Abstract
• Background and Aims Chiropterophillous and ornithophillous characteristics can form part of a single reproductive strategy in plants that have flowers with diurnal and nocturnal anthesis. This broader pollination strategy can ensure seed set when pollinators are scarce or unpredictable. This appears to be true of hummingbirds, which presumably pollinate Marginatocereus marginatus, a columnar cactus with red nocturnal and diurnal flowers growing as part of dense bat-pollinated columnar cacti forests in arid regions of central Mexico. The aim of this study was to study the floral biology of M. marginatus, and evaluate the effectiveness of nocturnal vs. diurnal pollinators and the contribution of each pollinator group to overall plant fitness.
• Methods Individual flower buds were marked and followed to evaluate flower phenology and anthesis time. Flowers and nectar production were measured. An exclusion experiment was conducted to measure the relative contribution of nocturnal and diurnal pollinators to seed set.
• Key Results Marginatocereus marginatus has red hermaphroditic flowers with nocturnal and diurnal anthesis. The plant cannot produce seeds by selfing and was pollinated during the day by hummingbirds and during the night by bats, demonstrating that both pollinator groups were important for plant reproduction. Strong pollen limitation was found in the absence of one of the pollinator guilds.
• Conclusions Marginatocereus marginatus has an open pollination system in which both diurnal and nocturnal pollinators are needed to set seeds. This represents a fail-safe pollination system that can ensure both pollination, in a situation of low abundance of one of the pollinator groups (hummingbirds), and high competition for nocturnal pollinators with other columnar cacti that bloom synchronously with M. marginatus in the Tehuacan Valley, Mexico.
Keywords: Bats, columnar cacti, fail safe pollination, hummingbirds, pollination
INTRODUCTION
The presence of characteristics that belong to different plant pollination systems has been documented for a number of plant species (i.e. Buzato et al., 1994; Sazima et al., 1994; Liu et al., 2002; Muchhala, 2003). Extended anthesis is a baseline characteristic of such plants, enabling both nocturnal and diurnal visitors to pollinate flowers while feeding on nectar. This results in an enhancement of the seed set and represents a level of flexibility in the pollination system that enables plants to be able to set seed when either pollinator group is scarce or absent (Muchhala, 2003). This shared pollination can be viewed as a step in the evolution between pollination syndromes, as discussed by Buzato et al. (1994) and Sazima et al. (1994), but also as an evolutionary endpoint (Muchhala, 2003).
Columnar cacti have been characterized as hermaphrodite plants that depend closely on animals for reproduction (Gibson and Nobel, 1986; Valiente-Banuet et al., 1996, 1997a, b, 2002; Rojas-Martínez et al., 1999). Valiente-Banuet et al. (1996, 2002) reported that the majority of species of columnar cacti (Tribe Pachycereeae) are bat pollinated (60–72 %), this being the probable ancestral type of pollination for this group (Fleming, 2002). These species have tubular, strong flowers, presenting dull colours, strong, unpleasant odours and nocturnal anthesis. They form a specialized mutualism with bats, which are responsible for 100 % of fruit production of columnar cacti growing in tropical deserts (Sosa and Soriano, 1993; Petit, 1995; Valiente-Banuet et al., 1996, 1997a, b, 2002; Nasar et al., 1997; Rojas-Martinez et al., 1999; Arizmendi et al., 2002). In central Mexico cacti form dense forests (up to 1800 individuals per hectare); 4–8 species of columnar cacti flower synchronously between February and June (Valiente-Banuet et al., 1996). One such columnar species, Marginatocereus marginatus, has red tubular flowers, smaller than those reported for other columnar cacti, and exhibits both nocturnal and diurnal anthesis (Bravo-Hollis, 1978). This species, along with two species of Rathbunia, is considered to form evolved clades in the phylogeny of columnar cacti, diverging from the ancestral type mainly with regard to their means of pollination syndrome (Gibson and Nobel, 1986). M. marginatus is a Mexican endemic columnar cactus distributed mainly from south-central to the north-east Mexico (Bravo-Hollis, 1978).
The purpose of this study was to document the floral biology and breeding system of M. marginatus in the Tehuacán Valley, as representing a unique case of hummingbird pollination. Floral biology, flower morphology, mating system and pollinators were studied in the valley, where hummingbirds are represented by just a few species that are not abundant throughout the year (Arizmendi and Espinosa de los Monteros, 1996).
MATERIALS AND METHODS
Study site
The study was conducted from March to May, 1997, at two localities in the semi-arid valley of Zapotitlán de las Salinas (18°20′N, 97°28′W), a local basin of the Tehuacán Valley in the state of Puebla, México. The region's arid conditions result from the rain shadow produced by the Sierra Madre Oriental range to the north-east (Smith, 1965). Average annual rainfall is 380 mm, and annual mean temperature is 21 °C with very rare frosts (García, 1973). Arid tropical scrub vegetation predominates, with giant columnar cacti constituting the most important physiognomic elements (Valiente-Banuet et al., 2000). Tropical deciduous forest predominates at Cerro Cutá (elevation approx. 1700 m), where most of the sampling for this study was conducted. The site is characterized by a predominance of the plant families Caesalpinaceae, Fabaceae and Mimosaceae, as represented by the species Mimosa lacerata, M. luisana, Prosopis laevigata, Senna holwayana, Zapoteca formosa, Acacia sericea, A. coulteri, Ceiba parviflora, Ipomoea arborescens and Caesalpinia melanadenia. Also present are Fouquieria formosa, Bursera biflora, Myrtillocactus geometrizans, Opuntia pilifera, Marginatocereus marginatus, Yucca periculosa, Plumeria rubra and Pittocaulon praecox (Osorio-Beristain et al., 1996).
Studied species
Marginatocereus marginatus is a non-branched or slightly branched columnar cactus (Cactaceae: Tribe Pachycereeae). Bravo-Hollis (1978) described two varieties for the species. The first, var. marginatus, is a smaller plant (3–6 m in height) that inhabits Mexican central highlands from Tamaulipas to Queretaro. It has white–yellow flowers. The second, var. gemmatus, is a taller plant (5–12 m) with red flowers, and has been reported in arid lands from Puebla to Guerrero. In the Tehuacan Valley it reaches 6 m in height and forms a minor component in dense, mixed columnar cacti forest.
Floral biology
Fifty flowers from 36 individual plants were used to describe the floral biology in M. marginatus. Twenty-eight flowers from 18 plants were measured when fully open to determine floral dimensions (total length, distance to the top of the nectar chamber from corolla opening, diameter of corolla and corolla opening). Twenty-four flowers from 15 plants were marked and followed through an entire flowering cycle to determine opening and closing times, pollen availability and stigma turgency as a measure of flower receptivity (Dafni, 1992; Valiente-Banuet et al., 2004). The condition of each flower was recorded every 2 h over the duration of the flowering cycle, about 72 h.
Fourteen flowers from eight plants were measured for nectar accumulation between floral opening and closure over a period of 3 d. Flowers were bagged using mesh bags to prevent visitation before they opened. Nectar was collected using 1-mL insulin syringes, and sugar concentration was determined using a field refractometer (ATAGO N1, Tokyo, Japan; Brix value 0–32 %). Concentration of sugars was measured from five of these flowers.
Breeding system
To determine the breeding system and differential effectiveness of floral visitors, 116 flower buds were marked and bagged from a total of 29 individual plants with mesh exclusion nets. Flowers were then assigned to the following treatment groups: (1) un-bagged, but marked natural pollination as a control (55 flowers from ten plants); (2) non-manipulated self-pollination (25 flowers from 11 plants)–bags were left in place for the full duration of floral opening; (3) hand self-pollination (ten flowers from eight plants)–flowers were hand-pollinated using fresh pollen from a flower on the same plant; (4) diurnal pollination (37 flowers from 13 plants)–when flowers opened, they were exposed to daytime floral visitors by the removal of the bag from 0600 to 1600 h, after which the flowers were again bagged; (5) nocturnal pollination (13 flowers from seven plants)–when flowers opened, they were exposed to nighttime visitors by the removal of the bag from 2100 to 0600 h, after which the flowers were re-bagged; and (6) hand outcross pollination (21 flowers from 16 plants)–flowers were hand-pollinated using fresh pollen from a flower on another plant.
Following experimental manipulation, flowers were left bagged and monitored until abortion or fruit production. In the event of fruit production, immature fruits were bagged with predator-resistant mesh exclusions and monitored until fruit and seed maturity, at which time the fruits were removed from the plant, dissected and the number of mature seeds recorded. Fruit set among pollination treatments was analysed by one-way ANOVA (JMP 3.1, Sall and Lehman, 1996).
Floral visitors
To identify flower visitors, three mist-nets (9 m long × 3 m tall) were placed among flowering individuals of M. marginatus on six occasions between March and April (1997). Nets were opened at approx. 0600 h (just prior to sunrise) and closed the following day at sunrise for a combined total of 432 net hours. Nets were monitored every 30 min for captured animals. A black light nocturnal insect trap was also used on four nights in March and April.
A pollen preparation was made for each captured animal by rubbing a cube of fuchsine-stained jelly (Beattie, 1971) on the animal's body to collect any adhering pollen grains. The cube was then placed on a microscope slide, melted and covered with a coverslip for later examination under a microscope. Pollen samples were compared with reference samples made directly from flowers of M. marginatus. The presence of pollen was considered as proof of flower visitation, and frequency of pollen indicated extent of use by the animal species, measured as the number of individuals in each pollinator species that bore pollen of M. marginatus. For identification purposes, pollen of other columnar cacti blooming synchronously with M. marginatus was collected, mainly of Stenocereus pruinosus and Neobuxbaumia mezcalaensis, both of which have pollen that can be differentiated from M. marginatus by their much larger size.
RESULTS
Flowers were present from mid-February until May, while fruits were produced between March and June, just prior to the start of the rainy season. With effective pollination, fruits are present until as late as August.
Floral biology
Flowers of M. marginatus are tubular and hermaphroditic, externally red with perianth parts adjacent to the floral aperture typically paler in colour. Mean flower dimensions are detailed in Table 1. Flowers began opening at approx. 2100 h, and were fully opened between 0130 and 0230 h. Pollen was available from approx. 0100 h until approx. 1000 h. Receptive stigmas were observed in approx. 8 % of the surveyed flowers as early as 0330 h, and by approx. 0700 h stigmas of most flowers were receptive. In most cases stigmas remained receptive until mid-morning (0900–1000 h), although turgid stigmas were observed on some plants as late as 1350 h. Flowers rarely began to close as early as 1130 h, but by 1300 h most flowers were closing or already closed. All flowers were closed by approx. 1630 h. Flowers only opened once, and did not re-open after closing.
Table 1.
Floral dimensions of Marginatocereus marginatus
| Character | Mean ± s.e. | n | Range |
|---|---|---|---|
| Inner diameter (mm) | 6·0 ± 0·166 | 26 | 5–7 |
| Outer diameter (mm) | 15·7 ± 0·42 | 22 | 12–20 |
| Total length (mm) | 34·7 ± 0·65 | 28 | 25–41 |
| Distance to nectar chamber (mm) | 22·3 ± 0·51 | 28 | 18–27 |
| Stamen height (mm) | 8·02 ± 0·31 | 40 | 5–12 |
| Pistil length (mm) | 22·88 ± 0·53 | 28 | 18–28 |
| Pistil length/total length | 0·66 ± 0·016 | 28 | 0·53–0·8 |
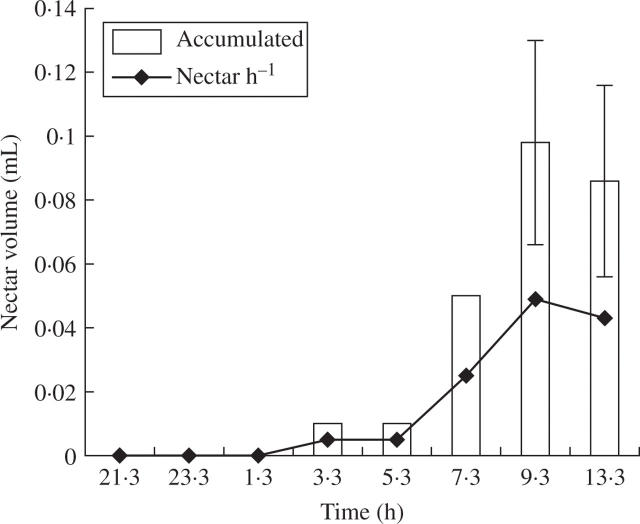
Nectar production starts during the night but with minimal production (0·01 mL at 0330 h); peak nectar accumulation occurred between 0915 and 1015 h, when the volume of floral nectar averaged 0·098 mL (s.e. = 0·013, n = 6). Mean sugar concentration during this period was 25·48 % (s.e. = 0·54, n = 5). Daily nectar accumulation (measured at 1300 h, just before flowers closed) averaged 0·087 mL (s.e. = 0·018, n = 3; Fig. 1).
Fig. 1.
Nectar accumulation (bars) and nectar produced per hour (lines) in Marginatocereus marginatus in the Tehuacan Valley, Mexico (data are shown as mean and s.e.).
Breeding system
Un-manipulated flowers to which pollinators were denied access did not produce fruits, indicating the absence of spontaneous auto-fertilization. Diurnal fruit set was very low, with only one mature fruit produced from the 36 flowers used (2·8 %). The lone fruit contained 159 mature seeds. Fruit set was similarly low in the nocturnal treatment (8·33 %), in which only two fruits were formed, containing a mean of 94·5 (s.e. = 20·56, n = 2) seeds. Hand-outcrossed flowers produced six fruits (28·5 %) containing a mean of 123·42 seeds (s.e. = 16·49, n = 6). In the control treatment, an average of 102·12 seeds were produced per fruit (s.e. = 9·21, n = 25) and a fruit set of 45·4 %, values not significantly different from hand-outcrossed flowers (F3,57 = 7·32, P = 0·0003; Table 2).
Table 2.
Fruit set, number of fruits and seeds produced by the different pollination treatments of Marginatocereus marginatus at the Tehuacan Valley, Mexico March–May, 1997
| Treatment | No. of flowers/plants (see methods) | No. of fruits produced | Fruit set (%) | Mean no. of seeds per fruit + s.e. (range) |
|---|---|---|---|---|
| Diurnal | 37/13 | 1 | 2·8 b | 159 |
| Nocturnal | 24/16 | 2 | 8·33 b | 94·5 + 20·56 (74–115) |
| Non-manipulated self-pollination | 25/11 | 0 | 0 | – |
| Hand self-pollination | 10/8 | 0 | 0 | – |
| Control | 55/10 | 25 | 45·4 a | 102·12 + 9·21 (43–171) |
| Manual outcross | 21/16 | 6 | 28·57 a | 123·42 + 16·49 (88–191) |
Same letters in fruit set indicate similar groups in statistical analysis (F3,57 = 7·32, P = 0·0003).
Floral visitors
Three species of hummingbirds were observed visiting M. marginatus flowers, Amazilia violiceps, Cynanthus sordidus and Cynanthus latirostris, all of which are resident in the valley (Arizmendi and Espinosa de los Monteros, 1996). At night two bat species, Choeronycteris mexicana and Leptonycteris curasoae, and one moth species were detected visiting M. marginatus flowers, but only the hummingbirds and bats bore pollen on their throats and foreheads (Table 3). Hummingbirds presented the highest frequencies of individuals bearing pollen (Table 3).
Table 3.
Frequency of pollen presence in the bodies of different visitors to flowers of Marginatocereus marginatus
| Taxa | n | Individuals bearing pollen of M. marginatus (%) |
|---|---|---|
| Choeronycteris mexicana | 128 | 4·68 |
| Leptonycteris curasoae | 151 | 1·32 |
| Nocturnal moth | 18 | 0 |
| Amazilia violiceps | 3 | 33·3 |
| Cynanthus latirostris | 4 | 75 |
| Cynanthus sordidus | 27 | 18·51 |
DISCUSSION
The flowers of M. marginatus have the fundamental characteristics associated with pollination by hummingbirds: tubular red flowers, producing moderate amounts of nectar with sugar concentration in the range preferred by these birds (Baker and Baker, 1975; Gibson and Nobel, 1986). However, M. marginatus flowers present nocturnal and diurnal anthesis. This is atypical among other columnar cacti inhabiting the Tehuacán Valley, most of which are pollinated by bats, with nocturnal anthesis and chiropterophilous flowers (Valiente-Banuet, 1996, 1997a, b, 2002).
Very low fruit set was observed in both nocturnal and diurnal pollination treatments, suggesting a strong pollinator limitation not observed for the nocturnally pollinated columnar cacti in the valley (Valiente-Banuet et al., 1996, 1997a, b, 2002). Remarkably, when flowers were exposed to both nocturnal and diurnal pollinators, fruit set increased, reaching values comparable with the hand-outcrossed treatments. This suggest that this columnar cactus has evolved towards a more open, complementary pollination system (sensu Tilman, 1980) to enhance reproductive success in a locality where hummingbirds are not particularly abundant or diverse (Arizmendi and Espinosa de los Monteros, 1996). This can be viewed as a ‘fail-safe’ pollination system where plants expand their anthesis period, thus promoting a wider group of visitors and enhancing fruit production. A similar situation occurs in both latitudinal extremes of columnar cacti distribution (Fleming, 2002). In Peru, Sahley (1996) described the pollination of Weberbauerocereus weberbaueri, the flowers of which present a bat pollination syndrome and were pollinated by an endemic bat (Platalina genovensium). These bats moved locally under conditions of severe drought, and flowers then were less abundant. Under these conditions hummingbirds become the effective pollinators of W. weberbaueri. Sahley also described that the flowers of this species exhibit great variation in colour, from pure white to pink–red, and also in total length, width and style length. In the northern limit of the distribution of columnar cacti, in the Sonoran Desert, Fleming (2002) stated that of the four species studied, three were pollinated by bats, birds and diurnal insects to different extents. In this case, most of the floral visitors were migrants that fluctuate extensively on a year-to-year and site-to-site basis, favouring a bet-hedging pollination strategy among ancestral night-blooming cacti. These cacti have extended their anthesis time, remaining open and secreting nectar by day to attract a wide array of visitors, therefore enhancing their probability of pollination (Fleming et al., 1996; Fleming, 2002). These above two cases highlight the probable evolution of an ancestral specialized pollination system to a more open system, under conditions when abundance of the original pollinator varies.
In the Tehuacan Valley the columnar cactus Polaskia chichipe exhibits an open pollination system, in which bats, hummingbirds and diurnal insects are responsible for fruit production, although the diurnal pollinators are the more efficient (Otero-Arnaiz et al., 2003). Polaskia chichipe has diurnal flowers that extend anthesis into the night, especially when flowering during winter when flowers remain open all night. This may be related to the low abundance of diurnal pollinators during winter (Otero-Arnaiz et al., 2003).
In Marginatocereus marginatus, flowers opened at night and remained opened until the following mid-day, and nocturnal pollinators produced three times more fruit set then diurnal animals. In both this and Otero et al.'s study, a strong pollinator limitation was found. The data presented here support the suggestion that the tendency of presenting a more open and generalized pollination system is related to seasonal availability and predictability of pollinators (Arizmendi et al., 1996; Fishbein and Venable, 1996; Valiente-Banuet et al., 1996, 2002; Waser et al., 1996; Rojas-Martinez et al., 1999; Mayfield et al., 2001; Fleming, 2002; Muchhala, 2003).
Acknowledgments
We thank Juan Arroyo for the revision of an earlier version of this manuscript during a visit supported by CYTED. We thank Dr Giles Cassidy Thelen, University of Montana, for revisions to the English text. Financial support was provided by DGAPA IN-208301.
LITERATURE CITED
- Arizmendi MC, Espinosa de los Monteros A. 1996. Avifauna de los bosques de cactáceas columnares del Valle de Tehuacán, Puebla. Acta Zoologica Mexicana 67: 25–46. [Google Scholar]
- Arizmendi MC, Dominguez CA, Dirzo R. 1996. The role of an avian nectar robber and of hummingbird pollinators in the reproduction of two plant species. Functional Ecology 10: 119–127. [Google Scholar]
- Arizmendi MC, Valiente Banuet A, Rojas-Martínez A, Dávila P. 2002. Columnar cacti and the diets of nectar-feeding bats. In: Fleming T, Valiente-Banuet A, eds. Columnar cacti and their mutualists: evolution, ecology and conservation. Tuscon: University of Arizona Press, 264–283.
- Baker HG, Baker I. 1975. Studies in nectar-constitution and pollinator–plant coevolution. In: Gilbert LE, Raven HM, eds. Animal plant coevolution. Austin: University of Texas Press, 101–138.
- Beattie AJ. 1971. A technique for the study of insect-borne pollen. Pan Pacific Enthomologist 47: 82. [Google Scholar]
- Bravo-Hollis H. 1978. Las cactáceas de México, vol. I. México: Universidad Nacional Autónoma de México.
- Buzato S, Sazima M, Sazima I. 1994. Pollination of three species of Aboutilon (Malvaceae) intermediate between bat and hummingbird flower syndromes. Flora 189: 327–334. [Google Scholar]
- Dafni, A. 1992. Pollination ecology: a practical approach. New York: Oxford University Press.
- Fishbein M, Venable DL. 1996. Diversity and temporal change in the effective pollinators of Asclepias tuberose. Ecology 77:1061–1073. [Google Scholar]
- Fleming TH. 2002. Pollination biology of four species of Sonoran Desert columnar cacti. In: Fleming T, Valiente-Banuet A, eds. Columnar cacti and their mutualists: evolution, ecology and conservation. Tuscon: University of Arizona Press, 207–225.
- Fleming TH, Tuttle MD, Horner MA. 1996. Pollination biology and the relative importance of nocturnal and diurnal pollinators in three species of Sonoran desert columnar cacti. The Southwestern Naturalist 41: 257–269. [Google Scholar]
- García E. 1973. Modificaciones al sistema de clasificación climática de Köppen. México: Instituto de Geografía, Universidad Nacional Autónoma de México.
- Gibson AC, Nobel PS. 1986. The cactus primer. Cambridge, MA: Harvard University Press.
- Liu AZ, Li DZ, Wang H. 2002. Ornithophilous and chiropterophilous pollination in Musa itinerans (Musacea), a pioneer species in tropical rain forest of Yunnan, southwestern China. Biotropica 34: 254–260. [Google Scholar]
- Mayfield MM, Waser NM, Price MV. 2001. Exploring the ‘most effective pollinator principle’ with complex flowers: bumble-bees and Ipomopsis aggregata. Annals of Botany 88: 591–596. [Google Scholar]
- Muchhala N. 2003. Exploring the boundary between pollination syndromes: bats and hummingbirds as pollinators of Burmeistera cyclostigmata and B. tenuifolia (Campanulaceae). Oecologia 134: 373–380. [DOI] [PubMed] [Google Scholar]
- Nasar JM, Ramírez N, Linares O. 1997. Comparative pollination biology of Venezuelan columnar cacti and the role of nectar feeding bats in their sexual reproduction. American Journal of Botany 84: 918–927. [PubMed] [Google Scholar]
- Osorio-Beristain O. 1996. Análisis de la vegetación del cerro Cutac y sus alrededores. Tesis Licenciatura en Biología, Facultad de Ciencias, U.N.A.M. México.
- Otero-Arnaiz A, Casas A, Bartolo C, Perez-Negrón E, Valiente Banuet A. 2003. Evolution of Polaskia chichipe (Cactaceae) under domestication in the Tehuacan Valley, Central Mexico: reproductive biology. American Journal of Botany 90: 593–602. [DOI] [PubMed] [Google Scholar]
- Petit S. 1995. The pollinators of two species of columnar cacti on Curaçao, Netherlands Antilles. Biotropica 27: 538–541. [Google Scholar]
- Rojas-Martínez A, Valiente-Banuet A, Arizmendi MC, Alcántara-Eguren A, Arita H. 1999. Seasonal distribution of the long nosed bat Leptonycteris curasoae in North America: does a generalized migration pattern really exist? Journal of Biogeography 26: 1065–1077. [Google Scholar]
- Sazima M, Sazima I, Buzato S. 1994. Nectar by day and night Siphocampylus sulfureus (Lobeliaceae) pollinated by hummingbirds and bats. Plant Systematics and Evolution 191: 237–246. [Google Scholar]
- Sahley CT. 1996. Bat and hummingbird pollination of an autotetraploid columnar cactus, Weberbauerocereus weberbaueri (Cactaceae). American Journal of Botany 83: 1329–1336. [Google Scholar]
- Sall J, Lehman A. 1996. JMP Stara statistics: a guide to statistical and data analysis using JMP and JMPIN software. Belmont, CA: Duxbury Press.
- Smith CE. 1965. Flora Tehuacan Valley. Fieldiana Botany 31: 107–143. [Google Scholar]
- Sosa M, Soriano PJ. 1993. Solapamiento de dieta entre Leptonycteris curasoae y Glossophaga longirostris (Mammalia: Chiroptera). Revista de Biología Tropical 41: 529–532. [Google Scholar]
- Tilman D. 1980. Resources: a graphical-mechanistic approach to competition and predation. American Naturalist 116: 362–393. [Google Scholar]
- Valiente-Banuet A, Arizmendi MC, Rojas-Martínez A, Domínguez-Canseco L. 1996. Ecological relationships between columnar cacti and nectar-feeding bats in Mexico. Journal of Tropical Ecology 12: 103–119. [Google Scholar]
- Valiente-Banuet A, Rojas-Martínez A, Arizmendi MC, Dávila P. 1997a. Pollination biology of two columnar cacti (and Neobuxbaumia mezcalaensis and Neobuxbaumia macrocephala) in the Tehuacán Valley, Central Mexico. American Journal of Botany 84: 452–455. [Google Scholar]
- Valiente-Banuet A, Rojas-Martínez A, Casas A, Arizmendi MC, Dávila P. 1997b. Pollination ecology of two winter-blooming giant columnar cacti in the Tehuacan Valley, Mexico. Journal of Arid Environments 37: 331–341. [Google Scholar]
- Valiente-Banuet A, Casas A, Alcántara A, Dávila P, Flores-Hernández N, Arizmendi MC, Villaseñor JL, Ortega-Ramírez J. 2000. La vegetación del Valle de Tehuacan-Cuicatlán. Boletín de la Sociedad Botánica de México 67: 25–74. [Google Scholar]
- Valiente-Banuet A, Arizmendi MC, Rojas-Martinez A, Casas A, Godinez-Alvarez H, Silva C, Davila P. 2002. Biotic interactions and population dynamics of columnar cacti. In: Fleming T, Valiente-Banuet A, eds. Columnar cacti and their mutualists: evolution, ecology and conservation. Tuscon: University of Arizona Press, 225–241.
- Valiente-Banuet A, Molina-Freaner F, Torres A, Arizmendi MC, Casas A. 2004. Geographic differentiation in the pollination system of the columnar cactus Pachycereus pecten-aboriginum. American Journal of Botany 91: 850–855. [DOI] [PubMed] [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems and why it matters. Ecology 77: 1043–1060. [Google Scholar]