Abstract
• Background and Aims A number of strawberry varieties were surveyed for their total ellagic acid concentration, and attempts were made to determine if ellagic acid and ascorbic acid concentrations of two strawberry cultivars could be increased by polythene reflective mulches.
• Methods After adjusting crop yields and cultivation using polythene mulches with two different PAR reflective capacities, field- and polytunnel-grown strawberries were analysed for ellagic acid and ascorbic acid concentrations by HPLC. Comparative measurements of yield and fruit quality were determined along with plant developmental changes.
• Key Results Ellagic acid concentration varied widely with strawberry cultivar (60–341 µg g−1 frozen weight), as did the ratio of conjugated ellagic acid : free ellagic acid. Also, there was significant year-to-year variation in total ellagic acid concentration with some cultivars. Mulches with different reflective capacities impacted on strawberry production; highly reflective mulches significantly increased growth and yield, the latter due to increases in fruit size and number.
• Conclusions Highly reflective mulches significantly increased total concentrations of ellagic acid and ascorbic acid relative to control in fruit of different cultivars. The potential of agronomic practices to enhance the concentration and amounts of these important dietary bioactive compounds is discussed.
Keywords: Ellagic acid, ellagitannins, ascorbic acid, Fragaria, PAR, everbearers, June bearers, light reflectance, reflective mulch, strawberries, vitamin C
INTRODUCTION
Strawberry production is a major part of the European and UK soft fruit industry with a home production value of £96m in 2003 (Defra, 2003). Research programmes aimed at improving the quality of strawberry fruit are important. Consistent and clear epidemiological evidence indicates the importance of increased consumption of fruit and vegetables in improving human health (Block et al., 1992; Steinmetz and Potter, 1996; Kris-Etherton et al., 2002). We are now beginning to attach increased importance to aspects of fruit quality related to the health benefits of fruit such as strawberry. These fruits contain high concentrations of potentially beneficial phytochemicals and antioxidants, such as ascorbic acid (vitamin C) and ellagic acid (Potter, 1997; Eastwood, 1999; Urquiaga and Leighton, 2000). Ascorbic acid, present in high concentrations, provides an essential nutrient with numerous associated health benefits (Block, 1991; Kalt, 2001; Olsson, 2004). Its well-known antioxidant activity is due to the ease with which it loses electrons thus acting as a reducing agent for a number of reactive oxidant species (Klein and Kurilich, 2000; Prior and Cao, 2000). Ascorbic acid also helps maintain enzyme configurations in the correct reduction state for catalytic activity, as well as having an involvement in deactivation of carcinogens (Kalt, 2001).
Ellagic acid is a naturally occurring polyphenolic secondary metabolite that accumulates in strawberry and raspberry fruits (Häkkinen et al., 1999; Vattem and Shetty, 2005). The concentration of ellagic acid is generally high in strawberry, ranging from 0·16 to 2·07 mg g−1 d. wt (Williner et al., 2003). Most of the ellagic acid in plants is present within the vacuoles, as water-soluble ellagitannins rather than as the free acid. The biosynthesis of ellagic acid is thought to proceed via gallic acid. By conjugation with carbohydrates, gallic acid units form gallotannins, and the coupling of galloyl units in gallotannins is believed to produce the hexahydroxydiphenic units of the ellagitannins (Seigler, 1998). These ellagitannins release free ellagic acid upon hydrolytic cleaving.
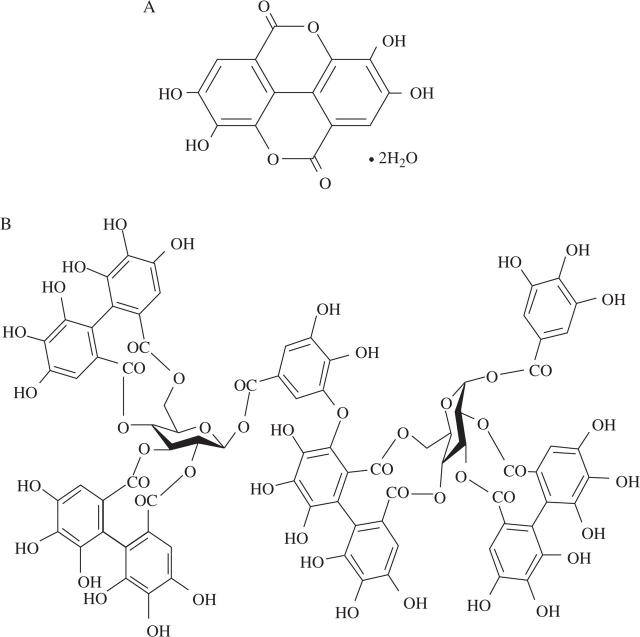
Evidence for this biosynthetic scheme has been provided by Niemetz and Gross (2003, and see references therein) from studies of Tellima grandiflora leaves. Both free ellagic acid and ellagitannins (see Fig. 1) have properties which suggest they can have positive effects on human health (phytonutrients). Ellagitannins and ellagic acid, like other phenolics, have been shown to significantly contribute to the antioxidative activity of red raspberries and other fruits (Kalt, 2001; Mullen et al., 2002; Vattem and Shetty, 2005). More recently, ellagic acid has been linked to health benefits by providing antimutagenic and anticarcinogen responses (Mandal and Stoner, 1990; Maas et al., 1991a; Clifford and Scalbert, 2000; Perchellet et al., 2002; Olsson et al., 2004; Vattem and Shetty, 2005). These are important characteristics considering current efforts to improve dietary habits and minimize cancer risks (Williner et al., 2003). However, the bioavailability of ellagic acid and ellagitannins is not well understood, and the potential anticancer and toxic effects need further study (Clifford and Scalbert, 2000).
Fig. 1.
The chemical structures of ellagic acid (A) and a sugar-conjugated ellagitannin (B).
Studies show that the concentration of ellagic acid in strawberry fruit varies 10-fold with cultivars (Maas et al., 1991b) and declines with season; most of the ellagic acid in ripe fruit is present in the achenes (Maas et al., 1991b; Williner et al., 2003). Although little is known about European strawberry cultivars, there is evidence that fruit ellagic acid concentrations can be influenced by environmental conditions. Wang et al. (1998, 2002) showed that different mulch types had a significant effect on the concentration of ellagic acid in strawberry fruit, while Häkkinen and Törrönen (2000) showed an effect of geographical origin and cultivation technique on fruit phenolic acids in strawberries.
The development of agronomic techniques to enhance production of useful secondary metabolites provides an opportunity to optimize currently available cultivars. Furthermore, such agronomic manipulations (e.g. the application of mild stress at defined points during the growing season) may have generic effects on a range of phytochemicals.
The initial aim was to carry out a survey of conjugated and free ellagic acid concentrations in a range of strawberry varieties grown in the UK. Total ellagic acid, i.e. free ellagic acid + conjugated ellagic acid, assumed to be released from ellagitannins, was measured. Also explored was the ability to enhance fruit ellagic acid concentration by altering crop load (number of fruit per plant). Manipulation of crop load can change the partitioning of resources, in particular the berry-to-berry competition for polyphenols such as ellagic acid. Stopar et al. (2002) showed that polyphenol concentration in Malus (apple) was increased by reducing crop load, although there was no effect of thinning on flavonoid and chlorogenic acid concentrations (Awad et al., 2001).
Finally, the aim was to determine whether ellagic acid and ascorbic acid concentrations of two strawberry cultivars (a short-day or June-bearer and a day-neutral or everbearer) can be enhanced through the use of light (photosynthetically active radiation, PAR) reflective mulches. These mulches are believed to enhance light interception; this may improve crop photosynthesis, solar heat transmission, nutrient uptake, soil moisture conservation, root and flower development, fruit set, fruit development, fruit maturity, fruit quality (colour), yield and disease control. Work with apple has shown the effectiveness of reflective films in increasing light penetration into the canopy, and this correlates with increasing anthocyanin concentration in fruit peel (Ju et al., 1999). Red colour development, at least in apple, is well documented as being light regulated (Siegelman and Hendricks, 1958; Lancaster, 1992). Several key enzymes (including UDPGalactose : flavonoid-3-glucosyltransferase, UFGalT) have been shown to be light inducible (Saure, 1990; Ju et al., 1995). Commercial strawberry production varies in its sophistication, but use of mulches (non-reflective) is extremely common. Reflective mulches offer potential practical benefits as well as the enhancement of bioactive compounds within fruits.
MATERIALS AND METHODS
Strawberry cultivars used to survey ellagic acid concentration
Fruit was collected at commercial ripeness from 45 different cultivars of field-grown strawberry plants in June–July (year 1) and from 17 cultivars in June–July (year 2), using plants from the breeding programme at East Malling. For each cultivar, several fruit (same hierarchical position) were collected from each of six separate plants to generate a representative pooled fruit sample. The fruit were frozen in liquid nitrogen and stored at −80 °C until analysis.
Manipulation of strawberry fruit number (expt 1)
Potted strawberry plants of the June-bearers ‘Elsanta’ and ‘Florence’ (R. W. Walpole Ltd, Norfolk, UK) were grown in a glasshouse. Four treatments were applied to each cultivar, and all treatments were replicated once in each of five blocks, with one replicate consisting of five plants. Plants were arranged according to a complete randomized block design. The treatments involved removing primary, secondary or tertiary flowers, as described below:
Treatment (flower sample)—fruit samples taken
Control (no flowers removed)—primary, secondary and tertiary berries
Primary flower removed—secondary and tertiary berries
Primary and secondary flowers removed—tertiary berries
All flowers except the primary removed—primary berries
Manipulation of strawberry environment through the use of reflective mulches (expt 2)
Two experiments were carried out, one with the June-bearing cultivar Elsanta, and a second with the everbearer cultivar Flamenco. Bare-rooted material (Darby Plants Ltd, Norfolk UK) was planted in commercial growing bags. Prior to planting, reflective mulches were applied to provide the following treatments:
Treatment 1 = hessian (commercial brown weave = ‘zero reflectance’)
Treatment 2 = black polythene
Treatment 3 = Extenday™ (ST200, J & D Wilkie Ltd)
Treatment 4 = Ultra-reflective (‘aluminized polythene’)
A 1-m2 sheet of the relevant mulch material was positioned over the top of each growing bag, with the overlapping edges clipped on each side to wires, suspended approx. 20 cm above the ground. This ensured that the mulch received any solar radiation that passed through the canopy. Once the mulches were in place, the bags were planted, on 3 Jun. 2003, at a commercial density of ten plants per bag.
Liquid fertilizer was continuously injected into the irrigation system using a dosatron. Standard Agrosol (Scotts UK Ltd) feed (1 kg dm−3 water). For the first month, Agrosol 313 (14 : 7 : 14, N : P : K) was used, switching to Agrosol F316 (13 : 5 : 28, N : P : K) once flowering had begun. The initial fertigation (nutrition and water given together) regime was 500 mL per bag (using three drippers, pressure-regulated to 2 L h−1) four times daily (0800, 1200, 1600 and 2000 h). In early July, this was increased to 1000 mL, four times daily, to compensate for higher plant water usage. This continued until mid-September, when the fertigation was reduced to 500 mL per event. When it was necessary to adjust the fertigation in response to environmental conditions, i.e. during periods of high temperatures, an extra irrigation event was applied manually. Pollination was carried out manually with a fine brush.
Environmental conditions in the polytunnel and treatment differences were logged (Data logger DL2E, Delta T Devices, Cambridge, UK) throughout the season. For all treatments, temperature probes (thermocouples for air temperature and thermistors in the compost) were mounted in three positions: on the mulch surface; directly under the mulch; and in the compost at a depth of 5 cm. The thermocouple on the mulch surface was not exposed to direct radiation; temperatures recorded here were expected to decline as the plant grew and shading increased. For each treatment, temperatures were meaned (n = 3) across three experimental blocks. Absolute PAR was continuously recorded with a series of quantum sensors mounted at fixed positions in the crop. All data were recorded at 1-min intervals and means logged over 30 min.
Spectral reflectance characteristic of the mulches
Spectra of mulch-reflected light were measured between 280 and 800 nm with a double-monochromator spectroradiometer (Model SR99-1 PC; Macam Photometrics, Livingstone, UK) equipped with a 10-cm-diameter integrating sphere. The light source was a 75-W xenon-arc lamp (LOT Oriel, Leatherhead, Surrey). Reflectance was measured against a Spectralon white standard (LOT Oriel). Normalized data show the spectral balance of reflected light independent of total reflectance.
Seasonal in situ measurements of mulch reflectance
Measurements of PAR were also used to determine seasonal changes in vegetative growth, using a multi-sensor (80 sensors) PAR ‘Ceptometer’ (AccuPAR, Decagon Devices Inc., USA). Records were made manually in the crop canopy at fortnightly intervals. For each bag, PAR readings were taken from three different positions: (1) incident radiation above the canopy, the probe held 0·5 m above the ground along the length of the bag; (2) incoming canopy radiation, the probe positioned in the leaf canopy, along the bag; and (3) radiation reflected from the mulch into the canopy, the probe held in same position as for (2) but with the sensors inverted. For (2) and (3), the probe was held in the same position in respect to the mulch surface and the growing bag for each sampling date. Reflected measurements are expressed relative to the incident radiation; on each measurement occasion five replicate blocks per treatment were measured and the treatment average calculated.
Experimental design
The polytunnel was divided into two, with each cultivar occupying half. In both halves, a randomized block design was used, with each experiment comprising five blocks (replicates), running east–west, arranged in four rows running north–south. Each block contained one bag per mulch type, i.e. each experiment contained 20 bags: five blocks × four treatments. One growing bag represented one experimental analysis unit.
Developmental records
Non-destructive measurements of vegetative growth were made from leaflet counts carried out fortnightly, using two plants per bag. All leaves that were judged to be at least 75 % fully expanded were counted. Runners were removed when appropriate and records were made of the total number collected per bag.
Leaf nitrogen concentration under optimal light conditions can be linked with plant growth potential; it can also be closely correlated with leaf chlorophyll concentration. Therefore, leaf ‘nitrogen content’ was monitored by measuring chlorophyll fluorescence (CCM-200, Opti-Sciences, USA) (to avoid nitrogen deficiency). Measurements were made fortnightly, using the youngest fully expanded leaf.
Volumetric measurements of compost moisture content (m3 m−3) were recorded manually to ensure water availability was sufficient and similar for all the different treatments, irrespective of the impact of different compost temperatures. Measurements were made using a ThetaProbe connected to a moisture meter (type HH2; Delta T Devices, Cambridge, UK) using the manufacturer's calibration for organic compost; taking three readings per bag, at fortnightly intervals.
Destructive analysis of dry matter partitioning
In early November, destructive records were made of the above-ground parts of ‘Flamenco’ plants; because ‘Elsanta’ ceased fruiting in August no analysis was carried out. The analysis involved counts of runners, fruits and trusses, as well as recording crown number and diameter, lamina area and fresh weight, petiole number and length, and dry weights of all plant parts.
Crop records and fruit quality measurements
Ripe fruit were harvested and size graded at least twice weekly from expt 2 until the end of October. Harvested fruit, per bag, was graded into four diameter size classes, i.e. >35 mm, 25–35 mm, 22–25 mm, and waste (includes any small, diseased, rotten and malformed fruit). The largest two classes constitute commercial class-1 fruit, with class-2 fruit being 22–25 mm. Once a week, fully ripe, medium-sized fruit were used to determine juice soluble solids (% Brix) with a digital refractometer (Palett 100, Atago & Co. Ltd, Tokyo, Japan).
Fruit samples were collected for chemical analysis (ellagic acid and ascorbic acid concentration) on several occasions during the growing season but for comparative purposes, samples of ‘Elsanta’ and ‘Flamenco’ fruit harvested in mid-July are presented. Approximately 50-g samples of medium-sized ripe fruit were collected from each bag prior to being frozen in liquid nitrogen and stored at −20 °C until required for analysis as described below.
Extraction and analysis of free and conjugated ellagic acid
Approximately 10 g of frozen fruit was macerated in 50 mL of cold methanol : water (80 : 20 v/v) containing 20 mg L−1 butylated hydroxytoluene as an antioxidant. The macerate was centrifuged at 1000 g for 10 min. The supernatant was collected and its volume measured. For free ellagic acid determination, 5 mL of the supernatant was passed through a Waters C18 (Milford, MA, USA) solid phase extraction cartridge pre-equilibrated with methanol : water (80 : 20 v/v). This rp C18 was used as a ‘filter’ to remove non-polar co-extractive (see manufactures' guides, etc.) demonstrated by a high level of sample coloration retained on the C18. All C18 were conditioned with methanol and pre-equilibrated with 80 % methanol. A 2·5-mL aliquot of the eluate was reduced to dryness in a centrifugal vacuum concentrator and redissolved in 0·8 mL methanol : water (20 : 10 v/v). For total ellagic acid determination, 3 mL of the supernatant was mixed with 1·8 mL of 3·9 m HCl aqueous solution, to give a final concentration of 1·5 m HCl in methanol : water (50 : 50 v/v). The mixture was capped in a glass reaction vial and heated at 100 °C for 2 h to hydrolyse the ellagitannins. After cooling, 2·5 mL of the resulting hydrolysate was passed through a Waters C18 solid-phase extraction cartridge pre-equilibrated with water, after washing with methanol to remove the HCl and non-polar hydrolysis products prior to HPLC. Ellagic acid was eluted from the cartridge with 5 mL of methanol : water (80 : 20 v/v).
Ellagic acid was analysed using a Waters Alliance 2690 HPLC system with a Waters 996 photodiode array detector. HPLC conditions were as follows: 20 µL injection volume, Phenomenex Luna C18 3µ 2 × 150 mm HPLC column fitted with a C18 guard cartridge, 35 °C column temperature, and a flow rate of 0·2 mL min−1. The mobile phase was (A) 0·8 % trifluoroacetic acid in aqueous solution at pH 1·5, and (B) acetonitrile, and the following gradient was used: 0–2 min, 90 % A and 10 % B; 2–12 min, linear increase to 60 % A and 40 % B; 12–20 min, 60 % A and 40 % B; 20–30 min, linear increase to 10 % A and 90 % B; 30–45 min, linear decrease to starting conditions, with a further 5 min equilibration. Ellagic acid was detected at 254 nm. Quantification was by external standard calibration curve, generated from ellagic acid standards (ellagic acid dehydrate) purchased from Avocado Research Chemicals Ltd, Heysham, Lancashire, UK.
The efficiencies of the extraction methods used for free and total ellagic acid were tested by repeatedly extracting the berry samples as described. For a high yield cultivar (‘EM1055WF’) 86·3 % of the free ellagic acid was present in the first extract, 11·7 % was present in the second extract, and 2·0 % was present in the third extract. For a low yield cultivar (‘St William's’) 97·1 % of the free ellagic acid was present in the first extract, 2·9 % was present in the second extract, and 0·0 % was present in the third extract. The efficiency of the extraction method for free ellagic acid, based on results from both cultivars, was 91·7 ± 5·4 % (mean ± s.e. of the mean).
Recovery of total ellagic acid was slightly less efficient than for free ellagic acid. For ‘EM1055WF’ 81·5 % of the total ellagic acid was present in the first extract, 15·6 % was present in the second extract, and 2·9 % was present in the third extract. For ‘St William's’, 88·9 % of the total ellagic acid was present in the first extract, 8·5 % was present in the second extract, and 2·6 % was present in the third extract. The efficiency of the extraction method for total ellagic acid was estimated to be 85·2 ± 3·7 % (mean ± standard error of the mean).
Extraction and analysis of total (oxidized + reduced) ascorbic acid
Approximately 20 g frozen weight of strawberry fruit was homogenized in a blender with 10 volumes of 25 mm ammonium acetate buffer pH 4·0. The homogenate was centrifuged at 24 000 g in a cooled (4 °C) ultracentrifuge for 15 min, and the volume of the supernatant measured. A 3-mL aliquot was added to 300 µL of a 5 % trifluoroacetic acid aqueous solution and 30 µL of a 500 mm solution of the reducing agent TRIS (2-carboxyethyl) phosphine hydrochloride and left at 4 °C for 30 min. Five hundred microlitres of the resulting solution were pipetted into a Whatman Mini-UniPrep HPLC vial incorporating a 0·45-µm polypropylene filter. HPLC conditions were as follows: Varian Polaris C18-A 3µ 150 × 2-mm column (C18 column with a polar embedded phase) fitted with a Metaguard 4·6-mm Polaris C18-A 3µ guard cartridge; column temperature 30 °C; mobile phase 0·5 % trifluoroacetic acid in aqueous solution (A) and methanol (B); gradient 0–10 min 100 % A, 10–15 min ramp to 50 % A + 50 % B, 15–25 min hold at 50 % A + 50 % B, 25–30 ramp to 100 % A, 30–45 min, equilibrate at 100 % A; flow rate 0·2 mL min−1. The Waters Alliance 2690 HPLC chromatographic system was again used for the analysis. Detection of ascorbic acid was at 245 nm, and quantitative analysis was by means of an external standard curve.
Statistical analysis
All experiments used replicated (see tables and figures of individual replication and degrees of freedom) and randomized designs. All statistical analyses were carried out using ANOVA to calculate F probabilities and the standard error of the difference of the means (s.e.d.) for treatment effects within cultivars.
RESULTS
Strawberry cultivar variation in fruit ellagic acid concentration
Tables 1 and 2 show the range of concentrations of ellagic acid in 45 strawberry cultivars analysed in year 1, and in 17 cultivars analysed in year 2. Total ellagic concentrations varied considerably over the 2 years. Interestingly, the white-fruited cultivars (‘Osmanli’, EM676WF, EM1055WF and EM1107WF) were generally all within the upper ranges, although the individual cultivar rankings differed between the years. For example, the white-fruited ‘Osmanli’ had highest total ellagic acid in year 1 (341 µg g−1). In year 2, however, ‘Osmanli’ had only 165 µg g−1 of total ellagic acid compared with 232 µg g−1 in ‘Honeoye’. This apparent year-to-year difference between cultivars was also evident with ‘Symphony’, ‘Cambridge Favourite’ and ‘Elsanta’.
Table 1.
Concentrations of ellagitannins, free ellagic acid and total ellagic acid in ripe strawberry fruit harvested from 45 different cultivars in June–July in year 1
| Concentration (μg g−1 frozen weight) |
||||
|---|---|---|---|---|
| Strawberry cultivar | Total ellagic acid | Free ellagic acid (EA) | Ellagitannins (ET) | Ratio ET : EA |
| Osmanli* | 341 | 11·3 | 330 | 29·2 |
| Nida | 322 | 21·4 | 301 | 14·1 |
| Laura | 243 | 21·1 | 222 | 10·5 |
| EM894-1 | 230 | ND | ND | ND |
| Florence | 226 | ND | ND | ND |
| ITA93-971-59 | 218 | 12·7 | 206 | 16·3 |
| Cigoulette | 217 | 6·7 | 210 | 31·3 |
| ITA91-355-3 | 208 | 7·9 | 200 | 25·4 |
| EM676WF* | 206 | 11·7 | 194 | 16·6 |
| Jaune | 201 | 5·0 | 196 | 38·8 |
| Sophie | 192 | 7·0 | 185 | 26·4 |
| EM1088 | 190 | 11·8 | 178 | 15·0 |
| EM1109WR | 186 | 15·3 | 171 | 11·2 |
| EM1089 | 181 | 16·2 | 165 | 10·2 |
| Premial | 177 | 8·0 | 169 | 21·0 |
| Coral | 176 | 23·0 | 153 | 6·7 |
| EM726 | 176 | ND | ND | ND |
| EM772-2 | 176 | ND | ND | ND |
| EM835-2 | 174 | ND | ND | ND |
| EM1097DC | 166 | 6·2 | 160 | 25·7 |
| Rosie | 155 | 6·4 | 149 | 23·4 |
| Emily | 155 | ND | ND | ND |
| Onda | 154 | 3·8 | 151 | 39·2 |
| Pandora | 154 | 10·6 | 143 | 13·5 |
| EM881-1 | 150 | ND | ND | ND |
| Cigaline | 149 | 12·9 | 136 | 10·5 |
| Bolero | 140 | 15·4 | 124 | 8·1 |
| Darselect | 139 | 4·1 | 135 | 33·1 |
| Marshmello | 135 | ND | ND | ND |
| Pegasus | 133 | 14·7 | 118 | 8·0 |
| Selva | 129 | 3·5 | 125 | 35·4 |
| Ciloe | 126 | 7·5 | 118 | 15·7 |
| Symphony | 124 | 10·9 | 113 | 10·4 |
| Aromel | 120 | 6·1 | 114 | 18·6 |
| Eros | 115 | 8·9 | 106 | 11·9 |
| Cambridge Favourite | 107 | 8·1 | 99·1 | 12·2 |
| Totem | 98·4 | 10·1 | 88·3 | 8·8 |
| Elsanta | 94·4 | ND | ND | ND |
| Calypso | 92·8 | 9·0 | 83·8 | 9·3 |
| Korona | 85·6 | ND | ND | ND |
| Honeoye | 84·4 | 10·9 | 73·5 | 6·7 |
| Elvira | 84·0 | 3·7 | 80·3 | 22·0 |
| Tango | 79·2 | 4·3 | 74·9 | 17·6 |
| Hapil | 60·0 | ND | ND | ND |
The cultivars are ordered by total ellagic acid concentration, in descending order.
ND, Not determined.
Cultivars which produce white fruit.
Table 2.
Concentrations of ellagitannins, free ellagic acid and total ellagic acid in ripe strawberry fruit harvested from 17 different cultivars in June–July in year 2
| Concentration (μg g−1 frozen weight) |
||||
|---|---|---|---|---|
| Cultivar | Total ellagic acid | Free ellagic acid (EA) | Ellagitannins (ET) | Ratio ET : EA |
| EM1055WF* | 255 | 9·1 | 246 | 27·0 |
| Florence | 250 | 8·2 | 241 | 29·6 |
| Symphony | 239 | 12·6 | 227 | 18·0 |
| EM1107WF* | 236 | 5·7 | 230 | 40·3 |
| Alice | 235 | 10·1 | 225 | 22·3 |
| Honeoye | 232 | 11·2 | 221 | 19·8 |
| Mira | 230 | 18·5 | 211 | 11·4 |
| Elsanta | 223 | 8·1 | 215 | 26·7 |
| Ciloe | 205 | 5·8 | 199 | 34·6 |
| Darselect | 194 | 5·5 | 188 | 34·1 |
| Evangaline | 191 | ND | ND | ND |
| Cambridge Favourite | 187 | 9·8 | 177 | 18·0 |
| Osmanli* | 165 | 9·7 | 155 | 16·0 |
| Oka | 155 | ND | ND | ND |
| Sophie | 138 | 9·0 | 129 | 14·4 |
| Onda | 134 | 4·4 | 129 | 29·1 |
| St William's | 102 | 5·5 | 96·1 | 17·4 |
The cultivars are ordered by total ellagic acid concentration, in descending order.
ND, Not determined.
Cultivars which produce white fruit.
Effect of crop load on berry ellagic acid concentration
Tables 3 and 4 show that manipulation of crop load had no statistically significant effect on fruit ellagic acid concentration in either of the cultivars tested, either between treatments or berry types within a treatment. The greatest changes were seen for treatment 4 (secondary and tertiary inflorescences removed), where the primary berries had approx. 14 % and approx. 10 % less total ellagic acid than control primary berries in ‘Elsanta’ and ‘Florence’, respectively. However, these differences were not statistically significant.
Table 3.
Effects of crop thinning treatments on strawberry fruit ellagic acid concentrations
| Cultivar and treatment* | Fruit type | Total ellagic acid concentration (μg g−1) | Total fruit f. wt (g) | Total ellagic acid yield (mg) | Total number of berries produced | Estimated mean ellagic acid (mg) per fruit |
|---|---|---|---|---|---|---|
| ‘Elsanta’ | ||||||
| 1. | Primary | 112† ± 13‡ | 71 ± 25 | 7·4 | 5·3 | 1·4 |
| Secondary | 106 ± 17 | 111 ± 13 | 12·2 | 11·0 | 1·1 | |
| Tertiary | 96 ± 20 | 107 ± 14 | 10·4 | 14·3 | 0·7 | |
| Overall totals (or mean) | (102) | 289 | 30·0 | 30·7 | 1·0 | |
| 2. | Secondary | 96 ± 14 | 83 ± 11 | 8·3 | 5·7 | 1·4 |
| Tertiary | 101 ± 32 | 46 ± 30 | 3·9 | 4·7 | 0·9 | |
| Overall totals (or mean) | (91) | 129 | 12·2 | 10·3 | 1·2 | |
| 3. | Tertiary | 92 ± 12 | 48 ± 26 | 3·9 | 4·3 | 0·8 |
| 4. | Primary | 96 ± 15 | 92 ± 16 | 9·2 | 5·7 | 1·6 |
| ‘Florence’ | ||||||
| 1. | Primary | 209 ± 18 | 111 ± 18 | 23·7 | 8·3 | 2·8 |
| Secondary | 198 ± 28 | 86 ± 24 | 15·9 | 10·0 | 1·6 | |
| Tertiary | 200 ± 37 | 13 ± 9 | 3·3 | 1·7 | 1·7 | |
| Overall totals (or mean) | (203) | 210 | 42·9 | 20·0 | 2·1 | |
| 2. | Secondary | 206 ± 22 | 67 ± 26 | 13·6 | 5·3 | 2·5 |
| Tertiary | 209 ± 16 | 13 ± 8 | 5·3 | 1·5 | 1·8 | |
| Overall totals (or mean) | (205) | 75·4 | 15·4 | 6·3 | 2·3 | |
| 3. | Tertiary | 197 ± 23 | 19·0 ± 12 | 4·1 | 2·7 | 1·4 |
| 4. | Primary | 188 ± 24 | 150 ± 24 | 29·2 | 7·7 | 3·7 |
n.s., Non-significant.
Treatments: 1, control—no flowers removed; 2, primary flowers removed; 3, primary and secondary flowers removed; 4, all flowers except primary removed.
Total ellagic acid concentration is expressed as μg g−1 frozen weight of fruit; total ellagic acid yield is estimated by multiplying the concentration by the total fresh weight (g) of the berries harvested. Data for primary, secondary and tertiary berries are the means of three replicates. For treatments 1 and 2, an overall treatment mean taking into account the total harvest for all berry types has been calculated.
Means are shown plus 1 s.e.m.
Table 4.
Statistical analysis of total ellagic acid concentration (μg g−1)
| Treatment* |
||||||||
|---|---|---|---|---|---|---|---|---|
| Fruit analysed | 1 | 2 | 3 | 4 | Cultivar F probability | Cultivar s.e.d. (d.f.) | Treatment F probability | Treatment s.e.d. (d.f.) |
| Primary | + | · | · | + | <0·001 | 12·69 (6) | n.s. | 20·06 (6) |
| Secondary | + | + | · | · | <0·001 | 14·79 (6) | n.s. | 23·38 (6) |
| Tertiary | + | + | + | · | <0·001 | 16·52 (9) | n.s. | 26·12 (9) |
+, Fruit included in the analysis.
Cultivar × treatments interactions were not significant.
Treatments: 1, control—no flowers removed; 2, primary flowers removed; 3, primary and secondary flowers removed; 4, all flowers except primary removed.
As the concentration of ellagic acid was not affected by treatment, the variation of total yield of ellagic acid (averaged over all berry types) with treatment was similar to the variation of total fruit harvest weight. Treatments 2–4 necessarily reduced total fruit numbers, and the mean total amount of ellagic acid per fruit was greater in treatments 2 and 4 than in the control (treatment 1), due to an increase in average fruit size.
Effects of reflective mulches on the growing environment
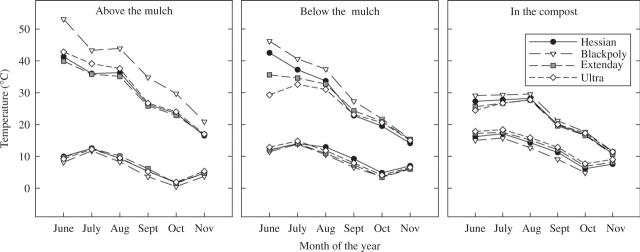
Combined temperature data, from both cultivars, collected from above or below the mulch and in the compost are summarized in Fig. 2. These data show the average maximum and minimum temperatures from June to November. Generally, as seasonal temperature declined, treatment differences were reduced. Temperatures directly above the mulch, which are most likely to impact on above-ground growth, were increased by the black polythene, while both reflective mulches showed lower early season temperatures directly below the mulch relative to the hessian and black polythene. Compost temperatures showed similar seasonal trends, but the differences in treatment mean maximum and minimum temperatures were less marked. However, daily compost temperature fluctuations showed distinct differences, both with and between treatments, particularly with respect to the black polythene: this resulted in warmer compost during the day, but lower night-time temperatures relative to the other treatments (data not shown).
Fig. 2.
The hourly mean average maximum and minimum temperatures for each month recorded throughout the growing season at three positions within the strawberry crop: air temperature above the mulch surface (5 cm); air temperature directly below the mulch on top of the compost; and soil temperature within the compost (at depth of 5 cm). Each data point represents data from three temperature sensors in three different experimental blocks. Data from both ‘Elsanta’ and ‘Flamenco’ are combined as there were no obvious cultivar differences.
To determine the impact of compost temperature on water availability for growth, compost moisture content, measured from July to November, ranged from 0·40 to 0·65 m3 m−3 for ‘Elsanta’, and 0·45 to 0·58 m3 m−3 for ‘Flamenco’; there was no consistent treatment effect (data not shown).
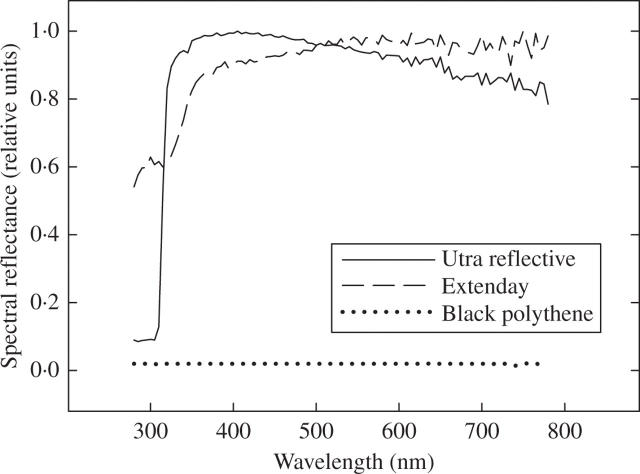
Measurements of mulch reflectance spectra
Reflectance spectra of the three plastic mulches were measured relative to the maximum reflectance for any film, in this case the ultra-reflective film (data not shown). Reflectance spectra are shown relative to each specific film (Fig. 3) to isolate the ‘shape’ of the reflectance spectrum from any difference in total reflectance. The Extenday and black polythene mulches have lower total reflectance than the ultra-reflective mulch, with a spectrum that is flat across the whole of the PAR and into the near infra-red. There is a progressive decline in reflectance with decreasing wavelength in the ultra-violet. For the ultra-reflective mulch, with its higher total reflectance, the spectrum is flat from near infra-red, through the PAR into the ultra-violet. There is, however, a sharp drop-off in reflectance below approx. 330 nm.
Fig. 3.
Reflectance spectra of the three plastic mulches relative to the maximum reflectance achieved for each specific mulch. This shows the ‘shape’ of the reflectance spectrum irrespective of any differences in total reflectance between mulches.
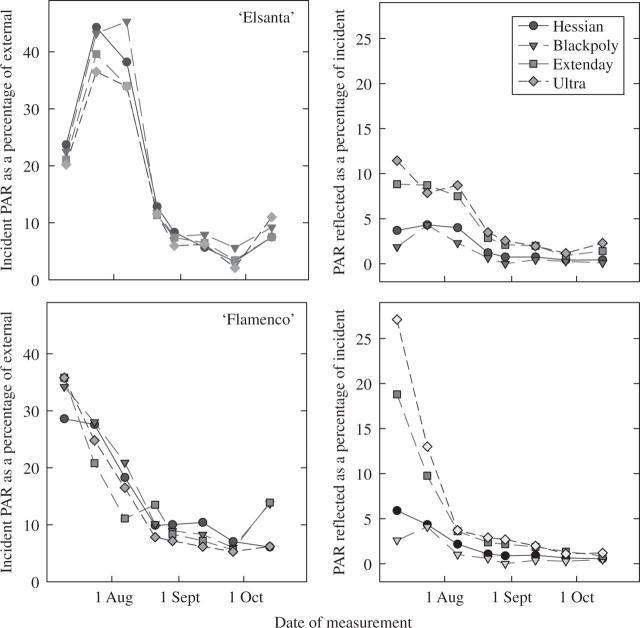
Figure 4 shows the reflected PAR measured in situ during the growing season. To exclude daily and seasonal changes in absolute PAR, measurements were expressed relative (as a percentage) to PAR above the crop (the external sensor). For both ‘Elsanta’ and ‘Flamenco’, the incident radiation declined during the season as the plant canopies expanded and intercepted a greater proportion of the PAR. Different seasonal changes were apparent with the two cultivars, due to initial differences in plant size, and the subsequent development patterns of June bearers and everbearers. More important were the treatment effects on PAR levels reflected back from the mulch. As well as marked seasonal differences, there were strong treatment effects on reflected PAR with the ultra-reflective and Extenday mulches reflecting 10–25 % of the incident radiation in early summer, depending on cultivar. Initially, the smaller ‘Flamenco’ plants intercepted less incident PAR.
Fig. 4.
The amount of incident radiation (PAR) detected close to ‘Elsanta’ (top) and ‘Flamenco’ (bottom) strawberry plants grown on a range of mulches with different reflective properties. Incident radiation is expressed directly in proportion to values of an external sensor to remove daily and seasonal changes during measurement. Reflected radiation is measured at the same position as the incident radiation, with the sensors inverted, and is expressed as a proportion of the incident radiation. Each data point represents the mean of four replicate blocks measured on each date.
Seasonal measurements showed that leaf ‘chlorophyll content’ increased for both ‘Elsanta’ and ‘Flamenco’, from approx. 12 to 25 units during July to November, but there were no treatment differences (data not shown).
Effects of reflective mulches on the distribution of dry matter within ‘Flamenco’ plants
Several plant organs showed statistically significant treatment differences in dry matter distribution (Table 5). Total petiole length, lamina area and fresh and dry weights, were increased by the ultra- and Extenday reflective mulch treatments. These effects were greatest for the Extenday mulch treatment. All of the other parameters measured indicated a greater, albeit not significant, allocation of dry matter and total biomass to plants within the Extenday treatment.
Table 5.
Analysis of plant dry matter distribution to crowns and leaves, and plant size for ‘Flamenco’ strawberries grown on a range of mulches with different reflective properties
| Mulch type |
Statistical analysis |
||||||
|---|---|---|---|---|---|---|---|
| Hessian | Black polythene | Extenday™ | Ultra-reflective | F probability | s.e.d. | d.f. | |
| Crown number | 54 | 52 | 64 | 48 | 0·05 | 5·26 | 12 |
| Total crown diameter (mm) | 507 | 454 | 573 | 464 | 0·074 | 44·3 | 12 |
| Petiole number | 328 | 334 | 380 | 350 | 0·284 | 27·6 | 12 |
| Total petiole length (mm) | 3554 | 3953 | 4538 | 3947 | 0·042 | 297·0 | 12 |
| No. of non-fruiting trusses | 33 | 33 | 39 | 40 | 0·728 | 7·6 | 12 |
| Lamina area (cm2) | 14734 | 15637 | 17687 | 16127 | 0·063 | 981·4 | 12 |
| Lamina f. wt (g) | 357 | 372 | 434 | 400 | 0·047 | 25·4 | 12 |
| Lamina d. wt (g) | 98 | 100 | 121 | 110 | 0·044 | 7·7 | 12 |
| Total vegetative d. wt (g) | 233 | 237 | 282 | 253 | 0·110 | 20·1 | 12 |
Plants were harvested at the end of the growing season (3 Nov.) and values are the means from 50 plants.
All data are mean values per bag (i.e. total of ten plants).
Effects of reflective mulches on leaf growth of ‘Elsanta’ and ‘Flamenco’
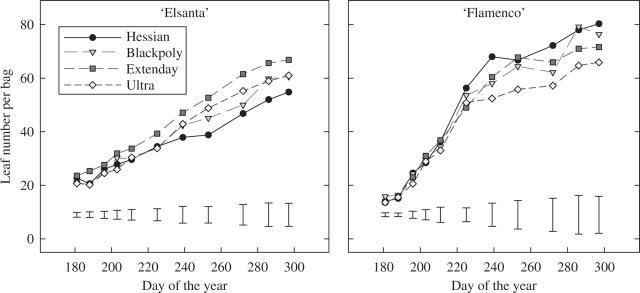
To determine dynamic changes in vegetative growth, frequent counts of leaf number were made. Initially, ‘Flamenco’ plants were smaller (approx. 15 leaves) relative to the ‘Elsanta’ (approx. 21 leaves) (Fig. 5). However, ‘Flamenco’ grew vigorously throughout July and early August, with leaf production exceeding that of ‘Elsanta’ by late July. Despite cultivar differences in growth and development of leaf number there were no statistically significant treatment effects (Fig. 5).
Fig. 5.
Seasonal change (day of the year) in leaf number for ‘Elsanta’ (left) and ‘Flamenco’ (right) strawberry plants grown on a range of mulches with different reflective properties. The bars represent the s.e.d. for each measurement date.
Effects of reflective mulches on productivity and seasonal cropping patterns of ‘Flamenco’ and the total crop weight and fruit number of ‘Elsanta’ and ‘Flamenco’
The effects of the reflective mulches on the seasonal cropping patterns for ‘Flamenco’ are shown, over a 17-week cropping season, in Fig. 6 (top). Mean weight of class-1 fruit is shown as an average weekly total harvested per bag, i.e. ten plants per bag. Seasonal variation in fruit harvested was considerable, with crop weight peaking at weeks 7 (18 Aug.) and 11 (15 Oct.). The early production peaks, particularly in week 7, were dominated by the Extenday and ultra-reflective treatments. By week 11, all treatments yielded similar crop weights and, from week 13, yields from the hessian treatment declined.
Fig. 6.
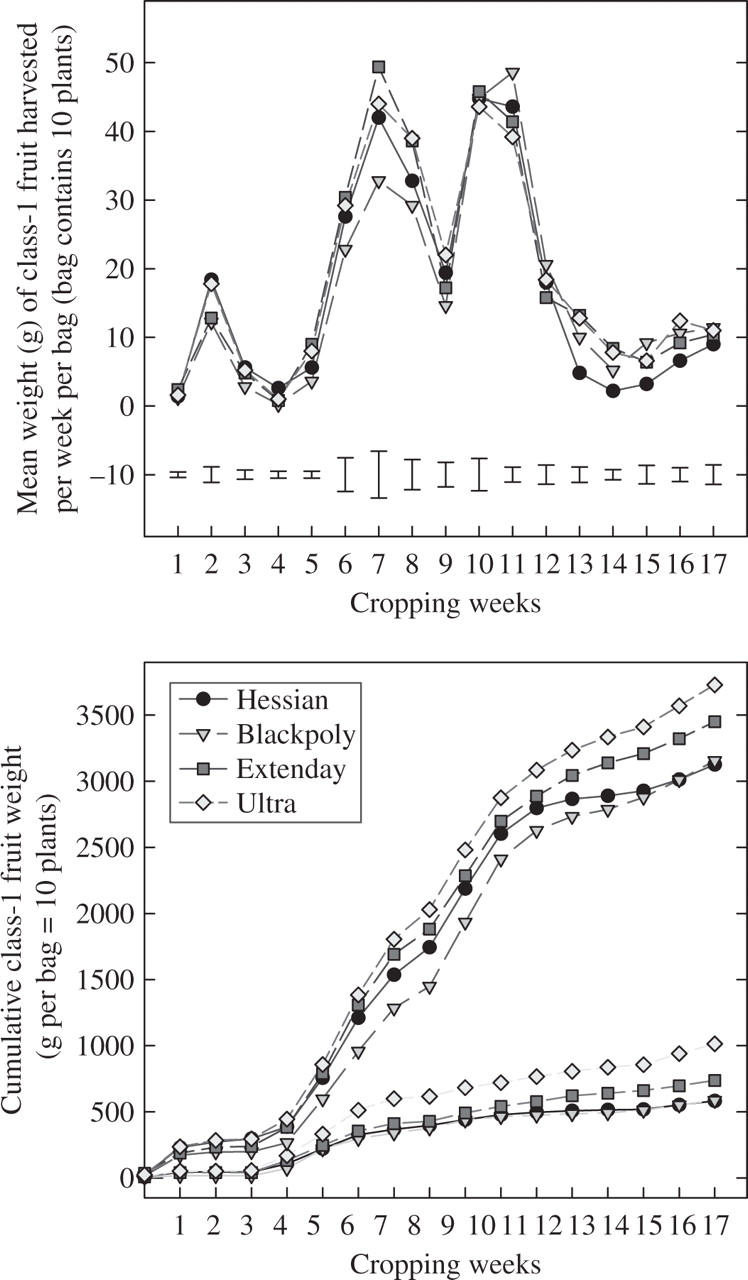
Seasonal changes in the weight of class-1 fruit from ‘Flamenco’ strawberry plants grown with a range of mulches with different reflective properties. The mean average weight of class-1 fruit harvested per week per bag (ten plants) and the cumulative weight of total class-1 fruit harvested throughout the growing season (top right) and the >35-mm class-1 fruit (bottom right).
When expressed as cumulative seasonal crop yields for total class-1 fruit, and separately for the very large class-1 fruit (>35 mm), the phasic nature of the cropping season and the treatment trends were apparent (Fig. 6, bottom). By week 5 (4 Aug.) the ultra-reflective and Extenday treatments had the highest rates of production, while the black polythene treatment had the lowest. By week 11, despite a decline in cropping with all treatments, the ultra-reflective and Extenday treatments still exhibited the highest fruit production rates.
The greater total mean crop yield for the everbearer ‘Flamenco’ compared with the June-bearer ‘Elsanta’ was, as expected, due to the differences in cropping season length (Table 6). Treatment differences were also apparent, with ultra-reflective mulch significantly increasing the total fruit weight for ‘Elsanta’ (25 %) and ‘Flamenco’ (16 %), and fruit number for ‘Flamenco’ (19 %). This increase in fruit production, for both cultivars, resulted from a significant increase in class-1 fruit (all fruit >25 mm in size) (Table 7).
Table 6.
Mean total season crop weight and fruit number harvested over the entire season from ‘Elsanta’ and ‘Flamenco’ strawberries grown on a range of mulches with different reflective properties
| Elsanta |
Flamenco |
|||
|---|---|---|---|---|
| Mulch type | Fruit weight (g) | Fruit number | Fruit weight (g) | Fruit number |
| Hessian | 1615* | 207 | 4095 | 485 |
| Black polythene | 1465 | 192 | 4050 | 505 |
| Extenday™ | 1698 | 202 | 4403 | 528 |
| Ultra-reflective | 2012 | 243 | 4759 | 575 |
| F probability | 0·025 | 0·199 | 0·05 | 0·033 |
| s.e.d. | 154 | 23·1 | 248·2 | 27·2 |
| d.f. | 12 | 12 | 12 | 12 |
Total average weight was from a bag containing ten plants.
Table 7.
Mean crop weight (g) harvested per bag (ten plants) with respect to fruit size class for ‘Elsanta’ and ‘Flamenco’ strawberries grown on a range of mulches with different reflective properties
| Elsanta |
Flamenco |
|||||
|---|---|---|---|---|---|---|
| Mulch type | Class 1* | Class 2 | Waste | Class 1 | Class 2 | Waste |
| Hessian | 1282 | 270 | 10·6 | 3126 | 839 | 52·3 |
| Black polythene | 1168 | 265 | 13·2 | 3315 | 709 | 44·6 |
| Extenday™ | 1435 | 215 | 15·8 | 3450 | 821 | 34·4 |
| Ultra-reflective | 1726 | 219 | 9·5 | 3729 | 855 | 36·2 |
| F probability | 0·006 | 0·355 | 0·384 | 0·02 | 0·447 | 0·149 |
| s.e.d. | 130·7 | 37·8 | 3·78 | 182·1 | 96·2 | 7·99 |
| d.f. | 12 | 12 | 12 | 12 | 12 | 12 |
Class 1, >25 mm; Class 2, 22–25 mm; Waste <22 mm and misshapes, etc.
Effects of reflective mulches on seasonal soluble solids (Brix %) of ‘Flamenco’
Values for soluble solids were highest in mid-July but then declined during August and September, only to increase back to July values in October (data not shown). The seasonal trends were generally consistent, with the only significant difference being in early September when the fruit grown with ultra-reflective and Extenday mulches had higher soluble solids. There was a seasonal link between fruit production and soluble solids; when the former was highest, soluble solids were lowest.
Effect of reflective mulches on fruit total ellagic acid and ascorbic acid concentration
Mulch type affected ellagic acid concentration in ‘Flamenco’ but not in ‘Elsanta’ (Table 8). The Extenday polythene mulch significantly increased fruit total ellagic acid concentration in ‘Flamenco’ by 40 % over the hessian mulch (P = 0·03). The ultra-reflective mulching increased ellagic acid concentration to a lesser extent: 20 % greater compared with hessian.
Table 8.
Total ellagic acid concentration in fruit from ‘Elsanta’ and ‘Flamenco’ strawberries grown on a range of mulches with different reflective properties
| Total ellagic acid concentration (μg g−1 frozen weight) |
||
|---|---|---|
| Mulch type | Elsanta | Flamenco |
| Hessian | 184* | 137 |
| Black polythene | 172 | 154 |
| Extenday™ | 179 | 191 |
| Ultra-reflective | 192 | 163 |
| F probability | 0·754 | 0·031 |
| s.e.d. | 19·4 | 16·0 |
| d.f. | 12 | 12 |
Fruit samples for analysis were taken in mid-July.
Data shown are the means of five replicate blocks.
Table 9 shows that, in contrast to the results reported for total ellagic acid, mulch type affected ascorbic acid concentration in ‘Elsanta’ but not in ‘Flamenco’. The Extenday mulch increased fruit ascorbic acid concentration in ‘Elsanta’ by 11 % over hessian and by 14 % over black polythene, and there was a significant treatment effect. Ultra-reflective mulching increased ascorbic acid concentration by slightly less than Extenday mulching (8 % greater than for hessian and 10 % greater than for black polythene).
Table 9.
Total ascorbic acid (vitamin C) concentration in fruit from ‘Elsanta’ and ‘Flamenco’ strawberries grown on a range of mulches with different reflective properties
| Total vitamin C concentration (μg g−1 frozen weight) |
||
|---|---|---|
| Mulch type | Elsanta | Flamenco |
| Hessian | 725* | 603 |
| Black polythene | 709 | 591 |
| Extenday™ | 804 | 567 |
| Ultra-reflective | 781 | 607 |
| F probability | 0·038 | 0·567 |
| s.e.d. | 32·6 | 30·0 |
| d.f. | 12 | 12 |
Fruit samples for analysis were taken in mid-July.
Data shown are the means of five replicate blocks.
DISCUSSION
The results from an initial survey of UK strawberry cultivars showed a large range in fruit total ellagic acid concentration—from 60 to 341 µg g−1 frozen weight. The present results are in the same range as those of Maas et al. (1991b), who reported ellagic acid concentrations between 0·43 and 4·64 mg g−1 d. wt in different US-grown strawberry cultivars (roughly equivalent to 43–464 µg g−1 f. wt assuming a 10 : 1 ratio of fresh weight : dry weight). Similarly, Häkkinen and Törrönen (2000) showed that ellagic acid concentrations in strawberries grown in Finland and Poland varied from 340 to 590 µg g−1 f. wt. Williner et al. (2003), however, measured lower ellagic acid concentrations of 16–46 µg g−1 f. wt in Argentine-grown strawberries. Differences between these results and those shown here, along with those of Maas et al. (1991b), may be due to the basis of data expression (fresh, dry and frozen weights), or perhaps the impact of cultural or climatic variation.
Berries from the white-fruited cultivars tended to be in the higher range of total ellagic acid concentration. Ellagic acid biosynthesis is believed to proceed from gallic acid, which is formed directly from shikimic acid (see Seigler, 1998; Niemetz and Gross, 2003). The biosynthesis of anthocyanins is also dependent on shikimic acid, although they are downstream in the biosynthetic pathway. It is unclear where a blockage in anthocyanin synthesis occurs in these white-fruited cultivars, or if the lack of colour is linked to a direct or indirect effect on channelling precursor into ellagitannin formation. Shikimic acid itself does not accumulate at high concentrations in strawberry fruit; Sturm et al. (2003) measured a range of 6–18 µg g−1 f. wt in ripe fruit. Preliminary investigations of ellagic acid concentrations in yellow or apricot-coloured raspberry cultivars, which were presumed to show a degree of anthocyanin-deficiency, did not show elevated ellagic acid concentrations (J. Le Mière and Y. Y. Ford, unpubl. res.).
Comparison of year-to-year variation in total ellagic acid concentration showed significant differences between cultivars. There were also considerable differences in the conjugated and free ellagic acid ratios for the different cultivars. Seasonal cultivar differences were principally caused by changes in conjugated ellagic acid. In all cases, the conjugated ellagic acid was present in greater quantities than the free acid, but the ratio of conjugated ellagic acid : free ellagic acid was not as great in strawberry as in raspberry, which had little free acid (J. Le Mière and Y. Y. Ford, unpubl. res.; Mullen et al., 2002). Seasonal variation in total ellagic acid suggests that its concentrations may be environmentally influenced; this suggests the possibility of agronomic manipulation. Other researchers have reported this seasonal variation in antioxidant concentration (EU COST 863, ‘Euroberry’).
As yet, it is not known whether ellagic acid biosynthesis occurs in its entirety in leaves and fruit of strawberry, or whether ellagic acid accumulation in the fruit is partly or wholly dependent on the supply of precursors from leaves (shikimic or gallic acid, or precursors upstream). Shikimic acid concentration increases during strawberry fruit ripening, but the increase is small and does not affect the overall decrease in total acids (Sturm et al., 2003). Ellagic acid concentration decreases during strawberry fruit ripening (Maas et al., 1991b; Williner et al., 2003), probably as a result of fruit cellular expansion (dilution). Manipulation of crop load, by flower removal, may alter berry-to-berry competition for leaf-derived assimilates. However, there was no significant effect of crop load on fruit ellagic acid concentration, and the total yield of ellagic acid per plant declined with severity of flower thinning. Thus, a decrease in fruit number per plant did not lead to an increased accumulation of ellagic acid in the remaining berries, suggesting that ellagic acid concentration is relatively tightly regulated in strawberry, irrespective of crop load.
The agronomic impacts of the two reflective mulches on fruit yield are important, particularly where the ultra-reflective mulch increased yields in the absence of enhanced vegetative growth (leaf number). Harvest costs are negatively influenced by increases in leaf canopy because the fruit are difficult to find. It is equally important when trying to enhance yields that fruit quality is not compromised; here reflective mulches enhanced fruit quality through an increased proportion of fruit in class 1. Fruit quality is not solely dependent on fruit size with several factors such as taste and texture also interacting. Increased yields in response to the use of mulches and reflective mulches are not in themselves novel; many studies have documented increases in yield for a number of crops (Dufault and Wiggans, 1981; Farias-Larios and Orozco-Santos, 1997; Csizinszky et al., 1999; Greer and Dole, 2003). However, improvements in crop quality are not often recorded and, as far as is known, this is the first study to describe dual benefits of reflective mulches on strawberry fruit yield and chemical composition. The type of mulch used in strawberry cultivation appears to have different responses with respect to the antioxidant altered and the cultivar used. Using reflective mulches significantly increased the concentration of total fruit ellagic acid (by between 20 % and 40 %) in ‘Flamenco’ and that of the ascorbic acid in ‘Elsanta’.
The present results show that antioxidant capacity, with respect to ellagic acid concentration, varies with season, but as yet it is not known why. This phenomenon is not solely associated with these experiments; other researchers within Europe also report such variation (R. Nestby and E. Krüger-Steden, pers. comm.). More importantly, these results suggest that opportunities exist for developing agronomic practices to manipulate growth, to enhance yields and to optimize fruit bioactive compounds.
Acknowledgments
We gratefully acknowledge funds provided by the Worshipful Company of Fruiterers to support J. Le Mière, and those from the Department for Environment Food and Rural Affairs to undertake the experiments involving light reflective mulches.
LITERATURE CITED
- Awad MA, De Jager A, Dekker M, Jongen WMF. 2001. Formation of flavonoids and chlorogenic acid in apples as affected by crop load. Scientia Horticulturae 91: 227–237. [Google Scholar]
- Block G. 1991. Epidemiological evidence regarding vitamin C and cancer. American Journal of Clinical Nutrition 54: 131S–131S. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. 1992. Fruit, vegetables and cancer prevention: a review of the epidemiological evidence. Nutrition and Cancer 18: 1–29. [DOI] [PubMed] [Google Scholar]
- Clifford MN, Scalbert A. 2000. Ellagitannins—nature, occurrence and dietary burden. Journal of the Science of Food and Agriculture 80: 1118–1125. [Google Scholar]
- Csizinszky AA, Schuster DJ, Polston JE. 1999. Effect of ultraviolet-reflective mulches on tomato yields and on the silverleaf whitefly. HortScience 34: 911–914. [Google Scholar]
- Defra. 2003. Basic Horticultural Statistics for the United Kingdom, Calendar and Crop Years 1992/93–20002/03. National Statistics, Defra PB 8889.
- Dufault RJ, Wiggans SC. 1981. Response of sweet peppers to solar reflectors and reflective mulches. HortScience 16: 65–67. [Google Scholar]
- Eastwood MA. 1999. Interaction of dietary antioxidants in vivo: how fruit and vegetables prevent disease? Quarterly Journal of Medicine 92: 527–530. [DOI] [PubMed] [Google Scholar]
- Farias-Larios J, Orozco-Santos M. 1997. Colored polyethylene mulches increases fruit quality and yield in watermelon and reduces insect pest populations in dry tropics. Gartenbauwissenschaft 62: 255–260. [Google Scholar]
- Greer L, Dole JM. 2003. Aluminium foil, aluminium-painted, plastic and degradable mulches increase yields and decrease insect-vectored diseases of vegetables. HortTechnology 13: 276–284. [Google Scholar]
- Häkkinen SH, Törrönen AR. 2000. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: influence of cultivar, cultivation site and technique. Food Research International 33: 517–524. [Google Scholar]
- Häkkinen S, Heinonen M, Kärenlampi S, Mykkänen H, Ruuskanen J, Törrönen R. 1999. Screening of selected flavonoids and phenolic acids in 19 berries. Food Research International 32: 345–353. [Google Scholar]
- Ju Z, Liu C, Yuan Y. 1995. Activities of chalcone synthase and UDPGal : flavonoid-3-o-glycosyltransferase in relation to anthocyanin synthesis in apple. Scientia Horticulturae 63: 175–185. [Google Scholar]
- Ju Z, Duan Y, Ju Z. 1999. Effects of covering the orchard floor with reflective films on pigment accumulation and fruit coloration in ‘Fuji’ apples. Scientia Horticulturae 82: 47–56. [Google Scholar]
- Kalt W. 2001. Health functional phytochemicals of fruit. Horticultural Reviews 27: 269–315. [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. 2002. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine 113: 71S–88S. [DOI] [PubMed] [Google Scholar]
- Klein BP, Kurilich AC. 2000. Processing effects on dietary antioxidants from plant food. HortScience 35: 580–584. [Google Scholar]
- Lancaster JE. 1992. Regulation of skin color in apples. Critical Reviews of Plant Science 10: 487–502. [Google Scholar]
- Maas JL, Galletta GJ, Stoner GD. 1991a. Ellagic acid, an anticarcinogen in fruits, especially in strawberries: a review. HortScience 26: 10–14. [Google Scholar]
- Maas JL, Wang SY, Galletta GJ. 1991b. Evaluation of strawberry cultivars for ellagic acid content. HortScience 26: 66–68. [Google Scholar]
- Mandal S, Stoner GD. 1990. Inhibition of N-nitrosobenzyl-methylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis 11: 55–61, [DOI] [PubMed]
- Mullen W, McGinn J, Lean ME.J, MacLean M, Gardner P, Duthie GG, et al. 2002. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. Journal of Agricultural and Food Science 50: 5191–5196. [DOI] [PubMed] [Google Scholar]
- Niemetz R, Gross GG. 2003. Oxidation of pentagalloylglucose to the ellagitannin, tellimagrandin II, by a phenol oxidase from Tellima grandiflora leaves. Phytochemistry 62: 301–306. [DOI] [PubMed] [Google Scholar]
- Olsson ME, Gustavsson K-E, Andersson S, Nilsson A, Duan, R-D. 2004. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidants levels. Journal of Agricultural and Food Chemistry 52: 7264–7271. [DOI] [PubMed] [Google Scholar]
- Perchellet JP, Gali HU, Perchellet EM, Laks PE, Bothari V, Hemingway RW, et al. 1994. Anti-tumor promoting effects of gallotannis, ellagitannins and flavornoids in mouse skin in vivo. In: Food phytochemicals for cancer prevention 1: Fruits and vegetables. Washington, DC: American Chemical Society Symposium Series 546, 303–327.
- Potter JD. 1997. Cancer prevention: epidemiology and experiment. Cancer Letters 114: 7–9. [DOI] [PubMed] [Google Scholar]
- Prior RL, Cao G. 2000. Antioxidant phytochemicals in fruit and vegetables: diet and health implications. HortScience 35: 588–592. [Google Scholar]
- Saure MC. 1990. External control of anthocyanin formation in apple. Scientia Horticulturae 42: 181–218. [Google Scholar]
- Siegelman HW, Hendricks SB. 1958. Photocontrol of anthocyanin synthesis in apple skin. Plant Physiology, 33: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigler DS. 1998. Plant secondary metabolism. Boston: Kluwer Academic Publishers, 193–197.
- Steinmetz KA, Potter JD. 1996. Vegetables, fruit and cancer prevention: a review. Journal of the American Dietary Association 96: 1027–1039. [DOI] [PubMed] [Google Scholar]
- Stopar M, Bolcina U, Vanzo A, Vrhovsek U. 2002. Lower crop load for cv. Jonagold apples (Malus×domestica Borkh.) increases polyphenol content and fruit quality. Journal of Agricultural and Food Chemistry 50: 1643–1646. [DOI] [PubMed] [Google Scholar]
- Sturm K, Koron D, Stampar F. 2003. The composition of fruit of different strawberry varieties depending upon maturity stage. Food Chemistry 83: 417–422. [Google Scholar]
- Urquiaga I, Leighton F. 2000. Plant polyphenol antioxidants and oxidative stress. Biological Research 33: 55–64. [DOI] [PubMed] [Google Scholar]
- Vattem DA, Shetty K. 2005. Biological functionality of ellagic acid: a review. Journal of Food Biochemistry 29: 234–266. [Google Scholar]
- Wang SY, Galletta GJ, Camp MJ, Kasperbauer MJ. 1998. Mulch types affect fruit quality and composition of two strawberry genotypes. HortScience 33: 636–640. [Google Scholar]
- Wang SY, Zheng W, Galletta GJ. 2002. Cultural system affects fruit quality and antioxidant capacity in strawberries. Journal of Agricultural and Food Chemistry 50: 6534–6542. [DOI] [PubMed] [Google Scholar]
- Williner MR, Pirovani ME, Güemes DR. 2003. Ellagic acid content in strawberries of different cultivars and ripening stages. Journal of the Science of Food and Agriculture 83: 842–845. [Google Scholar]