Abstract
• Background Flowers have a species-specific, limited life span with an irreversible programme of senescence, which is largely independent of environmental factors, unlike leaf senescence, which is much more closely linked with external stimuli.
• Timing Life span of the whole flower is regulated for ecological and energetic reasons, but the death of individual tissues and cells within the flower is co-ordinated at many levels to ensure correct timing. Some floral cells die selectively during organ development, whereas others are retained until the whole organ dies.
• Triggers Pollination is an important floral cell death trigger in many species, and its effects are mediated by the plant growth regulator (PGR) ethylene. In some species ethylene is a major regulator of floral senescence, but in others it plays a very minor role and the co-ordinating signals involved remain elusive. Other PGRs such as cytokinin and brassinosteroids are also important but their role is understood only in some specific systems.
• Mechanisms In two floral cell types (the tapetum and the pollen-tube) there is strong evidence for apoptotic-type cell death, similar to that in animal cells. However, in petals there is stronger evidence for an autophagous type of cell death involving endoplasmic reticulum-derived vesicles and the vacuole. Proteases are important, and homologues to animal caspases, key regulators of animal cell death, exist in plants. However, their role is not yet clear.
• Comparison with Other Organs There are similarities to cell death in other plant organs, and many of the same genes are up-regulated in both leaf and petal senescence; however, there are also important differences for example in the role of PGRs.
• Conclusions Understanding gene regulation may help to understand cell death in floral organs better, but alone it cannot provide all the answers.
Keywords: Programmed cell death, flowers, petal, tapetum, pollen-tubes, senescence, ethylene, apoptosis, autophagy, ricinosomes, metacaspases, chromoplasts
INTRODUCTION: WHAT DO WE MEAN BY PROGRAMMED CELL DEATH IN FLORAL ORGANS?
Recently there has been much controversy over the use of the terms ‘senescence’ and ‘programmed cell death’ (PCD), especially with regard to leaves (Thomas et al., 2003; van Doorn and Woltering, 2004). In flowers it seems to me that the distinction is largely unnecessary. The deterioration of a flower is certainly programmed, is not a reversible process and inevitably leads to cell death. Thus, I have used the terms essentially interchangeably, using PCD more often when discussing the death of individual cell types, and senescence for whole organs.
WHY DO FLOWERS DIE, AND WHY DO THEY LAST LONGER IN SOME SPECIES THAN OTHERS?
Selective removal of reproductive structures is not unique to plants. Although sperm cells continue to be produced throughout male adult life, 99·9 % of human oocytes are removed by PCD (Tilly, 2001), perhaps ensuring that the costs of female reproduction are tightly regulated to benefit the survival of progeny to adulthood. However, unlike in mammals, both male and female reproductive structures in plants are only retained while they are needed, and are developed de novo, in perennial species, the following season. The duration of the flower is species-specific and carefully tailored to its ecological requirements. This is important because firstly the flower can be a substantial sink on the plant's resources, and as such is energetically expensive to maintain beyond its useful life (Ashman and Schoen, 1994). In addition, its architecture has been exploited by pathogens that use the stigma as a point of entry, and thus the flower poses an added risk of pathogen attack (Shykoff et al., 1996). Another important reason for floral death after pollination is to remove it from the population so that it does not compete for pollinators with the remaining blooms. One of the key triggers for petal death is pollination, which initiates a series of physiological events, orchestrated by plant growth regulators (PGRs). Ethylene is a clear regulator of petal senescence in some species (Stead and van Doorn, 1994); however, in other species including lilies such as Hemerocallis (daylilies) and Alstroemeria it appears to play little or no part (Woltering and van Doorn, 1988; Wagstaff et al., 2005). How petal senescence in these species is triggered and orchestrated remains unknown. Given the failure to find a common regulator for these species, and their taxonomic diversity, it seems likely that several inter-related mechanisms may be at play. Resource allocation has been one trigger proposed, and indeed removal of lower flowers in a cyme can lead to increased longevity of the first flower (Chanasut et al., 2003). However, this is clearly not a full explanation for all ethylene-insensitive species.
An important feature of floral death is that the different floral organs play very different roles. Hence, their life span needs to be appropriately co-ordinated. Likewise, the purpose and fate of the dying cells depends on the organ and tissues involved. At a whole organ level, petals, anthers and stigma are no longer required following pollination, whereas the ovary will mature to contain the developing seeds. In many species there is also a mechanism for rescuing resources from the degenerating organs such as petals, and diverting them to other parts of the plant such as the developing ovary (Stead and van Doorn, 1994). At a tissue and cell level, the situation is even more complex as there is a requirement for some reproductive tissues and cells to die to ensure correct development. For example, the tapetum must degenerate for pollen to develop properly, and synergid cells must die to allow fertilization. However, the fate of the dead cells is very different. In the case of the tapetum, cell contents are used to form the coat of the pollen grains, whereas removal of synergid cells is required for fertilization to occur (Christensen et al., 2002). Some types of cell death in floral organs also depend on specific genetic interactions. PCD occurs as a result of incompatible pollination events (Thomas and Franklin-Tong, 2004), and also as a result of defects in pollen development displayed in cytoplasmic male sterile lines (Balk and Leaver, 2001). Thus, important questions with regard to cell death in reproductive organs are: How do the cells perceive and respond to death signals, or, put more teleologically, how do they know when to die? Are the primary signals processed in the same way by the different organs and cells? Is the type of PCD in floral organs also found in other plant tissues and organs? I restrict myself here to considering the organs that make up the mature flower (Fig. 1). Although fruit ripening and seed maturation include further examples of PCD, these will not be considered here.
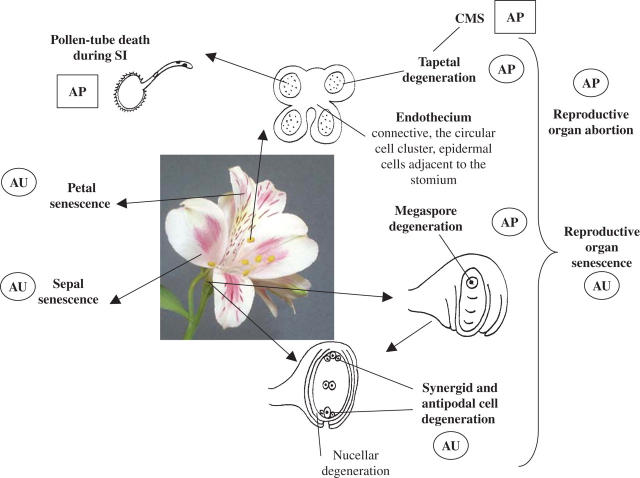
Fig. 1.
Sites of programmed cell death in floral organs. SI, self-incompatibility; CMS, cytoplasmic male sterility; AU, autophagous-like mechanism; AP, apoptotic-like mechanism; square indicates strong evidence, circle indicates weaker evidence. Bold text indicates cells and tissues discussed in the text.
HOW DO THE CELLS KNOW WHEN TO DIE?
In some species pollination dramatically shortens floral life span. For example, orchid flowers will last several months but senesce rapidly once pollinated. In several species, including Petunia, tobacco, carnation and orchids, senescence is mediated by the evolution of ethylene following contact between pollen and the stigmatal surface, which precedes fertilization (O'Neill, 1997). However, the exact nature of the primary signal resulting in ethylene evolution has not been established, although other PGRs and low-molecular-weight compounds have been implicated (O'Neill, 1997). In carnations, ethylene produced from the pollinated stigma is translocated, via the style and ovary, to the petals. Here it up-regulates ethylene biosynthetic genes and induces the production of ethylene in the petals (ten Have and Woltering, 1997). Once initiated, the evolution of ethylene becomes autocatalytic (Woodson and Lawton, 1988). This strongly suggests that promoters of the ethylene biosynthetic genes respond to ethylene and contain ethylene-responsive elements (EREs). To date, this has not been verified although an ERE from a senescence- and ethylene-regulated gene in carnation bears similarities to the ERE from an ethylene-responsive fruit-ripening gene, E4, suggesting commonality of transcription factors in these two processes (Deikman, 1997).
The response to ethylene is regulated by the production of ethylene receptors but how this regulation is achieved is not clear. In tomato an ETR1 (‘ethylene-resistant’)-type ethylene receptor was not transcribed in young flowers or senescent flowers, but only in mature flowers (Payton et al., 1996). Furthermore, ethylene receptor expression may itself be regulated by ethylene production. In pea, transcripts of an ERS (‘ethylene response sensor’)-type ethylene receptor were reduced when un-pollinated flowers were treated with an inhibitor of ethylene biosynthesis (Orzaez et al., 1999). So the balance between receptor production and ethylene sensitivity is clearly regulated at several levels.
Notably, in species in which ethylene is a major regulator, ethylene-independent signals are also present. Disruption of ethylene signalling or biosynthesis in carnation and petunia results in delayed floral death, but the flowers do eventually die (Michael et al., 1993). Perhaps it is these endogenous signals that are active in species where ethylene is not a major regulator. Several global transcriptomic studies (e.g. Alstroemeria: Breeze et al., 2004; Iris: van Doorn et al., 2003) have attempted to reveal the genes or pathways regulating floral degeneration in these species; however, no clear patterns have yet emerged. Verifying the role of genes in these species is hampered by the lack of genome sequences and, often, lack of efficient transformation systems. Another possibility is that senescence and PCD are regulated post-transcriptionally, as argued by Thomas et al. (2003). Perhaps a complex network of both transcriptional and post-transcriptional control is involved, as is found in other fundamental cellular processes such as the cell cycle. If the underlying ethylene-independent life span control in ethylene-sensitive species is common to ethylene-insensitive species, then models such as Arabidopsis and Brassica or tomato and petunia may offer better species in which to investigate these control networks. This would be a neat solution to a difficult problem. Langston et al. (2005) used this approach to study DNA fragmentation in petunia, showing that the ethylene induction of a 43-kDa nuclease (PhNUC1) was delayed in 35S:etr1-1 plants but not eliminated. Nine thousand expressed sequence tags (ESTs) have been recently generated from a global transcriptomic analysis of petunia floral senescence (D. Clarke, University of Florida, pers. comm.) and it will be interesting to see what proportion of these genes are up-regulated in 35S:etr1-1 lines. Likewise, in Arabidopsis, transcriptomic and perhaps proteomic analysis of petal senescence in etr1-1 lines may be a fruitful line of enquiry. However, if ethylene-independent regulation turns out to be species-specific then it is important to continue work with the diverse species currently being studied to appreciate the range of networks employed.
Another important question is whether the same signal differentially regulates PCD in floral organs. In some cases the answer is yes: for example in tobacco, ethylene regulates petal senescence (Rieu et al., 2003); however, at the same time ovary tissues continue to develop. So how is a primary signal such as ethylene transduced to ensure the co-ordinated life and death of different floral organs? Presumably this is through differential signal translocation or differential signal perception. Petal margins often degenerate before the centre and cross-sections of developing petals reveal that while the epidermal cells are still functional, mesophyll cells have largely degenerated even before the flower is fully open (Wagstaff et al., 2003). So is there a gradient here of a diffusible signal, or of receptors or other intracellular mediators of the cell-death signal? In some cases this signalling differential is very distinct: in the Arabidopsis gfa2 mutant, synergids fail to undergo PCD, but antipodal PCD is not affected (Christensen et al., 2002).
Ethylene is not the only PGR stimulating PCD in floral organs: some links to other PGRs are reviewed in Wu and Cheung (2000). Mutation of gibberellic acid biosynthetic genes anther ear 1 and dwarf results in failure to abort stamens on maize female flowers. Mutation of a gene associated with brassinosteroids (TS) (‘tasselseed’) results in feminization of male flowers, and application of jasmonate (JA) enhances petal senescence in some species (Porat and Halevy, 1993), although this effect may be indirect, through ethylene signalling (Stead et al., in press). Elevating cytokinin levels in petunia delayed flower senescence; however, this may also be indirect through changes in sugar transport (Lara et al., 2004). So are all these PGRs involved in floral PCD in all species? Or are there important quantitative or even qualitative species-specific differences in their effects? Perhaps metabolomic approaches to measure endogenous levels of PGRs, coupled with a more extensive use of mutants, may begin to address these questions.
IS THERE JUST ONE PCD MECHANISM OPERATING IN FLORAL ORGANS?
van Doorn and Woltering (2005) have recently categorized plant PCD into three types: apoptotic, autophagic and neither apoptotic nor autophagic. In animal cells four types of apoptosis have been described (Orrenius et al., 2003), three of which involve cytochrome c release from the mitochondrion controlled by a family of proteins (Bcl-2) that interact with the mitochondrial membrane to facilitate or inhibit this process. Cytochrome c then activates a family of cysteine aspartate-specific proteases (caspases), which both regulate and effect PCD. Apoptotic PCD in animals is characterized by cytological features, including chromatin and nuclear condensation and marginalization followed by DNA fragmentation into nucleosomal units known as DNA laddering, nuclear blebbing and formation of membrane inclusions known as apoptotic bodies (Cohen, 1993). The apoptotic bodies are then engulfed by neighbouring living cells.
In the tapetum and pollen-tubes, there is compelling evidence to support an important role for the mitochondrion and involvement of caspases. This suggests a mechanism similar to animal apoptosis, although caution must be exercised in drawing too close a parallel, as engulfment of cellular remains by other cells does not occur in plants (van Doorn and Woltering, 2005). Following its nutritive role during pollen development, the tapetum degenerates. This is characterized by chromatin condensation in Lobivia rauschii and Tillandsia albida (Papini et al., 1999), and by DNA fragmentation in barley anthers (Wang et al., 1999). In Brassica oleracea, Brassica napus, Digitalis purpurea and a cultivated form of Fuchsia there is also nuclear blebbing (A. D. Stead, Royal Holloway, University of London, unpubl. data). Furthermore, in PET1 cytoplasmic male sterility (CMS) of sunflower, there is cytochrome c release into the cytosol followed by changes in cell morphology, loss of outer mitochondrial membrane integrity and a fall in the respiratory control ratio (Balk and Leaver, 2001). Assuming that CMS is just anticipating a normal event (quite a major assumption), it might be concluded that PCD in the tapetum is apoptotic; however, we still do not know how it is triggered. Studies of nuclear mutations resulting in tapetal degeneration may be helpful: morphological changes were charted in a rice male sterile mutant (Ku et al., 2003), including cytoplasmic shrinkage, membrane blebbing, vacuolation, changes in mitochondrial morphology and early DNA fragmentation. However, cytochrome c leakage and respiratory control ratio were not measured in this system. Further studies on PCD in non-CMS tapetal cells would be helpful in this context.
Another example of apoptotic-like PCD in floral organs is in the death of the pollen-tube during self-incompatible pollination interactions. In Papaver, Thomas and Franklin-Tong (2004) showed that self-incompatibility (SI) stimulated increases in cytosolic [Ca2+], which in turn activated release of cytochrome c into the cytosol and induced caspase-3-like activity. The caspase-3-like activity cleaved poly(ADP-ribose) polymerase (PARP), a classic substrate for caspase-3 enzymes, and was also inhibited by the peptide Ac-DEVD-CHO, which blocked DNA fragmentation and pollen-tube growth.
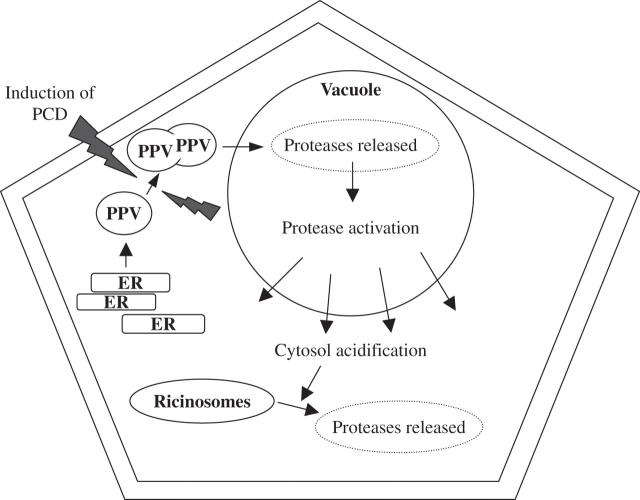
Another form of animal PCD is autophagy, in which vesicles (autophagosomes) containing proteins and organelles are transported to the hydrolase-packed lysosome. Here the contents are digested to generate monomeric building blocks (Klionsky and Emr, 2000). Evidence for autophagic PCD in petals comes from the identification of organelles that deliver proteases to the vacuole. The hypothesis is that the vacuole may be playing a homologous role to the animal lysosome. Healthy epidermal Arabidopsis leaf cells contain plant-specific, endoplasmic reticulum (ER)-derived compartments containing precursors of cysteine proteinases known as protease precursor vesicles (PPVs) (Hayashi et al., 2001). On application of stress, the PPVs fuse with each other and with the vacuole, delivering protease precursors to the vacuole, which then effect the maturation of other proteases and participate in the disassembly of cellular components during senescence. One senescence-induced cysteine protease found in Arabidopsis PPVs is vacuolar processing enzyme-γ (VPE γ). The PPVs deliver VPE γ to the vacuole where it is required for the processing of a number of proteins. VPEs are one of the two groups of plant proteases which are candidates for plant caspases, the other being metacaspases (Sanmartín et al., 2005). Modelling of VPEs shows tertiary structure homology to caspases (Sanmartin et al., 2005), with VPE γ being closest in structure to caspase-8. VPE γ also shows caspase-1 activity and binds to caspase-1-specific inhibitors (Rojo et al., 2004). VPEs are up-regulated during leaf and cotyledon senescence, stress (Kinoshita et al., 1999), and during pathogen defence (Rojo et al., 2004). So is this mechanism active in floral organs? Papain-class proteases identified from fruit, senescent petals and ovaries, and degenerating endosperm all contain a conserved Asn residue in the protein precursor that is probably a target for cleavage, resulting in maturation of the enzyme (Kinoshita et al., 1999). As VPEs target Asn residues, VPEs may activate these proteases within the vacuole of senescent tissues. Bringing together the different strands of evidence, there seems to me to be a good argument for VPEs performing a regulatory role in many if not all the forms of PCD seen in floral organs.
Similar organelles to PPVs have been isolated from Ricinus communis endosperm known as ricinosomes. These organelles develop as the cells undergo PCD, and rupture releasing their cargo of proteases directly into the cytosol. Acidification triggers ricinosome rupture; thus, release of proteases from ricinosomes may be activated after acidification of the cytosol following vacuolar leakage (Schmid et al., 1999). Ricinosomes contain CysEP, a type of cysteine endoprotease with a C-terminal KDEL motif (Gietl et al., 1997), which directs proteins to the ER lumen. KDEL-containing cysteine proteases have been identified in a number of senescent floral organs including Hemerocallis (daylily) petals (Valpuesta et al., 1995) and in Pisum sativum senescent ovaries (Cercos et al., 1999). In senescent daylily petals the KDEL-containing protease is within vesicles similar to ricinosomes (Schmid et al., 1999) so ricinosome-mediated PCD may be a feature of petal PCD. However, ricinosome-like vesicles are clearly distinct from the PPVs in that they are presumed to act downstream of the vacuolar leakage, and to deliver their cargo into the cytosol rather than the vacuole (Fig. 2). At least two metacaspases (AtMCP2f and AtMCP1a) are also thought, based on array data, to be induced in senescing flowers (Sanmartín et al., 2005), but their location is unknown. Confirmation of their role in planta will be important.
Fig. 2.
Tentative model for the role of protease precursor vesicles (PPVs) and ricinosomes in cell death. Derived from the endoplamsic reticulum (ER), PPVs fuse with each other and then the vacuole when programmed cell death (PCD) is induced (by extracellular or intracellular signals). Proteases are released from the PPVs, which activate further proteases leading to vacuolar membrane leakage. Consequent acidification of the cytosol induces ricinosome breakdown, releasing further proteases which degrade cytosolic proteins.
DO FLORAL ORGANS AND OTHER PLANT TISSUES DIE IN THE SAME WAY?
A functional categorization of PCD can be made on the basis of the fate of the cell contents. Remobilization is central to leaf, sepal and petal senescence (Thomas et al., 2003) and in a different way also to tapetal PCD. But endothecium, synergid, antipodal cell or pollen-tube PCD is the selective death of unwanted cells.
In green tissues, the chloroplast is seen by some (Thomas et al., 2003) as the key participant in the senescence process, and an early sign of senescence in green tissues is conversion of chloroplasts to gerontoplasts. Sepals, the floral organ that most closely resembles leaves, senesce in a similar way: in broccoli, sepal chlorophyll degradation is the first visual sign of senescence (Page et al., 2001). Petals are, however, not usually green, and an early step in their development is a conversion of chloroplasts to chromoplasts. This conversion has been compared with the chromoplast/gerontoplast transition (Thomas et al., 2003) with the inference that petals are most similar to senescent leaves. This agrees with the very early cell death seen in flowers (Wagstaff et al., 2003) presumably associated with nutrient remobilization. However, in-silico comparison of transcriptome changes in senescent Arabidopsis leaves and petals indicates that 25–30 % of genes share similar patterns of expression (Stead et al., in press). A comparison of transcriptome changes during petal senescence and fruit ripening would be interesting. Complex networks of ethylene, JA and salicylic acid operate in leaves (He et al., 2001), which may differ from those in petals, as ethylene does not have the same dramatic effects on leaves seen in petals (Grbić and Bleeker, 1995). At a subcellular level morphological changes to subcellular compartments during PCD are shared by many different cell types and tissues (Rogers, 2005). VPEs are found in leaves, roots and flowers; ricinosomes are seen in seed and petal tissues; and caspase activity is detected in pollen-tubes undergoing SI, and in many non-floral tissues during natural senescence and also during pathogen responses (Sanmartín et al., 2005).
CONCLUSIONS: WHAT DO WE REALLY WANT TO KNOW?
Progress in our understanding of PCD in plants has been rapid in the last 10 years, but the key regulators of some types of floral organ senescence, such as petal senescence in ethylene-insensitive species, remain obscure. It is also unclear whether regulation of petal senescence and PCD in these species is similar or divergent. The latter is an important question to resolve before a good model for these species can be developed. Even in ethylene-regulated petal senescence, the primary signal initiating the ethylene cascade remains elusive and needs pinning down, and again it is not at all certain that it will prove to be common to all species. Work on leaf senescence is suggesting that the search for a ‘master-switch’ gene or genes of plant senescence may be futile, and that a complex network of endogenous and exogenous signals tips the balance towards death. Floral senescence appears much more tightly regulated developmentally, so should we be looking for a master regulator here? Transcriptome analyses have not revealed any obvious candidates, but perhaps we should not stop looking yet. Another area that seems under-researched is our understanding of the promoters of floral-senescence genes. Genomic approaches and new developments in bio-informatics are powerful tools for developing this area and exploring how far transcription factors are shared between leaf senescence, floral senescence, fruit ripening and localized PCD. This will help to reveal the upstream regulatory networks, even if a master-switch is not the answer. Proteomics and metabolomics may also help to understand post-transcriptional regulatory networks, and define better the role of PGRs. Transgenics and mutants are powerful tools, and perturbation of specific pathways such as manipulation of PGR levels using inducible promoters will also contribute to our understanding of the roles of PGRs in floral senescence and PCD. At a cellular level plant PCD seems poised on the edge of major advances in unravelling protease cascades and intracellular signalling events. We can start to build a picture of the types of mechanisms operating in different cell types (Fig. 1, Table 1); however, it is far from complete. So we are still some way from a systems biology approach that might describe the whole process.
Table 1.
Comparison of signalling and possible mechanisms of programmed cell death (PCD) in floral organs
| Floral Organ | Intercellular signals | Intracellular signals and possible mechanisms for PCD | References |
|---|---|---|---|
| Sex organ abortion | GA and brassinosteroids | ? | Wu and Cheung (2000) |
| Tapetum in cytoplasmic male sterility lines | Mitochondrial dysfunction | Cytochrome c release, followed by loss of mitochondrial function | Balk and Leaver (2001) |
| Synergids | Pollination in some species | Requires mitochondrial function? | Christensen et al., 2002 |
| Petal senescence | Ethylene in some species | Ca2+/phosphatase signalling, reactive oxygen species increases | Porat and Halevy (1993), Kinoshita et al. (1999), Orzàez et al. (1999), Schmid et al. (1999), Wagstaff et al. (2003), Lara et al. (2004) |
| Jasmonate (via ethylene?) | Activation of vacuolar lytic enzymes through vacuolar processing enzyme (caspase-1 activity) | ||
| Cytokinin (via sugar transport) | Activation of vesicle-bound proteases following vacuolar leakage | ||
| Pollen-tube | During self-incompatiblity interactions | Increased Ca2+, resulting in cytochrome c release and caspase-3 activity | Thomas and Franklin-Tong (2004) |
Acknowledgments
Many thanks to Carol Wagstaff and Tony Stead for their many helpful comments on the manuscript.
LITERATURE CITED
- Ashman T-L, Schoen DJ. 1994. How long should flowers live? Nature 371: 788–791. [Google Scholar]
- Balk J, Leaver CJ. 2001. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. The Plant Cell 13: 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze E, Wagstaff C, Harrison E, Bramke I, Rogers H, Stead A, et al.2004. Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnology Journal 2: 155–168 [DOI] [PubMed] [Google Scholar]
- Cercos M, Santamaria S, Carbonell J. 1999. Cloning and characterization of TPE4A, a thiol-protease gene induced during ovary senescence and seed germination in pea. Plant Physiology 119: 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanasut U, Rogers HJ, Leverentz MK, Griffiths G, Thomas B, Wagstaff C, et al. 2003. Increasing flower longevity in Alstroemeria. Postharvest Biology and Technology 29: 325–333. [Google Scholar]
- Christensen CA, Gorsich SW, Brown RH, Jones LG, Brown J, Shaw JM, et al. 2002. Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. The Plant Cell 14: 2215–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JJ. 1993. Apoptosis. Immunology Today 14: 126–130. [DOI] [PubMed] [Google Scholar]
- Deikman J. 1997. Molecular mechanisms of ethylene regulation of gene transcription. Physiologia Plantarum 100: 561–566. [Google Scholar]
- van Doorn WG, Woltering EJ. 2004. Senescence and programmed cell death: substance or semantics? Journal of Experimental Botany 55: 2147–2153. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. 2005. Many ways to exit? Cell death categories in plants. Trends in Plant Science 10: 117–122. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Balk PA, van Houwelingen AM, Hoeberichts FA, Hall RD, Vorst O, et al.2003. Gene expression during anthesis and senescence in Iris flowers. Plant Molecular Biology 53: 845–863. [DOI] [PubMed] [Google Scholar]
- Gietl C, Wimmer B, Adamec J, Kalousek F. 1997. A cysteine endopeptidase isolated from castor bean endosperm microbodies processes the glyoxysomal malate dehydrogenase precursor protein. Plant Physiology 113: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić V, Bleecker AB. 1995. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant Journal 8: 595–602. [Google Scholar]
- ten Have A, Woltering EJ. 1997. Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Molecular Biology 34: 89–97. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa NK, Nishimura M, et al. 2001. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiology 42: 894–899. [DOI] [PubMed] [Google Scholar]
- He YH, Tang WN, Swain JD, Green AL, Jack TP, Gan SS. 2001. Networking senescence-regulating pathways by using Arabidopsis enhancer trap lines. Plant Physiology 126: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Yamada K, Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I. 1999. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant Journal 19: 43–53. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. 2000. Cell biology—Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SJ, Yoon H, Suh HS, Chung YY. 2003. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta 217: 559–565. [DOI] [PubMed] [Google Scholar]
- Langston BJ, Bai S, Jones ML. 2005. Increases in DNA fragmentation and induction of a senescence-specific nuclease are delayed during corolla senescence in ethylene-insensitive (etr1-1) transgenic petunias. Journal of Experimental Botany 56: 15–23. [DOI] [PubMed] [Google Scholar]
- Lara MEB, Garcia MCG, Fatima T, Ehness R, Lee TK, Proels R, et al. 2004. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16: 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MZ, Savin KW, Baudinette SC, Graham MW, Chandler SF, Lu C-Y, et al. 1993. Cloning of ethylene biosynthetic genes involved in petal senescence of carnation and petunia, and their antisense expression in transgenic plants. In: Pech JC, Latche A, Balague C, eds. Cellular and molecular aspects of the plant hormone ethylene. Dordrecht: Kluwer, 298–303.
- O'Neill SD. 1997. Pollination regulation of flower development. Annual Review of Plant Physiology and Plant Molecular Biology 48: 547–574. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. 2003. Regulation of cell death: the calcium-apoptosis link. Nature Reviews Molecular Cell Biology 4: 552–565. [DOI] [PubMed] [Google Scholar]
- Orzaez D, Blay R, Granell A. 1999. Programme of senescence in petals and carpels of Pisum sativum L. flowers and its control by ethylene. Planta 208: 220–226. [DOI] [PubMed] [Google Scholar]
- Page T, Griffiths G, Buchanan-Wollaston V. 2001. Molecular and biochemical characterization of postharvest senescence in broccoli. Plant Physiology 125: 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini A, Mosti S, Brighigna L. 1999. Programmed-cell death events during tapetum development of angiosperms. Protoplasma 207: 213–221. [Google Scholar]
- Payton S, Fray RG, Brown S, Grierson D. 1996. Ethylene receptor expression is regulated during fruit ripening, flower senescence and abscission. Plant Molecular Biology 31: 1227–1231. [DOI] [PubMed] [Google Scholar]
- Porat R, Halevy AH. 1993. Enhancement of petunia and dendrobium flower senescence by jasmonic acid methyl-ester is via the promotion of ethylene production. Plant Growth Regulation 13: 297–301. [Google Scholar]
- Rieu I, Wolters-Arts M, Derksen J, Mariani C. 2003. Ethylene regulates the timing of anther dehiscence in tobacco. Planta 217: 131–137. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. 2005. Cell death and organ development in plants. Current Topics in Developmental Biology 71: 225–261. [DOI] [PubMed]
- Rojo E, Martin R, Carter C, Zouhar J, Pan SQ, Plotnikova J, et al. 2004. VPE gamma exhibits a caspase-like activity that contributes to defense against pathogens. Current Biology 14: 1897–1906. [DOI] [PubMed] [Google Scholar]
- Sanmartín M, Jaroszewski L, Raikhel NV, Rojo E. 2005. Caspases, regulating death since the origin of life? Plant Physiology 137: 841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Gietl C. 1999. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proceedings of the National Academy of Sciences of the USA 96: 14159–14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykoff JA, Bucheli E, Kaltz O. 1996. Flower lifespan and disease risk. Nature 379: 779–780. [Google Scholar]
- Stead AD, van Doorn WG. 1994. Strategies of flower senescence—a review. In: Scott RJ, Stead AD, eds. Molecular and cellular aspects of plant reproduction. Cambridge: Cambridge University Press, 215–238.
- Stead AD, van Doorn WG, Wagstaff C, Jones ML. Floral senescence—fundamental and applied aspects. In: Ainsworth C, ed. Flowering and its manipulation. Oxford: Blackwell Publishing. In press.
- Thomas H, Ougham HJ, Wagstaff C, Stead AD. 2003. Defining senescence and death. Journal of Experimental Botany 54: 1127–1132. [DOI] [PubMed] [Google Scholar]
- Thomas S, Franklin-Tong VE. 2004. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429: 305–309. [DOI] [PubMed] [Google Scholar]
- Tilly JL. 2001. Commuting the death sentence: how oocytes strive to survive. Nature Reviews Molecular Cell Biology 2: 838–848. [DOI] [PubMed] [Google Scholar]
- Valpuesta V, Lange NE, Guerrero C, Reid MS. 1995. Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Molecular Biology 28: 575–582. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B, et al. 2003. Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytologist 160: 49–59. [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Chanasut U, Harren FJM, Laarhoven LJ, Thomas B, Rogers HJ, et al. 2005. Ethylene and flower longevity in Alstroemeria: relationship between tepal senescence, abscission and ethylene biosynthesis. Journal of Experimental Botany 56: 1007–1016. [DOI] [PubMed] [Google Scholar]
- Wang, M, Hoekstra S, Van Bergen S, Lamers GEM, Oppedijk BJ, Van der Heijden MW, et al. 1999. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Molecular Biology 39: 489–501. [DOI] [PubMed] [Google Scholar]
- Woltering EJ, van Doorn WG. 1988. Role of ethylene and senescence of petals: morphological and taxonomical relationships. Journal of Experimental Botany 39: 1605–1616. [Google Scholar]
- Woodson WR, Lawton KA. 1988. Ethylene-induced gene expression in carnation petals. Relationship to autocatalytic ethylene production and senescence. Plant Physiology 87: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H-M, Cheung AY. 2000. Programmed cell death in plant reproduction. Plant Molecular Biology 44: 267–281. [DOI] [PubMed] [Google Scholar]