Abstract
• Background and Aims Floral nectar concentration and chemical composition of 26 plant species native to the temperate forest of southern South America are reported and the relationships with the flower type are evaluated.
• Methods Nectar concentration was measured with a hand refractometer and sugar composition was analysed by gas–liquid chromatography. Plant species were classified into flower type categories based not only on floral features but also on data from the literature and field observations on their pollinators.
• Key Results Most data on nectar are new reports at the generic and/or specific level. Plant species in which more than one population was studied showed significant among-population variation in nectar sugar concentration and composition. Results showed a weak relationship between nectar traits and flower type. Many species had nectar containing 50 % or more sucrose (17 of 26 species), independent of the main pollinator.
• Conclusions Considering that (a) nectar characteristics did not show a clear association with different flower types or with plant taxonomic membership, and (b) different populations of the same species showed large variability in sugar composition, the results suggest that other factors (e.g. historical and environmental) could be involved in determining the sugar composition of the highly endemic plant species from this region.
Keywords: Nectar sugar composition, sugar concentration, flower type, pollination, hummingbirds, diurnal insects, nocturnal insects, temperate forest of southern South America
INTRODUCTION
Biotic pollination, the most common mutualism in terrestrial communities, represents an important source of reciprocal change between plants and animals (Willson et al., 1996). Over evolutionary time, this interaction has contributed to the modelling of many characteristics in both flowers and pollinators (e.g. Faegri and van der Pijl, 1979; Baker and Baker, 1983; Johnson and Steiner, 2000). From the plant side, selection forces should act to maximize their attraction to pollinators, which transfer compatible pollen and enhance ovule fertilization.
To attract pollinators, plants offer different types of rewards. However, floral nectar represents the main plant reward for many pollinators and thus a putative primary selection target (e.g. Percival, 1961; Baker and Baker, 1975, 1983; Kevan and Baker, 1999). Nectar is basically a sugar solution composed of one disaccharide (sucrose) and two hexoses (glucose and fructose). Particularly, nectar is expected to diverge among different plant lineages and thus differ among taxa because different pollinators show preferences for solutions of different viscosity and/or sugar composition (e.g. Baker and Baker, 1975; Martínez del Rio et al., 1992; Temeles and Kress, 2003). If this is true, convergent nectar features (i.e. concentration and/or sugar composition) present in plant taxa from different lineages may represent adaptations to the behaviour, morphology and nutrition requirements of a particular pollinator type. On the other hand, divergent nectar features can be found in plant taxa from the same lineage that have maintained a close relationship with different pollinator guilds.
Two main trends can be outlined based on worldwide data from different floras: (1) nectar sugar concentration is generally higher in insect- than vertebrate-pollinated species (e.g. Cruden et al., 1983; Gottsberger et al., 1984; Freeman and Worthington, 1985; Proctor et al., 1996); and (2) nectar sugar composition of species pollinated by hummingbirds, moths and long-tongued bees is dominated by sucrose, whereas nectar of species pollinated by passerines, short-tongued bees and neotropical bats is dominated by hexoses (Baker and Baker, 1983, 1990; Elisens and Freeman, 1988; Martínez del Rio et al., 1989, 1992; van Wyk, 1993; Proctor et al., 1996; Baker et al., 1998; Galetto and Bernardello, 2003; Nicolson and Fleming, 2003; Dupont et al., 2004). However, several authors have pointed out that nectar characteristics can be highly conservative traits and that some species differing in pollinator type may show similar nectar sugar composition due to their close phylogenetic relationship (Elisens and Freeman, 1988; van Wyk, 1993; van Wyk et al., 1993; Galetto et al., 1998; Hingston and Mc Quillan, 2000; Perret et al., 2001; Galetto and Bernardello, 2003).
The temperate forest of southern South America extends from 35°S to 55°S latitude and from the Pacific Ocean to the eastern slopes of the Patagonian Andes. This forest is characterized by one of the highest incidences of biotic pollination, particularly bird pollination, compared with other temperate biomes worldwide (Armesto and Rozzi, 1989; Riveros, 1991; Willson et al., 1996; Aizen and Ezcurra, 1998). In this biome, 85 % of the genera of woody plants are visited and presumably pollinated by animals, and nearly 20 % are pollinated by a single resident hummingbird, Sephanoides sephaniodes (Smith-Ramírez, 1993; Armesto et al., 1996; Fraga et al., 1997). The high incidence of pollination mutualisms is similar to what is found in more tropical latitudes but, unlike their tropical counterparts, the flora of the temperate forest of South America interacts with a relatively low number of animal pollinators (Smith-Ramírez, 1993; Armesto et al., 1996; Aizen et al., 2002). In addition, the flora of this region exhibits one of the highest rates of endemisms of any continental flora with many exclusive genera and even families (Aizen and Ezcurra, 1998). Thus, this temperate flora makes it an interesting system for evaluating relationships between nectar sugar concentration, nectar sugar composition and pollinator type.
In this paper, the following are analysed: (a) the nectar sugar concentration and composition of 26 species native to the temperate forest of southern South America; and (b) the relationships between nectar characteristics and flower types (based on flower morphology and main pollinators); also the amount of inter-population variation in nectar traits is assessed for some of the species. This is the first work of this kind in the temperate forest of southern South America, and the nectar-sugar composition data compiled in this study are first reports for most species.
MATERIALS AND METHODS
Nectar was sampled from natural populations of 26 animal-pollinated plant species (out of 18 families) native to the temperate forest of South America, during the spring and summer seasons of 1997–2000. All species begin flowering during the austral spring with the exception of Tristerix corymbosus that flowers from autumn to spring, and through the winter (Table 1). The 26 species included in this study were selected to represent the entire range of life forms (trees, shrubs, vines, herbs, epiphytes and hemiparasites), most of the flower types (which reflect associations with different pollinator assemblages), and the proportion of bird- and insect-pollinated genera suggested by Aizen and Ezcurra (1998) for this biome (i.e. about 30 and 70 %, respectively). Plant species, populations studied, and the number of individuals and flowers sampled per population are listed in Table 1.
Table 1.
The species studied, sample size and reproductive traits of 26 species from the temperate forest of southern South America
| Cod | Family/species | P | Locality | Ph | I | F | GF | C | S | D |
|---|---|---|---|---|---|---|---|---|---|---|
| Alstroemeriaceae | ||||||||||
| 1 | Alstroemeria aurea Graham | 1 | Otto Hill | D–F | 5 | 5 | H | Y | Z | D |
| Amaryllidaceae | ||||||||||
| 2 | Rhodophiala mendocina (Phil.) Rav. | 1 | Low Chall-huaco Valley | D–J | 5 | 3 | H | Y | A | D |
| Asclepiadaceae | ||||||||||
| 3 | Cynanchum diemii T. Mey. | 1 | Lake Escondido | O–N | 4 | 5 | V | Y–G | A | S |
| Asteraceae | ||||||||||
| 4 | Mutisia decurrens Cav. | 1 | Low Chall-huaco Valley | D–A | 5 | 5 | V | O | A | D |
| 2 | Otto Hill | 5 | 5 | |||||||
| 5 | Mutisia spinosa Ruiz & Pav. | 1 | Otto Hill | D–A | 5 | 5 | V | L | A | D |
| 2 | Traful | 3 | 5 | |||||||
| 6 | Perezia prenanthoides Less. | 1 | Low Chall-huaco Valley | J–F | 4 | 5 | H | S | A | S |
| Berberidaceae | ||||||||||
| 7 | Berberis buxifolia Lam. | 1 | Llao–Llao Hill | S–N | 1 | 5 | S | Y | A | S |
| 2 | Otto Hill | 2 | 5 | |||||||
| 8 | Berberis darwinii Hook. | 1 | Llao–Llao Hill | S–J | 2 | 5 | S | O | A | S |
| 2 | Puerto Blest | 2 | 5 | |||||||
| Bignoniaceae | ||||||||||
| 9 | Campsidium valdivianum (Phil.) Skottsb. | 1 | Puerto Blest | A–D | 5 | 5 | V | R | A | D |
| 10 | Eccremocarpus scaber Ruiz & Pav. | 1 | Lake Traful | O–F | 5 | 5 | V | R | Z | D |
| Desfontainiaceae | ||||||||||
| 11 | Desfontainia spinosa Ruiz & Pav. | 1 | Puerto Blest | J–M | 5 | 5 | S | R–Y | A | D |
| Elaeocarpaceae | ||||||||||
| 12 | Aristotelia chilensis (Molina) Stuntz | 1 | Otto Hill | N–D | 1 | 5 | T | W–R | A | S |
| Escalloniaceae | ||||||||||
| 13 | Escallonia rubra (Ruiz & Pav.) Pers. | 1 | Puerto Blest | D–J | 5 | 5 | S | R | A | D |
| Fabaceae | ||||||||||
| 14 | Lathyrus multiceps Clos | 1 | Otto Hill | N–D | 5 | 5 | H | S | Z | D |
| 15 | Vicia nigricans Hook. & Arn. | 1 | Low Chall-huaco Valley | O–J | 5 | 5 | H | P | Z | D |
| Gesneriaceae | ||||||||||
| 16 | Asteranthera ovata (Cav.) Hanst. | 1 | Puerto Blest | D–A | 4 | 5 | E | R | Z | D |
| 17 | Mitraria coccinea Cav. | 1 | Puerto Blest | D–A | 5 | 5 | E | O–R | A | D |
| Grossulariaceae | ||||||||||
| 18 | Ribes magellanicum Poir. | 1 | Otto Hill | O–D | 4 | 5 | S | Y | A | S |
| 2 | Lake Escondido | 1 | 5 | |||||||
| 3 | Llao–Llao Hill | 3 | 5 | |||||||
| Loranthaceae | ||||||||||
| 19 | Tristerix corymbosus (L.) Kuijt | 1 | Peninsula San Pedro | M–N | 5 | 5 | P | R | A | D |
| 2 | Llao–Llao Forest | 5 | 5 | |||||||
| Onagraceae | ||||||||||
| 20 | Fuchsia magellanica Lam. | 1 | Puerto Blest | N–My | 5 | 5 | S | R–Pu | A | D |
| 2 | Stream La Virgen | 4 | 5 | |||||||
| 21 | Oenothera odorata Jacq. | 1 | 237 Route (10 km) | N–A | 5 | 5 | H | Y | A | D |
| Philesiaceae | ||||||||||
| 22 | Lapageria rosea Ruiz & Pav. | 1 | Puyehue (Chile) | 2 | 5 | V | R | A | D | |
| Proteaceae | ||||||||||
| 23 | Embothrium coccineum J.R. Forst. & G. Forst. | 1 | Lake Quillen | O–J | 4 | 5 | S | R | Z | D |
| 2 | Traful | 5 | 5 | |||||||
| 3 | Piltriquitron Hill | 5 | 5 | |||||||
| 4 | Villa la Angostura | 5 | 5 | |||||||
| 5 | Otto Hill | 5 | 5 | |||||||
| 6 | Puerto Blest | 5 | 5 | |||||||
| 7 | Bariloche Airport road | 5 | 5 | |||||||
| 8 | Lake Espejo | 5 | 5 | |||||||
| 9 | Puyehue (Chile) | 5 | 5 | |||||||
| 24 | Lomatia ferruginea (Cav.) R. Br. | 1 | Puerto Blest | D–F | 5 | 5 | T | Y–P | Z | S |
| Scrophulariaceae | ||||||||||
| 25 | Ourisia poeppigii Benth. | 1 | Otto Hill | O–J | 3 | 5 | H | R | A | D |
| Verbenaceae | ||||||||||
| 26 | Diostea juncea (Gillies & hook.) Miers | 1 | Low Chall-huaco Valley | N–F | 5 | 5 | S | S | A | D |
Cod, species code number; P, population number; Locality, sampling site; Date, sampling date; Ph, flowering period (J, January, F; February; M, March; A, April; My, May; S, September; O, October; N, November; D, December); I, number of individuals sampled per population; F, number of flowers sampled per individual; GF, growth form (T, tree; S, shrub; E, epiphyte; V, vine; H, herbaceous; P, hemiparasite); C, flower colour (G, green; L, lilac; O, orange; P, pink; Pu, purple; R, red; S, sky-blue; Y, yellow; WR, wine-red); S, flower symmetry (A, actinomorphic; Z, zygomorphic); D, flower depth [D, deep (corolla >1 cm); S, shallow (corolla ≤1 cm)].
Nectar was extracted with capillary glass tubes from one to five flowers per individual and placed together on Whatman No. 1 chromatography paper (i.e. nectar samples obtained from different flowers of an individual were pooled for chromatographic analysis). The number of flowers sampled per plant was variable because of differences in the number of open flowers available among individuals of the same species and among species (Table 1). Likewise, the number of plants sampled per population was variable because of differences in the availability of flowering individuals (Table 1). Only recently opened flowers were sampled and nectar extraction was always carried out on sunny days around noon, although some species can be pollinated during the night.
Nectar sugar concentration in sucrose equivalents [% sugar = (sugar mass/total mass) × 100] was measured with a hand refractometer (Reichert-Jung; range 0–50 %) only for samples with volumes ≥1 µl because of the reading threshold of the refractometer. Nectar sugar composition was analysed using gas–liquid chromatography. Nectar was lyophilized and silylated following Sweeley et al. (1963). Derivatives were then injected into a Konik KNK 3000-HRGS gas–liquid chromatograph equipped with a Spectra-Physics SP 4290 data integrator, a flame ionization detector and an OV1 2 m column. Nitrogen was the carrier gas (30 ml min−1) and the temperature programme used was 208 °C for 1 min, 1 °C min−1 until 215 °C, 8 °C min−1 until 280 °C, and maintained for 5 min. Chromatographic sugar analyses were repeated at least twice for each sample in order to control for experimental errors. The sugar ratio (r) was calculated as r = sucrose/(fructose + glucose) following Baker and Baker (1983). These authors proposed four sugar ratio categories: sucrose dominant (r > 0·999), sucrose rich (0·999–0·5), hexose rich (0·499–0·1) and hexose dominant (r < 0·1).
To evaluate the occurrence of relationships between pollinators and nectar characteristics (as suggested by Baker and Baker, 1983), the main flower type for each plant species was determined. Our flower-type concept is relatively similar to the ‘pollination syndrome’ classification (Wyatt, 1983; Proctor et al., 1996), but not only floral characteristics were taken into account but also all information available on their actual associated pollinators or flower visitors. For this purpose, data on flowering phenology, and flower colour, symmetry, depth, and shape were compiled from Brion et al. (1988), Correa (1969–1988) and from field observations made by the authors. Information on the flower-visiting fauna of these species was compiled from published records (Riveros, 1991; Ruffini, 1992; Smith-Ramírez, 1993; Forcone et al., 1997; Aizen and Ezcurra, 1998; Bernardello et al., 1999; Aizen et al., 2002; Vázquez and Simberloff, 2003) and unpublished field observations (C. Morales, pers. comm.; M. A. Aizen and V. R. Chalcoff, pers. obs.). Despite using all existing available sources, pollinator information for most plant species should be considered as best educated guesses because of a lack of data on actual measures of pollen transfer. However, it is felt that the present classification into broad categories reflects the main trends in plant–pollinator associations in the temperate forest of South America. According to this, species were classified as hummingbird-pollinated (mostly species with red and tubular corollas), diurnal short-tongued insect-pollinated (mostly species with shallow corollas ≤1 cm visited by small bees and flies), diurnal long-tongued insect-pollinated (species with corolla tubes >1 cm but not red, and visited by bumblebees and other large bees) and nocturnal insect-pollinated (species releasing strong odour and/or with nocturnal anthesis and visited by moths and/or sphingids) (Table 2). Only these four flower-type categories were considered because the number of plant species analysed in this study was not large enough to carry out a more detailed classification.
Table 2.
Nectar concentration, sugar proportions, sugar ratio (r) and flower type (FT) of 26 species from the temperate forest of southern South America
| Species | P | % Conc. | S | F | G | Uk | r | FT |
|---|---|---|---|---|---|---|---|---|
| Alstroemeriaceae | ||||||||
| Alstroemeria aurea | 1 | 40·7 | 47·3 ± 21·5 | 18·5 ± 4·2 | 34·2 ± 19·25 | – | 0·89 | DLTI |
| Amaryllidaceae | ||||||||
| Rhodophiala mendocina | 1 | 12 ± 2·64 | 1·4 ± 2·23 | 38·7 ± 7·21 | 59·9 ± 7·74 | – | 0·01 | DLTI |
| Asclepiadaceae | ||||||||
| Cynanchum diemii | 1 | 48·5 ± 2·12 | 94·9 ± 5·31 | 2·7 ± 2·96 | 2·4 ± 2·37 | – | 18·61 | NI |
| Asteraceae | ||||||||
| Mutisia decurrens | 1 | 41·9 ± 5·48 | 45·8 ± 13·44 | 26·5 ± 6·14 | 27·7 ± 8·94 | – | 0·85 | DLTI |
| 2 | 25 | 54·6 ± 0·01 | 19·3 ± 0·02 | 26·1 ± 0·04 | – | 1·2 | ||
| Overall mean | 33·5 ± 11·95 | 50·2 ± 6·22 | 22·9 ± 5·11 | 26·9 ± 1·11 | – | 1·01 | ||
| Mutisia spinosa | 1 | 41·3 ± 1·06 | 71·3 ± 8·92 | 16·3 ± 5·68 | 12·4 ± 6·15 | – | 2·48 | DLTI |
| 2 | 50·3 ± 5·3 | 73·1 ± 8·21 | 19·2 ± 4·22 | 7·7 ± 3·98 | – | 2·72 | ||
| Overall mean | 45·8 ± 6·36 | 72·1 ± 1·27 | 17·8 ± 2·03 | 10·1 ± 3·31 | – | 2·58 | ||
| Perezia prenanthoides | 1 | 42·7 ± 6·52 | 52 ± 13·6 | 22·7 ± 8·52 | 25·3 ± 6·85 | – | 1·08 | DSTI |
| Berberidaceae | ||||||||
| Berberis buxifolia | 1 | Nd | 58·8 ± 0·26 | 10·8 ± 0·47 | 30·4 ± 0·74 | – | 1·43 | DSTI |
| 2 | Nd | 80·3 ± 5·54 | 8·2 ± 6·17 | 11·5 ± 0·62 | – | 4·08 | ||
| Overall mean | 69·6 ± 15·16 | 9·5 ± 1·78 | 20·9 ± 13·37 | – | 2·29 | |||
| Berberis darwinii | 1 | 42·5 | 91 ± 5·7 | 2 ± 1·97 | 3·9 ± 4·2 | 3·1 ± 0·47 | 15·42 | DSTI |
| 2 | 30 | 91·5 ± 3·51 | 1·2 ± 0·24 | 2·6 ± 0·44 | 4·7 ± 2·82 | 24·08 | ||
| Overall mean | 36·3 ± 8·83 | 91·2 ± 0·35 | 1·6 ± 0·54 | 3·3 ± 0·96 | 3·9 ± 1·15 | 18·61 | ||
| Bignoniaceae | ||||||||
| Campsidium valdivianum | 1 | 23·4 ± 2·88 | 46 ± 10·4 | 25·4 ± 5·17 | 28·6 ± 9·6 | – | 0·85 | HUM |
| Eccremocarpus scaber | 1 | 27 ± 4·32 | 65·1 ± 11·41 | 23·4 ± 8·01 | 11·5 ± 4·03 | – | 1·87 | HUM |
| Desfontainiaceae | ||||||||
| Desfontainia spinosa | 1 | 20·6 ± 3·41 | 76·3 ± 10·03 | 19·4 ± 9·3 | 4·3 ± 4·77 | – | 3·22 | HUM |
| Elaeocarpaceae | ||||||||
| Aristotelia chilensis | 1 | Nd | 17·1 ± 0·05 | 41·4 ± 0·42 | 41·5 ± 0·37 | – | 0·2 | DSTI |
| Escalloniaceae | ||||||||
| Escallonia rubra | 1 | 51·7 ± 11·4 | 26·4 ± 15·84 | 39·4 ± 8·18 | 34·2 ± 10·24 | – | 0·36 | HUM |
| Fabaceae | ||||||||
| Lathyrus multiceps | 1 | Nd | 58·6 ± 5·98 | 21·8 ± 3·54 | 19·6 ± 6·74 | – | 1·42 | DLTI |
| Vicia nigricans | 1 | 29·5 ± 9·25 | 45·8 ± 28·32 | 34·4 ± 19·03 | 19·8 ± 12·29 | – | 0·84 | DLTI |
| Gesneriaceae | ||||||||
| Asteranthera ovata | 1 | 29·7 ± 5·48 | 86·4 ± 5·42 | 10·8 ± 4·82 | 2 ± 1·49 | 0·8 ± 1·41 | 6·75 | HUM |
| Mitraria coccinea | 1 | 30·9 ± 7·43 | 78·9 ± 8·89 | 17·3 ± 8·24 | 2·8 ± 2·2 | 1 ± 0·71 | 3·93 | HUM |
| Grossulariaceae | ||||||||
| Ribes magellanicum | 1 | 16 ± 2·82 | 54·5 ± 8·36 | 23·3 ± 6·71 | 22·2 ± 1·66 | – | 1·2 | DSTI |
| 2 | Nd | 90·9 ± 1·52 | 5·8 ± 1 | 3·3 ± 0·53 | – | 9·99 | ||
| 3 | 13 | 57·1 ± 0·52 | 23·1 ± 0·9 | 19·8 ± 1·18 | – | 1·33 | ||
| Overall mean | 14·5 ± 2·12 | 67·5 ± 20·3 | 17·4 ± 10·04 | 15·1 ± 10·28 | – | 2·08 | ||
| Loranthaceae | ||||||||
| Tristerix corymbosus | 1 | 30·8 ± 6·3 | 55·3 ± 6·71 | 19·1 ± 3·13 | 25·6 ± 4·32 | – | 1·24 | HUM |
| 2 | 29·7 ± 5·46 | 45 ± 7·08 | 19·6 ± 2·95 | 35·4 ± 4·13 | – | 0·82 | ||
| Overall mean | 30·3 ± 0·77 | 50·2 ± 7·27 | 19·4 ± 0·31 | 30·4 ± 6·95 | – | 1·01 | ||
| Onagraceae | ||||||||
| Fuchsia magellanica | 1 | 23·9 ± 2·74 | 66 ± 21·61 | 21·8 ± 15·58 | 12·2 ± 7·03 | – | 1·94 | HUM |
| 2 | 25·3 ± 0·5 | 65·5 ± 11·17 | 21 ± 2·41 | 13·5 ± 8·97 | – | 1·9 | ||
| Overall mean | 24·6 ± 0·98 | 65·8 ± 0·38 | 21·3 ± 0·55 | 12·9 ± 0·93 | – | 1·92 | ||
| Oenothera odorata | 1 | 40·7 ± 0·57 | 95·1 ± 3·58 | 3 ± 1·74 | 1·9 ± 1·9 | – | 19·41 | NI |
| Philesiaceae | ||||||||
| Lapageria rosea | 1 | 27 ± 5·65 | 91·8 ± 8·11 | 3 ± 2·53 | 5·2 ± 5·57 | – | 11·2 | HUM |
| Proteaceae | ||||||||
| Embothrium coccineum | 1 | 35·3 ± 0·35 | 67·8 ± 14·87 | 16·7 ± 6·91 | 15·5 ± 7·96 | – | 2·1 | HUM– |
| 2 | 22·3 ± 1·06 | 75·6 ± 6·86 | 10·1 ± 2·96 | 14·2 ± 3·9 | – | 3·12 | (P) | |
| 3 | 24 ± 5·56 | 65 ± 5·29 | 12·5 ± 6·29 | 22·5 ± 3·54 | – | 1·86 | ||
| 4 | 47·4 ± 3·97 | 97·5 ± 1·44 | 1·1 ± 0·53 | 1·4 ± 0·68 | – | 39 | ||
| 5 | 45·2 ± 2·96 | 66·1 ± 11·25 | 15·5 ± 5·54 | 18·4 ± 6·11 | – | 1·95 | ||
| 6 | 20·9 ± 10·47 | 88 ± 7·54 | 5·7 ± 4·04 | 6·3 ± 3·6 | – | 7·33 | ||
| 7 | 21·6 ± 6·42 | 85·3 ± 3·66 | 6·4 ± 1·64 | 8·3 ± 2·24 | – | 5·8 | ||
| 8 | 42·3 ± 10·51 | 94·9 ± 2·84 | 2·1 ± 1·25 | 3 ± 1·61 | – | 18·23 | ||
| 9 | Nd | 38·4 ± 12·33 | 28·3 ± 19·24 | 33·3 ± 6·9 | – | 0·62 | ||
| Overall mean | 29·9 ± 9·79 | 75·4 ± 18·52 | 10·9 ± 8·52 | 13·7 ± 10·22 | – | 3·06 | ||
| Lomatia ferruginea | 1 | Nd | 36 ± 21·98 | 37·7 ± 14·49 | 26·3 ± 7·54 | – | 0·56 | DSTI |
| Scrophulariaceae | ||||||||
| Ourisia poeppigii | 1 | 28 | 34·9 ± 2·29 | 29·4 ± 0·66 | 35·7 ± 1·62 | – | 0·54 | HUM |
| Verbenaceae | ||||||||
| Diostea juncea | 1 | 34·8 ± 15·21 | 32·1 ± 18·41 | 30·2 ± 13 | 37·7 ± 7·66 | – | 0·47 | DLTI |
P, population number (see Table 1); % Conc., nectar concentration (mass/mass as percentage); sugar proportions for S, sucrose; F, fructose; G, glucose; Uk, unknown sugar; r, sugar ratio [S/(F + G)]; FT, flower-type categories (DLTI, diurnal long-tongued insects; DSTI, diurnal short-tongued insects; HUM, hummingbirds; NI, nocturnal insects; P, passerines).
Concentration and sugar percentage values are population means ± standard deviation.
Nd, no data available.
To analyse the effect of plant–pollinator association on nectar sugar concentration and nectar sugar composition, one-way ANOVAs with flower type as the independent variable was used followed by a posteriori Tukey test where appropriate. Because all variables were expressed as percentages or proportions, the arcsin root-square transformation following Sokal and Rohlf (1981) was used.
RESULTS
Overall characteristics of nectar
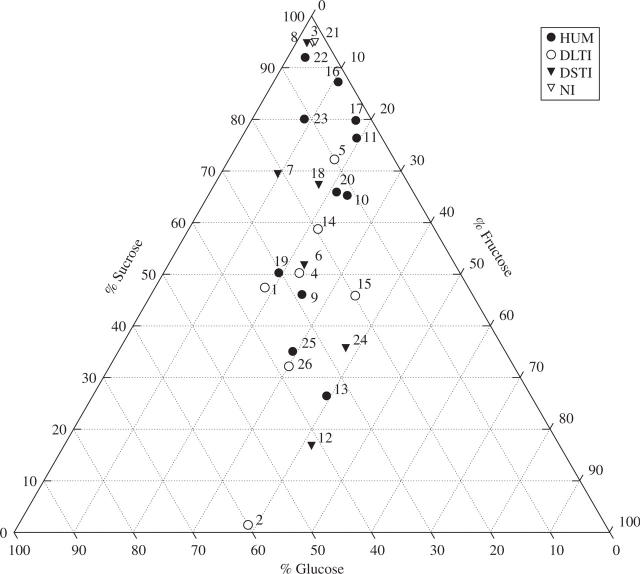
Mean nectar concentration (± standard deviation) for all species was 31·9 ± 10·23 %, ranging from 12 % (Rhodophiala mendocina) to 51·7 % (Escallonia rubra) (Table 2). The three most common sugars (sucrose, glucose and fructose) were found in the nectar of all the 26 species studied. The nectar composition of Asteranthera ovata, Berberis darwinii and Mitraria coccinea included a low percentage (<5 %) of an unknown sugar (Table 2). Nectar of 17 of the 26 species (i.e. 65·4 %) of the species was sucrose dominant (Table 2 and Fig. 1). It is interesting to see in Fig. 1 that the nectar of most species is distributed non-randomly from the top angle of the compositional triangle to the middle of the bottom axis (about 65 %) along the isoline of equal proportions of glucose and fructose.
Fig. 1.
Ternary diagram of sugar composition for the nectar of 26 species of the temperate forest of southern South America. The numbers refer to the species codes listed in Table 1. Symbols refer to flower-type categories (HUM, hummingbirds; DLTI, diurnal long-tongued insects; DSTI, diurnal short-tongued insects; NI, nocturnal insects. See Table 2).
Striking differences in nectar concentration and composition between species of different genera within the same family for species belonging to contrasting flower types were observed (Table 2). In the Onagraceae, Fuchsia magellanica is hummingbird-pollinated whereas Oenothera odorata is nocturnal insect-pollinated, the latter species showing a higher sugar concentration and proportion of sucrose. In addition, in the Proteaceae, Embothrium coccineum is hummingbird-pollinated whereas Lomatia ferruginea is diurnal short-tongued insect-pollinated, the first species having sucrose-dominant nectar.
The sample used in this study also included pairs of species of the same genus: Mutisia (Asteraceae) and Berberis (Berberidaceae) (each species is represented by two populations; Table 1). Species from these two genera were classified as diurnal long-tongued insect-pollinated, and diurnal short-tongued insect-pollinated, respectively. Nectar comparisons showed that M. decurrens and B. buxifolia had a lower proportion of sucrose than nectars of M. spinosa and B. darwinii (Table 2). In Berberis darwinii, a fourth unknown sugar that was not present in B. buxifolia was also detected (Table 2).
Intraspecific variation in nectar traits was evaluated in those species with two or more populations sampled (six species were represented by two populations, Ribes magellanicum by three populations, and Embothrium coccineum by nine populations; Table 1). Particularly, large interpopulation variability (i.e. CVs >50 %) in sugar composition was observed for B. darwinii, R. magellanicum and E. coccineum (Table 3).
Table 3.
Coefficient of variation [CV = (SD/species mean) × 100; data are from populations] for sugar concentration (% Conc.) and nectar proportion of sucrose (S), fructose (F) and glucose (G) for the eight species with more than one population studied
| N pop. | % Con | S | F | G | |
|---|---|---|---|---|---|
| Mutisia decurrens | 2 | 35·7 | 12·4 | 22·3 | 4·1 |
| Mutisia spinosa | 2 | 13·9 | 1·7 | 11·4 | 32·8 |
| Berberis buxifolia | 2 | Nd | 21·7 | 18·7 | 63·9 |
| Berberis darwinii | 2 | 24·3 | 0·38 | 33·7 | 29·1 |
| Ribes magellanicum | 3 | 14·6 | 30·1 | 57·7 | 68·1 |
| Tristerix corymbosus | 2 | 2·5 | 14·5 | 1·6 | 22·8 |
| Fuchsia magellanica | 2 | 3·9 | 0·58 | 2·6 | 7·2 |
| Embothrium coccineum | 9 | 32·7 | 24·6 | 78·2 | 74·6 |
N pop., number of populations (see Table 1).
The values in bold correspond to CVs >50 %.
Nd, no data available.
Nectar traits and flower types
Nectar concentration did not differ significantly among flower-type categories (one-way ANOVA, F3, 18 = 1·19, P = 0·34). Nevertheless, mean nectar concentration was comparatively lower for hummingbird-pollinated species (27·0 ± 3·6 %), intermediate for diurnal long-tongued and diurnal short-tongued insect-pollinated species (32·7 ± 11·7 % and 31·2 ± 14·8 %, respectively), and higher for nocturnal insect-pollinated species (44·6 ± 5·5 %) (Table 4).
Table 4.
Mean nectar concentration [% Conc.: (sugar mass/total mass) × 100], sugar proportions (S, sucrose; F, fructose; G, glucose), and sugar ratio [r, S/(F+G)] for the different flower type categories (DSTI, diurnal short-tongued insect-pollinated; DLTI, diurnal long-tongued insect-pollinated; HUM, hummingbird-pollinated; NI, nocturnal insect-pollinated)
| n | % Conc. | S | F | G | r | |
|---|---|---|---|---|---|---|
| DSTI | 6 | 31·16 ± 14·78 | 56·18 ± 27·42 | 21·73 ± 15·57 | 22·08 ± 12·68 | 4·14 ± 7·14 |
| DLTI | 7 | 32·72 ± 11·66 | 43·93 ± 22·4 | 26·33 ± 8·16 | 29·74 ± 16·27 | 1·03 ± 0·81 |
| HUM | 11 | 27·02 ± 3·59 | 63·93 ± 21·76 | 19·82 ± 10·04 | 16·26 ± 13·27 | 3·24 ± 3·26 |
| NI | 2 | 44·60 ± 5·52 | 95·00 ± 0·14 | 2·80 ± 0·21 | 02·20 ± 0·35 | 19·0 ± 0·56 |
Values are group means ± standard deviation.
n = number of species in each category group.
Hummingbird- and nocturnal insect-pollinated species showed higher sucrose proportions than diurnal short- and long-tongued insect-pollinated species (Table 4 and Fig. 1). Hummingbird-, diurnal short-tongued insect- and long-tongued insect-pollinated species showed a mean nectar sugar composition with comparable variabilities (Table 4). On the other hand, nocturnal insect-pollinated species showed lower variability for nectar sugar composition as well as for nectar concentration, although a low number of species was sampled in this group (Table 4). Significant differences were found among flower-type groups for the sugar ratio (r) (F3,22 = 7·66, P = 0·001), and sucrose percentage (F3,22 = 3·3, P = 0·039), but a posteriori tests showed that these differences could be attributed to the high sucrose content of the nocturnal insect-pollinated species in comparison with the other three remaining groups. In spite of this, the differences are basically among the nocturnal insect-pollinated group (sugar ratio of 19 and sucrose proportion of 95 %) and the diurnal long-tongued insect-pollinated group (sugar ratio of 1·03 and sucrose proportion of 43 %).
DISCUSSION
In general terms, the present results showed that average nectar concentration for species of the temperate forest of southern South America was low, particularly in comparison to the average nectar concentration found among species from different sites of the neighbouring Patagonian steppe [31·9 % for temperate forest in comparison to 44·6 and 42·4 % for steppe sites; authors' data, Forcone et al. (1997) and Bernardello et al. (1999), respectively]. This trend could be related to the large proportion of hummingbird-pollinated species of the temperate forest of southern South America, reflecting the higher incidence of ornithophily in this region compared with the steppe (Aizen and Ezcurra, 1998). The deep corolla tubes characteristic of hummingbird-pollinated flowers are usually associated with nectars of low concentration due to either lower evaporation than in more open flowers (Plowright, 1987) or the innate preferences, physical limitations and special requirements of hummingbirds for sugar solutions of low viscosity (Pyke and Waser, 1981, and references therein). However, when hummingbird-pollinated species were excluded to estimate the mean sugar concentration for insect-pollinated plant species of the temperate forest, a comparatively low nectar concentration (34·45 ± 11·85 %) was still obtained.
Nectar concentration is highly influenced by environmental factors, especially temperature and humidity (Rathcke, 1992). The differences in mean nectar concentration between forest plants and those from the Patagonian steppe (Forcone et al., 1997; Bernardello et al., 1999) can also be explained when considering the contrasting environmental particularities of these two regions. The relatively low mean nectar concentration of temperate forest species could be related to the lower mean maximum temperatures and higher precipitation characteristic of the forest environment in comparison with the nearby steppe (Barros et al., 1983).
In general terms, the present results show that nectar composition of species from the temperate forest of southern South America is mainly sucrose dominant. Only a low number of plant species had hexose-dominant (only Rodophiala mendocina) or hexose-rich nectars (Aristotelia chilensis, Escallonia rubra and Diostea juncea). This trend is again in contrast with the Patagonian steppe where nectars are mainly hexose rich or hexose dominant (Forcone et al., 1997; Bernardello et al., 1999).
Plant species of the temperate forest of southern South America did not show a clear-cut association between nectar concentration, sugar composition and pollinators. However, some weak trends resulted from the present analysis. For example, hummingbird- and nocturnal insect- pollinated species secrete nectars dominated by sucrose, whereas diurnal long- and short-tongued insect-pollinated flowers tend to produce nectar with a similar proportion between sucrose and hexoses (i.e. sucrose–hexose balanced nectars), suggesting a convergence in sugar composition of some species from different families according to the pollinators. Hummingbird flowers have been reported elsewhere to produce nectars with a relatively high proportion of sucrose (e.g. Cruden et al., 1983; Freeman et al., 1984; Gottsberger et al., 1984; Freeman and Worthington, 1985; Elisens and Freeman, 1988; Stiles and Freeman, 1993) and, in general, these birds prefer sucrose- over hexose-rich solutions (Hainsworth and Wolf, 1976; Stiles, 1976; Martínez del Rio, 1990). In the case of Sephanoides sephaniodes, the preference of this species for sucrose-rich solutions was experimentally corroborated by field experiments (Chalcoff, 2001).
It is interesting to point out that Ourisia poeppigii, a species with red tubular flowers but without field records of hummingbird visits (it was considered as a hummingbird-pollinated species based solely on the analysis of floral traits), showed the lowest relative proportion of sucrose among the sample of species with tubular red flowers from the temperate forest of South America. In addition, few records of hummingbird visits have been reported (Fraga et al., 1997) for Escallonia rubra, a species with a comparatively high nectar concentration with a low percentage of sucrose. Nevertheless, when these species are excluded from the analysis, the trends observed did not change. In addition, Ourisia poeppigii apparently has a high fruit and seed set, which suggests a highly autogamous breeding system, despite producing showy flowers (M. A. Aizen, pers. obs.).
In general, nectar traits were shown to be highly variable at any taxonomic scale. This is the case for the two species pairs analysed that were from different genera but belonging to the same family (e.g. Onagraceae and Proteaceae). Each member of these pairs of species is characterized by a particular nectar composition that seems to relate to its association with different pollinators. Nevertheless, pairs of congeneric species (Mutisia and Berberis) present divergent sugar ratios despite their association with similar pollinator assemblages (Table 2).
Variation at the intraspecfic level can be exemplified by the study of nectar traits in nine populations of E. coccineum. This species, a self-incompatible endemic tree of this forest, seems to have divergent bird-pollinator assemblages on the Chilean and Argentine side of the Andes (Fraga et al., 1997; Smith-Ramírez and Armesto, 1998). Accordingly, the present results show a divergent nectar composition pattern, which agrees with reported differences in nectar preferences by their two main pollinator types. The Chilean populations are reported as passerine-pollinated and with nectars dominated by hexoses (Smith-Ramírez and Armesto, 1998; M. Riveros, pers. comm.), and the nectar of the only Chilean population reported here is also dominated by hexoses. In contrast, the Argentine populations are mainly hummingbird-pollinated (Fraga et al., 1997; V. R. Chalcoff, pers. obs.) and their nectars are dominated by sucrose (Bernardello et al., 1999; and this study). In addition to hummingbirds, some diurnal insects have been reported to be pollinators of this species in Argentina, at the northern range of its distribution (Devoto et al., 2006). There is no actual evidence of flower visits by passerines in the Argentine populations, despite their high abundance during spring and summer (Amico and Aizen, 2005; M. A. Aizen, pers. obs.). Thus, nectar-sugar composition in this species seems to be closely related to different bird-pollinator assemblages on both sides of the Andes, and at least part of the regional variation in the nectar sugar composition could be explained by divergent selection mediated by these birds. However, a large amount of variation was still found in nectar traits among Argentine populations of E. coccineum that could be related to environmental plasticity, local selective factors and genetic drift. This large intraspecific variation in nectar sugar composition shows the underlying potential for evolutionary change in nectar traits under different selective pressures (cf. Schluter, 2000), a situation that merits further investigation.
Nevertheless, most of the present results showed that pollinators are not the only force modelling nectar traits, particularly sugar composition, in the flora of the temperate forest of southern South America. Other authors have pointed out a more complex evolutionary scenario which includes interactions with herbivores, nectar robbers, seed predators and/or seed dispersers influencing different reproductive traits in plants (e.g. Armbruster, 1997; Galen, 1999; Herrera, 2000; Aizen, 2003; Irwin et al., 2004). In the case of the flora of the temperate forest of southern South America, it is felt that its complex and ancient biogeographical history and its current development and occurrence on striking environmental gradients (Aizen and Ezcurra, 1998) may be important in understanding present patterns in nectar characteristics.
Acknowledgments
We thank C. Ezcurra and C. Morales for critically reading and making constructive comments on an earlier draft of this manuscript, and A. Jürgens, R. Wesselingh and D. Levin for their suggestions and comments that improved previous versions of this paper. We also thank Nahuel Huapi National Park authorities for allowing us to work in the park, and CONICET, SECyT (UNC) and FONCYT for financial support. Finally, we thank J. Grosfeld for providing the photograph for the content snapshots of this issue of Annals of Botany.
LITERATURED CITED
- Aizen MA. 2003. Influences of animal pollination and seed dispersal on winter flowering in a temperate mistletoe. Ecology 84: 2613–2627. [Google Scholar]
- Aizen MA, Ezcurra C. 1998. High incidence of plant–animal mutualisms in the woody flora of the temperate forest of southern South America: biogeographical origin and present ecological significance. Ecologia Austral 8: 217–236. [Google Scholar]
- Aizen MA, Vazquez DP, Smith-Ramírez C. 2002. Historia natural y conservación de los mutualismos planta-animal del bosque templado de Sudamérica austral. Revista Chilena de Historia Natural 75: 79–97. [Google Scholar]
- Amico GC, Aizen MA. 2005. Dispersión de semillas por aves en un bosque templado de Sudamérica austral: ¿quién dispersa a quién? Ecología Austral 15: 89–100.
- Armbruster WS. 1997. Exaptations link the evolution of plant–herbivore and plant–pollinator interactions: a phylogenetic inquiry. Ecology 78: 1661–1674. [Google Scholar]
- Armesto JJ, Rozzi R. 1989. Seed dispersal syndromes in the rain forest of Chiloé: evidence for the importance of biotic dispersal in a temperate rain forest. Journal of Biogeography 16: 219–226. [Google Scholar]
- Armesto JJ, Smith-Ramírez C, Sabag C. 1996. The importance of plant–bird mutualism in the temperate rainforest of southern South America. In: Lawford RG, Alaback PB, Fuentes E, eds. High latitude rainforest and associated ecosystems of the west coast of the Americas. New York: Springer, 248–265.
- Baker HG, Baker I. 1975. Studies of nectar-constitution and pollinator–plant coevolution. In: Gilbert LE, Raven PH, eds. Coevolution of animals and plants. Austin: University of Texas Press, 100–140.
- Baker HG, Baker I. 1983. Floral nectar sugars constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold Co., 117–141.
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany 39: 157–166. [Google Scholar]
- Baker HG, Baker I, Hodges SA. 1998. Sugar composition of nectars and fruits consumed by birds and bats in the tropics deciduous forests. Biotropica 30: 559–586. [Google Scholar]
- Barros VR, Cordon VH, Moyano CL, Mendez RJ, Forquera JC, Picio O. 1983. Cartas de precipitación de la zona oeste de las provincias de Río Negro y Neuquen. Universidad Nacional del Comahue. Centro Nacional Patagónico—CONICET, Facultad de Ciencias Agrarias, Cinco Saltos, Río Negro, Argentina.
- Bernardello G, Galetto L, Forcone A. 1999. Floral nectar chemical composition of some species from Patagonia. II. Biochemical Systematics and Ecology 27: 779–790. [Google Scholar]
- Brion C, Puntieri J, Grigera D, Calvelo S. 1988. Flora de Puerto Blest y sus alrededores. Universidad Nacional del Comahue, Centro Regional Universitario Bariloche, San Carlos de Bariloche.
- Chalcoff VR. 2001. Composición de azúcares de la flora ornitófila del Bosque Templado de Sudamérica Austral: efectos históricos y preferencias presentes de Sephanoides sephaniodes. Undergraduate Thesis, Universidad Nacional del Comahue, Argentina.
- Correa MN. 1969–1988. Flora Patagónica 2, 3, 4a, 4b, 5, 7. Colección Científica. INTA, Buenos Aires.
- Cruden RW, Hermann SM, Peterson S. 1983. Patterns of nectar production and plant-pollinator coevolution. In: Bentley B, Elias TS, eds. The biology of nectaries. New York: Columbia University Press, 80–125.
- Devoto M, Montaldo NH, Medan D. 2006. Mixed hummingbird–long-proboscid-fly pollination in ‘ornithophilous’ Embothrium coccineum (Proteaceae) along a rainfall gradient in Patagonia, Argentina. Austral Ecology, In press.
- Dupont YL, Hansen DM, Rasmussen JT, Olesen JM. 2004. Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Functional Ecology 18: 670–676. [Google Scholar]
- Elisens WJ, Freeman CE. 1988. Floral nectar sugar composition and pollinator type among new world genera in tribe Antirrhineae (Scrophulariaceae). American Journal of Botany 75: 971–978. [Google Scholar]
- Faegri K, van der Pijl L. 1979. The principles of pollination ecology. Oxford/New York: Pergamon Press.
- Forcone A, Galetto L, Bernardello L. 1997. Floral nectar chemical composition of some species from Patagonia. Biochemical Systematics and Ecology 25: 395–402. [Google Scholar]
- Fraga RM, Ruffini AE, Grigera D. 1997. Interacciones entre el picaflor rubí Sephanoides sephaniodes y las plantas del bosque Subantártico en el Parque Nacional Nahuel Huapi, Argentina. Hornero 14: 224–234. [Google Scholar]
- Freeman CE, Worthington RD. 1985. Some floral nectar-sugar compositions of species from southeastern Arizona and southwestern New Mexico. Madroño 32: 78–86. [Google Scholar]
- Freeman CE, Reid WH, Becvar JE, Scogin R. 1984. Similarity and apparent convergence in the nectar-sugar composition of some hummingbird-pollinated flowers. Botanical Magazine 145: 132–135. [Google Scholar]
- Galen C. 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience 49: 631–640. [Google Scholar]
- Galetto L, Bernardello G. 2003. Sugar nectar composition in angiosperms from Chaco and Patagonia (Argentina): an animal visitor's matter? Plant Systematics and Evolution 238: 69–86. [Google Scholar]
- Galetto L, Bernardello G, Sosa CA. 1998. The relationship between floral nectar composition and visitors in Lycium (Solanaceae) from Argentina and Chile: what does it reflect? Flora 193: 303–314. [Google Scholar]
- Gottsberger G, Schrauwen J, Linskens HF. 1984. Amino acids and sugars in nectar, and their putative evolutionary significance. Plant Systematics and Evolution 145: 55–77. [Google Scholar]
- Hainsworth FR, Wolf LL. 1976. Nectar characteristics and food selection by hummingbirds. Oecologia 25: 101–113. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 2000. Measuring the effects of pollinators and herbivores: evidence for non-additivity in a perennial herb. Ecology 81: 2170–2176. [Google Scholar]
- Hingston AB, Mc Quillan PB. 2000. Are pollination syndromes useful predictors of floral visitors in Tasmania? Austral Ecology 25: 600–609. [Google Scholar]
- Irwin RE, Adler LS, Brody AK. 2004. The dual role of floral traits: pollinator attraction and plant defense. Ecology 85: 1503–1511. [Google Scholar]
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Kevan PG, Baker HG. 1999. Insects on flowers. In: Huffaker CB, Elias AP, eds. Ecological Entomology, 2nd edn. New York: John Wiley and Sons, 553–584.
- Martínez del Rio C. 1990. Sugar preferences in hummingbirds: the influence of subtle chemical differences on food choice. The Condor 92: 1022–1030. [Google Scholar]
- Martínez del Rio C, Baker HG, Baker I. 1992. Ecological and evolutionary implications of digestive processes: bird preferences and the sugar constituents of floral nectar and fruit pulp. Experientia 48: 544–551. [Google Scholar]
- Martínez del Rio C, Karasov WH, Levey DJ. 1989. Physiological basis and ecological consequences of sugars preferences in Cedar waxwings. The Auk 106: 64–71. [Google Scholar]
- Nicolson SW, Fleming PA. 2003. Nectar as food for birds: the physiological consequences of drinking dilute sugar solutions. Plant Systematics and Evolution 238: 139–153. [Google Scholar]
- Percival MS. 1961. Types of nectar in angiosperms. New Phytologist 60: 235–281. [Google Scholar]
- Perret M, Chautems A, Spichiger R, Peixoto M, Savalainen V. 2001. Nectar sugar composition in relation to pollination syndromes in Sinningieae (Gesneriaceae). Annals of Botany 87: 267–273. [DOI] [PubMed] [Google Scholar]
- Plowright RC. 1987. Corolla depth and nectar concentration: an experimental study. Canadian Journal of Botany 65: 1011–1013. [Google Scholar]
- Proctor MP, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Oregon Timber Press.
- Pyke GH, Waser NM. 1981. The production of dilute nectars by hummingbird and honeyeater flowers. Biotropica 13: 260–270. [Google Scholar]
- Rathcke BJ. 1992. Nectar distributions, pollinator behavior, and plant reproductive success. In: Hunter MD, Ohgushi T, Price PW, eds. Effects of resource distribution on animal–plant interactions. New York: Academic Press, 113–138.
- Riveros M. 1991. Biologia reproductiva en especies vegetales de dos comunidades de la zona templada del sur de Chile, 40°S. PhD Thesis, Universidad de Chile.
- Ruffini AE. 1992. Interacciones entre Sephanoides sephaniodes (Molina) y las plantas que poliniza en el bosque de Nothofagus. Undergraduate Thesis, Universidad Nacional del Comahue, Argentina.
- Schluter D. 2000. The ecology of adaptative radiation. New York, NY: Oxford University Press.
- Smith-Ramírez C. 1993. Los picaflores y su recurso floral en el bosque templado de la isla de Chiloé, Chile. Revista Chilena de Historia Natural 66: 65–73. [Google Scholar]
- Smith-Ramírez C, Armesto JJ. 1998. Nectarivoría y polinización por aves en Embothrium coccineum (Proteaceae) en el bosque templado del sur de Chile. Revista Chilena de Historia Natural 71: 53–65. [Google Scholar]
- Sokal RR, Rohlf FJ. 1981. Biometry, 2nd edn. New York, NY: Freeman.
- Stiles FG. 1976. Taste preferences, color preferences, and flower choice in hummingbirds. The Condor 78: 10–26. [Google Scholar]
- Stiles FG, Freeman CE. 1993. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25: 191–205. [Google Scholar]
- Sweeley EC, Bentley R, Makita M, Wells WW. 1963. Gas liquid chromatography of trimethylsilyl derivatives of sugars and related substances. Journal of American Chemistry Society 85: 2497–2507. [Google Scholar]
- Temeles EJ, Kress WJ. 2003. Adaptation in a plant–hummingbird association. Science 300: 630–633. [DOI] [PubMed] [Google Scholar]
- Vázquez DP, Simberloff D. 2003. Changes in interaction biodiversity induced by an introduced ungulate. Ecology Letters 6: 1077–1083. [Google Scholar]
- Willson MF, Smith-Ramírez C, Sabag C, Hernandez JH. 1996. Mutualismos entre plantas y animales en bosques templados de Chile. In: Armesto JJ, Villagran C, Arroyo MTK, eds. Ecología de los Bosques Nativos de Chile. Santiago de Chile: Editorial Universitaria, 251–264.
- Wyatt R. 1983. Pollinator–plant interactions and the evolution of breeding systems. In: Real L, eds. Pollination biology. Orlando, FL: Academic Press, 51–86.
- van Wyk BE. 1993. Nectar sugar composition in Southern African Papilionoideae (Fabaceae). Biochemical Systematics and Ecology 21: 271–277. [Google Scholar]
- van Wyk BE, Whitehead CS, Glen HF, Hardy DS, van Jaarsveld EJ, Smith GF. 1993. Nectar sugar composition in the subfamily Alooideae (Asphodelaceae). Biochemical Systematics and Ecology 21: 249–253. [Google Scholar]