems likely that in P. rufescens, monomorphic enantiostyly also serves this role.
Acknowledgments
We would like to thank Prof. Spencer C. H. Barrett and Linley Jesson for their constructive suggestions before the research was undertaken and for valuable comments on the manuscript; Prof. Susanne S. Renner and W. John Kress for discussions and comments on the manuscript; Dr David Westcott for helping to improve the language of the revised manuscript; Hong Wang for identifying the plant, and Prof. Da-Rong Yang and Yan-Qiong Peng for identifying the insects. The research was supported by the key project of the Chinese Academy of Science (KSCX2-SW-105) and the National Natural Science Foundation of China Grant 30225007.
LITERATURE CITED
- Barrett SCH. 2002a. Sexual interference of the floral kind. Heredity 88: 154–159. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2002b. The evolution of plant sexual diversity. Nature Reviews Genetics 3: 274–284. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. 2000. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany 85 (Suppl. A): 253–265. [Google Scholar]
- Bowers KAW. 1975. The pollination ecology of Solanum rostratum (Solanaceae). American Journal of Botany 62: 633–638. [Google Scholar]
- Buchmann SL. 1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold, 73–113.
- Burtt BL. 1970. Studies in the Gesneriaceae of the Old World XXXI: some aspects of functional evolution. Notes from the Royal Botanic Garden Edinburgh 30: 1–10. [Google Scholar]
- Dulberger R. 1981. The floral biology of Cassia didymobotrya and C. auriculata (Caesalpiniaceae). American Journal of Botany 68: 1350–1360. [Google Scholar]
- Dulberger R, Ornduff R. 1980. Floral morphology and reproductive biology of four species of Cyanella (Tecophilaeaceae). New Phytologist 86: 45–56. [Google Scholar]
- Endress PK. 1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press.
- Fægri K, van der Piji L. 1979. The principles of pollination biology. New York, NY: Pergamon Press.
- Fenster CB. 1995. Mirror image flowers and their effect on outcrossing rate in Chamaecrista fasciculata (Leguminosae). American Journal of Botany 82: 46–50. [Google Scholar]
- Graham SW, Barrett SCH. 1995. Phylogenetic systematics of Pontederiales: implications for breeding system evolution. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, eds. Monocotyledons: systematics and evolution. London: Royal Botanic Gardens, Kew, 415–451.
- Harder LD. 1990. Pollen removal by bumble bees and its implications for pollen dispersal. Ecology 71: 1110–1125. [Google Scholar]
- Harder LD, Barclay RMR. 1994. The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Functional Ecology 8: 509–517. [Google Scholar]
- Harder LD, Barrett SCH. 1996. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall, 140–190.
- Harrison C, Möller MM, Cronk Q. 1999. Evolution and development of floral diversity in Streptocarpus and Saintpaulia. Annals of Botany 84: 49–60. [Google Scholar]
- Iyengar MO. 1923. On the biology of the flowers of Monochoria. Journal of Indian Botany Society 3: 170–177. [Google Scholar]
- Jesson LK, Barrett SCH. 2002a. The genetics of mirror-image flowers. Proceedings of the Royal Society of London, Series B—Biological Sciences 269: 1835–1839. [DOI] [PMC free article] [PubMed]
- Jesson LK, Barrett SCH. 2002b. Solving the puzzle of mirror-image flowers. Nature 417: 707. [DOI] [PubMed]
- Jesson LK, Barrett SCH. 2002c. Enantiostyly in Wachendorfia (Haemodoraceae): the influence of reproductive systems on the maintenance of the polymorphism. American Journal of Botany 89: 253–262. [DOI] [PubMed] [Google Scholar]
- Jesson LK, Barrett SCH. 2003. The comparative biology of mirror-image flowers. International Journal of Plant Science 164: S237–S249. [Google Scholar]
- Jesson LK, Kang J, Wagner SL, Barrett SCH, Dengler NG. 2003. The development of enantiostyly. American Journal of Botany 92: 183–195. [DOI] [PubMed] [Google Scholar]
- King MJ, Buchmann SL. 1996. Sonication dispensing of pollen from Solanum laciniatum flowers. Functional Ecology 10: 449–456. [Google Scholar]
- Lamond M, Vieth J. 1972. L'androcee synanthere du Rechsteinera cardinalis (Gesneriacees): une contribution au probleme des fusions. Canadian Journal of Botany 50: 1633–1637. [Google Scholar]
- Norusis MJ. 1999. SPSS for Windows +V9.0 base manual. Chicago, IL: SPSS Inc.
- Ornduff R, Dulberger R. 1978. Floral enantiomorphy and the reproductive system of Wachendorfia paniculata (Haemodoraceae). New Phytologist 80: 427–473. [Google Scholar]
- Todd JE. 1882. On the flowers of Solanum rostratum and Cassia chamaecrista. American Naturalist 16: 281–287. [Google Scholar]
- Wang WT, Pan KY, Li ZY, Weitzman AL, Skog LE. 1998. Gesneriaceae. In: Wu ZY, Raven PH, eds. Flora of China. Vol. 18. Scrophulariaceae through Gesneriaceae. Beijing: Science Press, 273–333.
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]
- Wiehler H. 1983. A synopsis of neotropical Gesneriaceae. Selbyana 6: 1–219. [Google Scholar]
- Wilson J. 1887. On the dimorphism of the flowers of Klachendorfia paniculata. Transactions and Proceedings of the Botanical Society of Edinburgh 17: 73–77. [Google Scholar]
- Zhu H, Wang H, Li BG, Sirirugsa P. 2003. Biogeography and floristic affinities of the limestone flora in Southern Yunnan, China. Annals of the Missouri Botanical Garden 90: 444–465. [Google Scholar]