Abstract
• Background Seventeen distinct pollination systems are known for genera of sub-Saharan African Iridaceae and recurrent shifts in pollination system have evolved in those with ten or more species. Pollination by long-tongued anthophorine bees foraging for nectar and coincidentally acquiring pollen on some part of their bodies is the inferred ancestral pollination strategy for most genera of the large subfamilies Iridoideae and Crocoideae and may be ancestral for the latter. Derived strategies include pollination by long-proboscid flies, large butterflies, night-flying hovering and settling moths, hopliine beetles and sunbirds. Bee pollination is diverse, with active pollen collection by female bees occurring in several genera, vibratile systems in a few and non-volatile oil as a reward in one species. Long-proboscid fly pollination, which is apparently restricted to southern Africa, includes four separate syndromes using different sets of flies and plant species in different parts of the subcontinent. Small numbers of species use bibionid flies, short-proboscid flies or wasps for their pollination; only about 2 % of species use multiple pollinators and can be described as generalists.
• Scope Using pollination observations for 375 species and based on repeated patterns of floral attractants and rewards, we infer pollination mechanisms for an additional 610 species. Matching pollination system to phylogeny or what is known about species relationships based on shared derived features, we infer repeated shifts in pollination system in some genera, as frequently as one shift for every five or six species of southern African Babiana or Gladiolus. Specialized systems using pollinators of one pollination group, or even a single pollinator species are the rule in the family. Shifts in pollination system are more frequent in genera of Crocoideae that have bilaterally symmetric flowers and a perianth tube, features that promote adaptive radiation by facilitating precise shifts in pollen placement, in conjunction with changes in flower colour, scent and tube length.
• Conclusions Diversity of pollination systems explains in part the huge species diversity of Iridaceae in sub-Saharan Africa, and permits species packing locally. Pollination shifts are, however, seen as playing a secondary role in speciation by promoting reproductive isolation in peripheral, ecologically distinct populations in areas of diverse topography, climate and soils. Pollination of Iridaceae in Eurasia and the New World, where the family is also well represented, is poorly studied but appears less diverse, although pollination by both pollen- and oil-collecting bees is frequent and bird pollination rare.
Keywords: Floral form, fragrance chemistry, guilds, keystone species, nectar chemistry, Coleoptera, Hymenoptera, Lepidoptera, Nectarinia
INTRODUCTION
Iridaceae, a family of some 1900 species in 65 genera, is more or less world-wide in distribution but with a marked concentration in sub-Saharan Africa, where there are an estimated 1190 species in 37 genera (our unpubl. data). The family is well known for the great variety of its flowers, especially among the southern African genera and species. In Africa, species diversity in Iridaceae increases dramatically southward, and over 1050 species are recorded from southern Africa, of which some 720 are restricted to the winter-rainfall zone in the extreme south-west of the subcontinent (Manning et al., 2002; our unpubl. data). Three of the four subfamilies of Iridaceae (Goldblatt, 1990) are represented in sub-Saharan Africa: Nivenioideae with Aristea, Geosiris (endemic to Madagascar) and three woody genera, Klattia, Nivenia and Witsenia; Iridoideae with Bobartia, Dietes, Ferraria and Moraea; and Crocoideae with 27 genera. All except Gladiolus and Romulea (Crocoideae) and Moraea and Dietes (Iridoideae) are endemic.
Preliminary observations by Scott Elliot (1890, 1891), Marloth (1898) and Vogel (1954) pointed to the existence of diverse pollination systems among the sub-Saharan African Iridaceae but the true extent of this diversity has only recently been fully revealed. Studies conducted over the past 15 years, mostly by us, often in collaboration with other workers (e.g. Goldblatt et al., 1995, 2000a, b, 2001, 2004b), and published in some 30 papers, have documented an extremely diverse pollination ecology in the southern African members of the family. It is now possible to provide an overview of this work, which documents the floral ecology and pollination of an important and, until recently, neglected group of plants in an under-studied part of the world. Although the majority of species of African Iridaceae are pollinated by Hymenoptera (mostly bees), the remaining species, in a variety of genera, are pollinated mainly, or solely, by insects in the orders Coleoptera (beetles), Diptera (short- and long-proboscid flies) and Lepidoptera (butterflies and moths), or by passerine birds (Nectarinidae). It is also now evident that pollination systems are predominantly specialist; plants rely on a single species or a few ecologically analogous species for pollination. By contrast, generalist species, which are pollinated by a range of pollinators from at least three pollinator groups, are rare among southern African Iridaceae. In consequence, almost all genera of any size exhibit a range of pollination systems, with similar patterns of floral variation having developed repeatedly within different genera (Bernhardt and Goldblatt, 2000, 2006).
Significantly, the diversity of pollination systems increases primarily with floral complexity and secondarily with genus size. Thus, Aristea (ca. 52 spp.), which has radially symmetric, mostly blue flowers, has three different pollination systems, whereas Sparaxis (15 species), with both zygomorphic and secondarily radially symmetric flowers, in a variety of colours, exhibits five different pollination systems, and Gladiolus, with a similar array of floral types but ca. 240 species, exploits seven different pollination systems, some of which have evolved multiple times.
As expected in predominantly specialist pollination systems, floral attractants and rewards correlate closely with pollinator profile, resulting in the development of distinct floral syndromes (fide Faegri and van der Pijl, 1971). Attractants are primarily perianth pigmentation, complemented by a range or floral odours in many species, but flower shape and tepal orientation, in particular functional floral symmetry, may be equally important for some pollinators. The reward to visitors in the majority of species is nectar, but in others it is pollen, and one species offers non-volatile oil. In the case of hopliine beetles (Scarabaeidae: Hopliinae), flowers provide a stable platform on which to congregate, and the value of pollen, which beetles sometimes consume, as a reward is uncertain.
PLANT AND FLORAL FORM
Iridaceae are mostly deciduous perennial herbs with underground perennating organs in the form of bulbs, corms or rhizomes, but almost all genera of Nivenioideae are evergreen. Among the latter are three genera that are true shrubs with woody stems, sometimes reaching to 2·5 m in height (Goldblatt, 1993). Plants produce one or more flowering stalks annually, and the inflorescence units are either rhipidia (laterally compressed, monochasial, umbellate cymes) or sessile flowers, usually arranged in spikes but occasionally solitary on branches (Goldblatt, 1990). Rhipidia may be solitary and contain one to several flowers, or may be arranged in racemes, spikes or corymbs, and thus a single flowering stalk may bear multiple flowers simultaneously, each from a different rhipidium. Spikes likewise may bear several flowers simultaneously. Flowers may be fugaceous, thus lasting less than a single day, e.g. Aristea and many Moraea species, or longer-lived, lasting two or more days, rarely more than five, as in all Crocoideae.
Flowers of all African Iridaceae are hermaphrodite and trimerous, thus consisting of two whorls of petal-like members (an outer and an inner series of tepals), with three stamens inserted opposite the outer tepals, and an inferior ovary of three united carpels sharing a common style. The style is three-forked apically, with each branch either developed into a broad entire or fringed lip, or comprising a short to long linear structure which may itself be divided. In Dietes and Moraea (Iridoideae) the style branches are dorsiventrally flattened and petaloid, each bearing a transverse stigmatic lobe on the abaxial surface (Fig. 1E and F), closely resembling the style branch of Iris. In Crocoideae the style divisions are usually slender, sometimes expanded apically or forked for half their length, or occasionally multifid. Receptive stigmatic surfaces may be confined to the distal portion of the style branches, e.g. in Babiana and Gladiolus, or the entire length of the branches may be stigmatic, e.g. in Hesperantha and Romulea. In Crocoideae and Nivenioideae the tepals are united basally in a variously developed hollow tube.
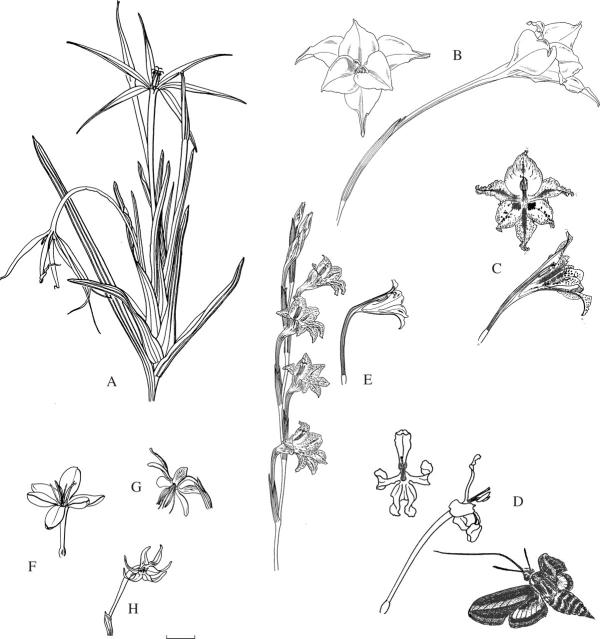
Fig. 1.

Flowers pollinated by bees, using either the large anthophorine system (A–F) or the pollen-collecting female bee system (G–J). (A) Gladiolus papilio, with arched dorsal tepal concealing the stamens and style branches. (B) G. alatus, arrows indicate flowers in male phase (style branches folded together and style not extended) or female phase (style extended, style branches unfolded and stigmatic tips expanded). (C) G. uysiae. (D) Tritonia watermeyeri, with tooth-like ridges on lower three tepals. (E) Moraea tripetala. (F) M. papilionacea. (G) Gladiolus stellatus. (H) G. quadrangulus. (I) Moraea bifida. (J) M. marlothii. I and J with the staminal column enlarged to show anthers in close proximity to stigmatic surfaces. Scale bar = 10 mm.
Flowers that last more than one day typically display closing movements, the tepals unfolding and closing again at specific times of the day. Thus, flowers pollinated by diurnal insects or birds open for all or part of the day, whereas those pollinated by night-flying moths usually open in the late afternoon or evening. Especially notable are species of Gladiolus and Hesperantha (Goldblatt and Manning, 2002; Goldblatt et al., 2004a), which show particularly complex opening and closing patterns. In Hesperantha diurnal opening is usually restricted to part of the day, morning or afternoon, whereas evening flowers open at specific times before or after sunset and close again in early or late evening. These closing movements not only prevent access to flowers by potential pollen or nectar thieves at certain times, but also protect the flowers from the elements, especially pollen-damaging moisture (Vlok, 2005). During unusually cold or wet weather, flowers will also close at times when they are normally open.
Most members of the latter two subfamilies secrete nectar from septal nectaries (Daumann, 1970; Rudall et al., 2003). By contrast, members of the Iridoideae bear so-called perigonal nectaries on the tepals. Secretion of non-volatile floral oil, found only in Tritoniopsis parviflora (Crocoideae) among the African Iridaceae, is accomplished by a secretory epithelium within the perianth (Manning and Goldblatt, 2002).
Flowers are radially symmetric in Iridoideae and Nivenioideae, but are ancestrally zygomorphic and bilabiate in Crocoideae with a prominent dorsal (adaxial) tepal, contrasting nectar guides on the lower tepals and arcuate (arching upward), unilateral stamens (Goldblatt et al., 2005c). The flower may be secondarily radially symmetric in some Crocoideae, a development that is either characteristic of entire genera, including Ixia and the Crocus–Romulea–Syringodea clade, or recurs repeatedly in a few species of some genera (Davies et al., 2004; Goldblatt et al., 2005c). Flowers of Geissorhiza and Hesperantha are radially symmetric in the majority of species but zygomorphic in a few, where the stamens are unilateral but declinate (arching downward), evidently a specialized feature.
Major adaptations of the crocoid flower are the development of a perianth tube, which may reach 50–100 mm, exceptionally 120 mm in length. The tube may be funnel-shaped or completely cylindrical. Associated with lengthening of the tube is the provision of greater amounts of nectar, which is always held in the proximal part of the tube and thus accessible only to visitors with longer mouthparts. A second type of adaptation to the tube is a decrease in the internal diameter, either by narrowing the entire tube or by thickening the walls, so that the interior of the tube tightly encloses the style. Nectar, when present, is then forced upward into the top of the tube. The tube of these species then serves a second purpose, as a stalk raising the sexual parts of the flower well above the ovary, a common adaptation in acaulescent species (Goldblatt et al., 1995). Alternatively, nectar may be absent and the tube may be vestigial (Aristea) or serve as a stalk promoting floral display (as in many Ixia species; Goldblatt et al., 2000a).
The flowers of Dietes, most species of Moraea and some of Ferraria function as meranthia, in the same way as in Iris where the meranthium flower has been understood since it was described by Hermann Müller in 1888 (Knuth, 1909; Proctor et al., 1996). In these genera the flower comprises three separate, gullet-like pollination units (Fig. 1E and F), each consisting of a platform provided by the limb of the outer tepal, a standard consisting of the style branch and its petaloid crests, and a gullet formed by the claw of the outer tepal and the closely opposed style branch. Although this type of flower is morphologically radially symmetrical, each pollination unit or meranthium is zygomorphic and bilabiate, and resembles the flowers of many Lamiaceae or Scrophulariaceae. Bees foraging for nectar probe the partial flowers (meranthium units) in order to reach nectar located at the base of each outer tepal, and in so doing brush against the concealed anther. During visits to other flowers, pollen is transferred to a stigmatic lobe that lies above the anther. Self-pollination and stigma clogging by self-pollen is prevented by the receptive adaxial part of the stigmatic lobe being pressed against the style branch as an insect exits the gullet. Although the meranthium units of these flowers can be said to be comparable with the shorter-tubed zygomorphic flowers of many Crocoideae, including, for example, Gladiolus and Sparaxis, they differ in lacking a true tube, and both short- and long-tongued bees are able to climb into the gullet and reach nectar at the base of the outer tepal (Goldblatt et al., 1989).
In many species of Moraea the elaborations of the flower, particularly the petaloid style branches, are only weakly developed or suppressed and a meranthium is not developed. These flowers, now with centrally placed, prominent stamens and style, are either bowl-shaped, with large tepal claws providing a floral cup, or stellate, in which case the tepal claws are short and the stamens and style are held above the outspread or reflexed tepals in a prominent column (Goldblatt and Bernhardt, 1999). Such flowers function as a single, actinomorphic bloom (Fig. 1I and J).
ATTRACTANTS: FLORAL SCENT, PIGMENTATION AND SHAPE
Perhaps the most striking feature of the flowers of sub-Saharan African Iridaceae is their sheer diversity of shape, colour and marking. Moreover, patterns of pigmentation are repeated across genera with remarkable consistency and are often correlated with other floral features, including type of scent produced (or absence of scent), perianth tube length, and nectar volume and concentration. The stability of these repeated suites of morphological, phenological and physiological features, or floral syndromes, and their multiple origins within the family, provide convincing evidence for the existence of modal optima (or adaptive peaks) in the pollination systems of Iridaceae (Manning and Goldblatt, 2005).
Floral odours
Iridaceae produce floral fragrances derived from a diversity of biosynthetic classes, including fatty acid derivatives, benzenoids and isoprenoids, often within a single genus (Manning and Goldblatt 2005). Attraction by floral display is usually complemented by scent production in most bee- and moth-pollinated flowers as well as those pollinated by short-proboscid flies, for example in Ferraria and Moraea. Floral odours are extremely varied across species, and sometimes within a species.
Bee-pollinated flowers typically produce a sweet, floral fragrance, reminiscent of violet or rose. Probably the best-known of the fragrant Iridaceae are the commercial hybrids derived from several bee-pollinated Freesia species, notably F. alba and F. caryophyllacea. In these species the terpenes linalool and ionone predominate (R. Kaiser, unpubl. data). Similarly, many bee-pollinated Gladiolus species, among them G. carinatus and G. virescens, also produce odours dominated by ionone, while linalool predominates in G. alatus, and geraniol or geraniol acetate, nerol and citronellol characterize G. jonquilliodorus, G. orchidiflorus and G. scullyi (Goldblatt et al., 1998b). Although floral odours are almost universal in bee-pollinated Gladiolus species of the southern African winter-rainfall zone, few bee-pollinated species elsewhere in Africa produce floral fragrance.
By contrast, Tritoniopsis parviflora, which is pollinated by oil-collecting bees in the genus Rediviva, produces a distinctive odour dominated by 3, 5-dimethoxy toluene, a compound also common among southern African oil-producing orchids of the genera Pterygodium and Disperis (Manning and Goldblatt, 2005). Vanilla-like floral fragrances are produced by some bee-pollinated Moraea species, including M. ciliata, M. fugax and M. macronyx. These fragrances are characterized by the presence of relatively large amounts of vanilline, in combination with jasmine lactone, phenylacetaldehyde, anisaldehyde and methyl anisate (R. Kaiser, unpubl. data).
Among moth-pollinated Gladiolus species examined for fragrance chemistry, linalool is the dominant compound in G. maculatus, G. recurvus and G. tristis, whereas eugenol dominates in G. liliaceus (Goldblatt and Manning, 2002). Similarly, the floral fragrance of Tritoniopsis nervosa, which is pollinated by sphinx moths, is also rich in linalool (Manning and Goldblatt 2005). Finally, the sapromyiophilous species M. lurida and M. ochroleuca produce odours of rotting flesh or fermenting fruit, as do those species of Ferraria pollinated by dung, flesh and game flies. In general, therefore, the floral odours produced by Iridaceae are consistent with those found in flowers of other plant families with the same pollination system.
The major floral types and their pollinators
Seven main pollination strategies and associated floral syndromes are evident within the sub-Saharan Iridaceae.
Bee flowers (Fig. 1).
Bee-pollination in African Iridaceae is diverse and may be subdivided into three separate, non-overlapping strategies.
Type 1. Pollination by large-bodied, long-tongued male and female anthophorine bees and native Apis mellifera is the most common system in the family (Table 1). It has been identified as the ancestral pollination system in a few genera through phylogenetic analysis, and its occurrence in nearly all genera suggests that it is the ancestral system for the African Iridaceae.
Table 1.
Comparative pollination systems of sub-Saharan African Iridaceae
| No. of species with each pollination system |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Long-p fly |
||||||||||||||
| Genus: with no. of species observed, and no. inferred plus observed/total in genus | Apid nectar | Apid pollen | Apid buzz | 1 | 2 | 3 | Scarab beetle | Scarab/bee | Moth | Butterfly | Bird | Short-p fly | Wasp | Generalist |
| Aristea 12, 52/52 | 0 | 43 | 4 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Babiana 44, 86/86 | 49 | 5 | 0 | 12 | 0 | 5 | 6 | 3 | 3 | 0 | 3 | 0 | 0 | 0 |
| Chasmanthe 2, 3/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Crocosmia 5, 7/8 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 |
| Duthieastrum 1, 1/1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ferraria 8, 9/13 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0 |
| Freesia 3, 15/15 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Geissorhiza 14, 17/85 | 6 | 4 | 0 | 1 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gladiolus 80, 213/240 | 115 | 4 | 0 | 0 | 13 | 15 | 0 | 1 | 25 | 9 | 29 | 0 | 0 | 0 |
| Hesperantha 25, 79/79 | 34 | 0 | 0 | 3 | 9 | 3 | 2 | 3 | 24 | 1 | 0 | 0 | 0 | 0 |
| Ixia 21, 52/52 | 17 | 0 | 4 | 0 | 0 | 6 | 19 | 4 | 0 | 0 | 0 | 0 | 0 | 2 |
| Klatta 2, 3/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Lapeirousia 20, 41/41 | 7 | 0 | 0 | 8 | 0 | 4 | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 15 |
| Melasphaerula 1, 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1* | 0 | 0 |
| Micranthus 2, 3/3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Moraea 38, 195/195 | 108 | 40 | 0 | 0 | 0 | 0 | 14 | 30 | 0 | 0 | 0 | 2 | 1 | 0 |
| Nivenia 5, 10/10 | 5 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pillansia 1, 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Radinosiphon 2, 1/2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Romulea 31, 75/75 | 0 | 47 | 0 | 3 | 0 | 1 | 8 | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| Savannosiphon 0, 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Sparaxis 13, 15/15 | 5 | 0 | 0 | 3 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Thereianthus 1, 2/7 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tritonia 7, 28/28 | 16 | 1 | 0 | 1 | 0 | 7 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tritoniopsis 21, 24/24 | 12† | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 7‡ | 0 | 0 | 0 |
| Watsonia 12, 51/51 | 17 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 1 | 2 | 25 | 0 | 0 | 0 |
| Witsenia 1, 1/1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Xenoscapa 1, 2/2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| S = 375, 988/1095 | 406 | 145 | 8 | 33 | 30 | 54 | 62 | 59 | 62 | 16 | 75 | 9 | 3 | 24 |
The long-proboscid fly pollination systems follow the definitions established by Goldblatt & Manning (2000b). Genera not included: Bobartia (15 species); Cyanixia (1); Devia (1); Dierama (44); Dietes (6); Syringodea (8); Zygotritonia (4). Apid nectar, pollination by large-bodied, long-tongued Apidae foraging for nectar; Apid pollen, pollination by female bees of various families actively collecting pollen; Apid buzz, vibratile pollination by female Apidae; Long-p fly, long-proboscid fly; 1, Prosoeaca peringueyi system; 2, Moegistorhynchus–Philoliche system; 3, Prosoeca ganglbauri system (including the three species using the Stenobasipteron wiedemannii pollination system); Short-p fly = short-proboscid fly. The passive pollination system that involves large-bodied, long-tongued Apid bees occasionally also includes tabanid and nemestrinid flies with short probosces which function exactly like Apid bees and we consider them part of the Apid nectar system. An exception is Nivenia binata, which is pollinated consistently by both long-proboscid flies and large anthophorine bees.
The only recorded pollinator of Melasphaerula is the bibionid fly Bibio sp. (Bibionidae), quite different from flies of Muscidae, Calliphoridae and Sarcophagidae of other species in this pollinator category.
In one species combined with oil secretion and pollination by oil-collecting bees (Rediviva).
Two of these bird-pollinated species are shared with the butterfly Aeropetes.
Flowers are typically zygomorphic, with a relatively short, funnel-shaped tube, and an enlarged dorsal tepal that is often arched forward, thus concealing the arcuate stamens and style (Fig. 1A–D), present in 46 % of the species under consideration. The flowers are often strongly scented, notably in Freesia, Gladiolus and Sparaxis. Less often, flowers are actinomorphic, as in Ixia latifolia and I. rapunculoides, but retain a funnel-like tube with a narrow basal portion. The floral reward is nectar, which is held within the lower part of the perianth tube and is thus accessible only to insects with mouthparts exceeding 5 mm in length. Perianth tubes are invariably longer and fairly narrow, thus permitting access only to the proboscis and tongue of the insect visitor. Flower colours are diverse, most often shades of blue to violet with white to yellow markings (nectar guides) edged in darker colour on the lower tepals. Other colours include pink, purple, yellow, orange and even red. Despite this variability, flowers generally retain a characteristic gullet form.
Pollen deposition and transfer occurs when the dorsum of a bee contacts anthers or stigmatic surfaces as it probes the perianth tube for nectar. Some unusual adaptations to ensure that the dorsum of a visiting bee brushes against the anthers or stigmatic surfaces include rigid spurs at the bases of the anthers in Gladiolus appendiculatus. These bar entry to the lower part of the perianth tube until pressed backward, when the anthers then tilt forward and swab pollen onto the dorsal part of a bee's thorax (Goldblatt et al., 1998b). In most species of Tritonia pollinated by bees, e.g. T. securigera and T. watermeyeri (Fig. 1D), the lower tepals each bear a median tooth-like ridge or callus (de Vos, 1983), which together direct a visiting bee upward to brush against the anthers or style branches, held immediately under the dorsal tepal, as it enters the flower.
Nectar in long-tongued bee flowers is typically of intermediate or high concentration (Table 2), mostly above 25 % sucrose equivalents and sometimes exceeding 50 %. Nectar is typically sucrose-dominant in Crocoideae but sucrose-rich in a few species. By contrast, hexose (fructose plus glucose)-dominant nectar characterizes all bee-pollinated Iridoideae (Table 3).
Table 2.
Nectar concentrations in percentage sucrose equivalents for genera of Crocoideae and Nivenioideae pollinated exclusively by one of the five pollination catergories listed
| Family/genus | Apid nectar | Long-proboscid fly | Moth | Bird | Butterfly | Reference |
|---|---|---|---|---|---|---|
| Crocoideae | ||||||
| Babiana | 22.0–42.3 | 22.0–31.7 | 26.0–31.7 | 21.0–25.5 | n/a | Manning & Goldblatt (1996), Goldblatt & Manning (in press) |
| Chasmanthe | n/a | n/a | n/a | 10.0–17.3 | n/a | Goldblatt et al. (2004a) |
| Crocosmia | 23.2 | n/a | n/a | 17.4–18.0 | 17.7–23.3 | Goldblatt et al. (2004a) |
| Geissorhiza | 26.3–>50 | 19.6–29.0 | n/a | n/a | n/a | Manning & Goldblatt (1997) |
| Gladiolus | 25.0–44.0 | 24.8–33.2 | 20.0–36.4 | 18.2–35.4 | 19.5–26.1 | Goldblatt & Manning (2004, and sources cited therein) |
| Hesperantha | 21.3–>50 | 26.4–28.5* 13.7–19.3† | (21.5–)32.0–48.0 | n/a | 15.4–20.8 | Goldblatt et al. (2004b) |
| Ixia | 28 | 23.7–29.8 | n/a | n/a | n/a | Goldblatt et al. (2000a) |
| Lapeirousia | 26.7–34.1 | 21.1–30.4 | 27.0–34.1 | n/a | n/a | Goldblatt et al. (1995) |
| Romulea | >50 | 20.0–23.5 | n/a | n/a | n/a | Goldblatt et al. (2002a) |
| Sparaxis | 24.3–41.5 | 28.2–28.5 | n/a | n/a | n/a | Goldblatt et al. (2000b) |
| Tritonia | 38.4 | 23.2–29.3 | n/a | n/a | n/a | Manning & Goldblatt (1997) and unpubl. data |
| Tritoniopsis | 23.8–44.0 | 29.0–32.0 | 23.8 | 24.2 | 3.5 | Manning & Goldblatt (2005) |
| Watsonia | 32.0–33.3 | 26.3–26.5 | n/a | 14.5–23.8 | n/a | Goldblatt et al. (1999) |
| Nivenioideae | ||||||
| Klattia | n/a | n/a | n/a | 13.2–15.0 | n/a | Goldblatt (1993) |
| Nivenia | 29.0 | 30.0–31.0 | n/a | n/a | n/a | Goldblatt (1993) |
| Witsenia | n/a | n/a | n/a | 12.0–13.5 | n/a | Goldblatt (1993) |
Data for Tritoniopsis species pollinated by both sunbirds and butterflies are not listed—the range for these species is 16.1–27.0. Nectar readings are based on sample sizes of at least five individuals and more often ten: only the major source(s) of data for each genus are provided.
Species of the southern African winter-rainfall zone.
Species of the southern African summer-rainfall zone.
Table 3.
Numbers of species in each of the four nectar/sugar ratio categories of Baker and Baker (1983) arranged by subfamily and pollinator group
| Ratio: sucrose/(glucose + fructose) |
||||
|---|---|---|---|---|
| Pollinator group | <0.1 | 0.1–0.49 | 0.5–0.99 | >0.99 |
| Crocoideae | ||||
| Sunbirds | 4 | 3 | 5 | 23 |
| Moths | 0 | 1 | 0 | 11 |
| Butterflies | 0 | 3 | 2 | 5 |
| Short-tongued bees | 0 | 0 | 0 | 1 |
| Long-tongued bees | 0 | 0 | 4 | 27 |
| Long-proboscid flies | 0 | 1 | 1 | 48 |
| Nivenioideae | ||||
| Sunbirds | 4 | 0 | 0 | 0 |
| Long-tongued bees | 0 | 0 | 1 | 0 |
| Long-proboscid flies | 0 | 0 | 3 | 0 |
| Iridoideae | ||||
| Short-tongued bees/wasps | 7 | 0 | 0 | 0 |
| Long-tongued bees | 5 | 0 | 0 | 0 |
| Short-proboscid flies | 2 | 0 | 0 | 0 |
HPLC sugar analysis was used to determine nectar sugars by B.-E. van Wyk, Rand Afrikaans University, Johannesburg. Data were compiled from the papers cited in Table 2. Exclusively beetle-pollinated species produce no nectar and are not included.
Type 2. In some bee-pollinated species pollen is the primary, or even sole, reward. Species with this type of flower are radially symmetric with a relatively short perianth tube and either with diverging stamens and an eccentric style (Aristea and Geissorhiza species) or with the style centrally placed and closely surrounded by the stamens, together forming a column (Moraea species, Gladiolus stellatus, Romulea species) (Fig. 1G–J). In a few instances, e.g. Sparaxis bulbifera, the perianth alone is radially symmetric, and the anthers and style are laxly unilateral (Goldblatt et al. 2000b). The anthers are prominently displayed and nectar is usually limited in quantity, often less than 0·5 mL, and is sucrose-dominant in Crocoideae but hexose-dominant in Iridoideae (Table 3). In bee-pollinated Aristea species no nectar is produced (Goldblatt and Manning, 1997a, b). Yellow trichomes at the base of the filaments in bee-pollinated species of Romulea may function as pseudopollen, prolonging the active life of the flower as a pollen resource and/or prolonging the foraging time. These trichomes are absent in Romulea species with other pollination systems.
Visitors to this type of flower are usually Apis workers, or female bees of other families, including Andrenidae, Apidae: Anthophorinae, Halictidae, Megachilidae, and occasionally Colletidae and Melittidae. Pollen deposition is active, with bees usually clasping the staminal column or the anthers and actively combing pollen into pollen baskets or other areas of pollen storage. This type of pollination is predominant and probably ancestral in Aristea and Romulea, and has evolved multiple times in Gladiolus and Moraea. In Gladiolus, which is ancestrally zygomorphic, it is accompanied by the development of radial symmetry (Goldblatt et al., 1998b). In Moraea, the ancestral Iris-type flower with its functionally bilabiate meranthium units is reduced in complexity such that the inner and outer tepals are similar in size and disposition, the elaborate style branches are reduced and the anthers are visible and often held well above the tepals in a column enclosing the style. In many species the anthers actually conceal the style branches and pollen may contact the stigmatic surfaces, but owing to self-incompatibility, selfing does not occur (Goldblatt, 1981).
Type 3. A third category of bee pollination is vibratile or buzz pollination (Table 1), which has thus far been demonstrated to occur in Iridaceae in only a few species of Ixia section Dichone. These flowers have a radially symmetric perianth, but nodding, unilateral stamens. The anthers open by slits at the base and must be vibrated by a visiting bee to release pollen. The flowers lack nectar and to date the only recorded visitors are female Amegilla (Apidae: Anthophorinae) bees (Goldblatt et al., 2000a). Four species of Aristea also have anthers opening by apical slits, in this case at the apex, and we infer vibratile pollination in these species, although there are examples of non-vibratile pollination in species with such anthers (Thompson et al., 2000).
Long-proboscid fly flowers (Figs 2 and 3).
Fig. 2.
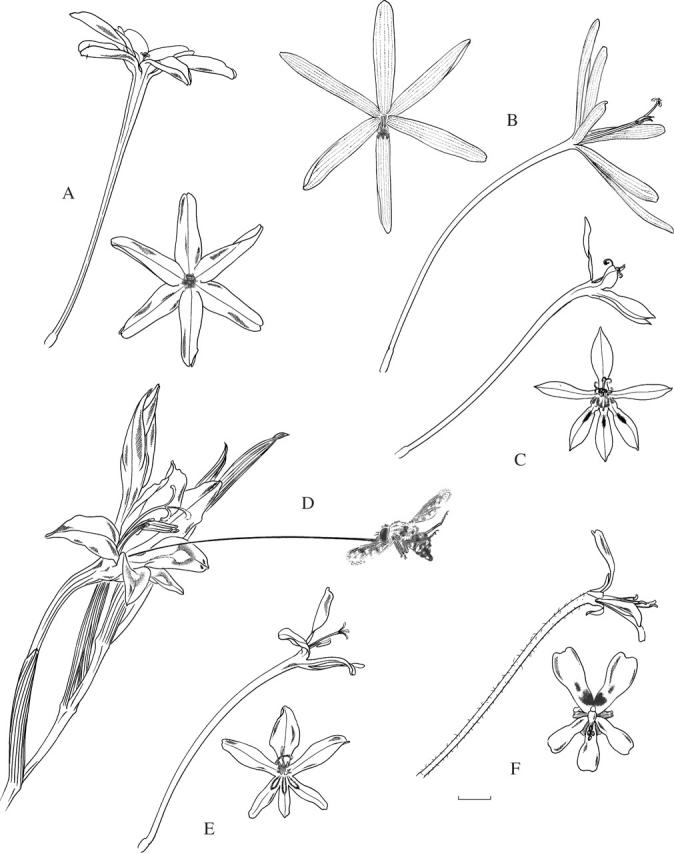
Lateral and frontal views of flowers pollinated by long-proboscid flies and the fly Moegistorhynchus longirostris. (A) Ixia paniculata. (B) Geissorhiza exscapa. (C) Lapeirousia anceps. (D) Gladiolus angustus. (E) Tritonia crispa. (F) Pelargonium moniliforme (Geraniaeae), to show similarity of flower form in another family belonging to this guild. Scale bar = 10 mm.
Fig. 3.
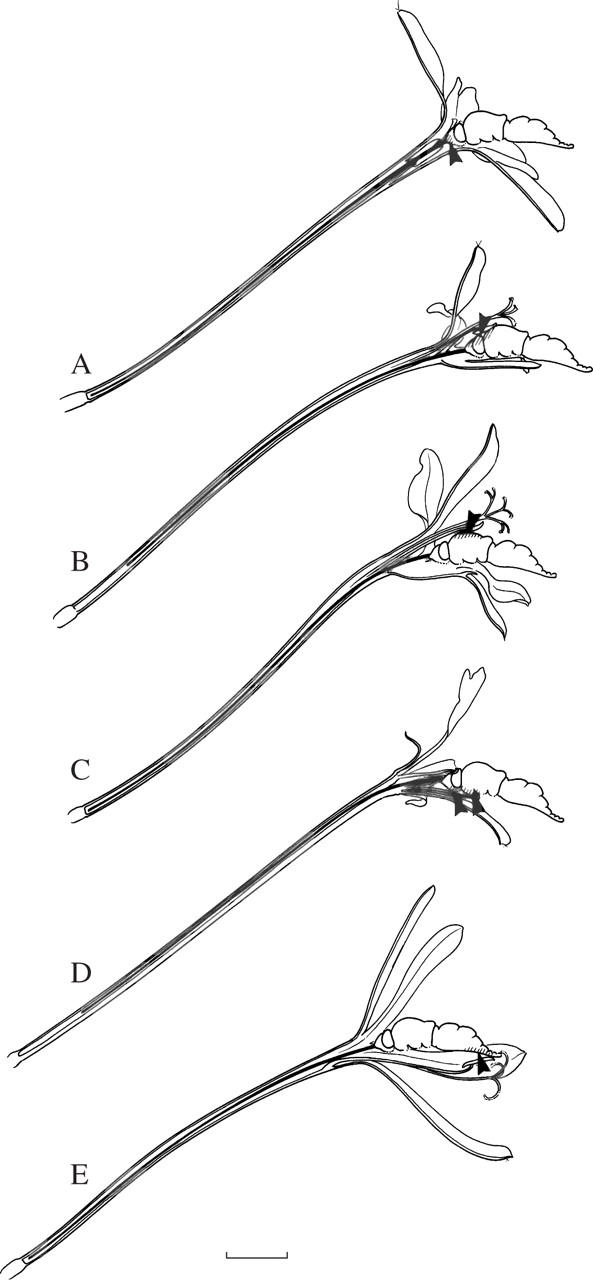
Differential pollen placement indicated by arrows and hatching on fly's body by different plant species of the Moegistorhynchus longirostris pollination guild. (A) Ixia paniculata (frons and base of proboscis). (B) Tritonia crispa (dorsal part of head). (C) Lapeirousia anceps (dorsal part of thorax). (D) Pelargonium appendiculatum (ventral part of head and thorax). (E) Geissorhiza exscapa (ventral part of abdomen). Scale bar = 1 cm.
An important pollinator group in southern Africa (Table 1), but so far unknown elsewhere in sub-Saharan Africa, long-proboscid flies (Nemestrinidae and Tabanidae) are loosely defined as having a proboscis at least 1·5 times as long as the body and more than 20 mm long (up to 100 mm in one instance) (Goldblatt and Manning, 2000a). The probosces in these insects do not retract, but project forward in Tabanidae or trail behind the insect in Nemestrinidae during flight. The flies are active foragers and consume relatively large quantities of nectar. Flowers usually have an elongate, more-or-less cylindrical perianth tube with an internal diameter of 1–1·5 mm. The perianth tube is typically slightly longer than the length of the pollinator proboscis (Goldblatt et al., 1995; Goldblatt and Manning, 2000a). Nectar, held within the lower portion of the tube, is sucrose-rich or sucrose-dominant (Table 3), and typically of intermediate concentration (Table 2), 20–29 % sucrose equivalents, but exceptionally up to 33·2 % in Gladiolus monticola. Hesperantha species of eastern southern Africa are exceptional in their low nectar concentrations, 13·7–19·3 % (Goldblatt et al., 2004b). The flowers are almost always unscented, and the few exceptions in the genus Babiana may represent recent entrants into this pollination system (Goldblatt and Manning, in press).
Unlike bee flowers, which show no adaptations to particular bee taxa, the different sets of floral features that are displayed by fly flowers are each associated with one of four guilds of long-proboscid flies. Each guild is ecologically or geographically distinct (Potgieter et al., 1999; Goldblatt and Manning, 2000a; Potgieter and Edwards, 2005).
Type 1. The Prosoeca peringueyi pollination system (Fig. 4). Restricted to the winter-rainfall west coast of South Africa and southern Namibia, this system comprises a guild of spring-flowering plant species with red to violet flowers with white to yellow markings, mostly in the families Iridaceae and Geraniaceae. They are pollinated by only two species of Nemestrinidae, Prosoeca peringueyi and P. sp. (Manning and Goldblatt, 1996; Goldblatt and Manning, 2000). At least 25 species of Iridaceae are pollinated exclusively by one of these two flies, among them 12 Babiana species (including B. dregei, B. curviscapa and B. framesii), Hesperantha latifolia and H. oligantha, Lapeirousia jacquinii, L. oreogena, L. silenoides and L. violacea, Romulea hantamensis and R. kamisensis, Sparaxis metelerkampiae and S. variegata, and Tritonia marlothii. Several species of Pelargonium with similarly coloured flowers also comprise part of the guild.
Fig. 4.

Pollination systems in Iridaceae I. Bee pollination. Apis mellifera (Apidae) on Moraea ciliata (top row, left); Rediviva sp. (Melittidae) on Moraea inclinata (top row, right). Long-proboscid fly pollination. Moegistorhynchus longirostris (Nemestrinidae) visiting Lapeirouisa anceps (middle row, left); Prosoeca sp. (Nemestrinidae) visiting Lapeirousia oreogena (middle row, centre); Prosoeca peringueyi (Nemestrinidae) visiting Lapeirousia pyramidalis subsp. regalis (middle row, right). Butterfly pollination. Aeropetes tulbaghia (Satyridae) on Tritoniopsis burchellii (bottom row, left). Bird pollination. Lesser double-collared sunbird, Nectarinia chalybea on Chasmanthe aethiopica (bottom row, right) (photographer: Colin Paterson-Jones).
Type 2. The Moegistorhynchus–Philoliche pollination system (Fig. 4). Operating along the western and south-western coasts and adjacent mountains of the southern African winter-rainfall zone, this second long-proboscid fly pollination system includes a guild of late spring- to summer-flowering plants. Species with white, cream or pale pink flowers usually with red markings are pollinated by one or more of several fly species in the Nemestrinidae (Moegistorhynchus longirostris, M. perplexus and M. sp.) and Tabanidae (Philoliche gulosa and P. rostrata). Some 35 species of Iridaceae, including Babiana brachystachys and B. tubulosa, Geissorhiza confusa, G. exscapa, several Gladiolus species (including G. angustus and G. floribundus), Lapeirousia anceps and L. fabricii, and Tritonia crispa, are pollinated exclusively by one or two of these fly species (Manning and Goldblatt, 1997; Manning et al., 1999). Other families with species using this pollination system include Ericaceae, Geranicaeae, Orchidaceae and even a single species of Proteaceae.
Type 3. The Prosoeca ganglbauri pollination system. The most widespread of the long-proboscid fly pollination systems, this system occurs throughout the highlands of eastern southern Africa and extends to the southern coast and adjacent mountains of southern Africa. It comprises a guild of late summer- and autumn-flowering plant species with white to pink (rarely blue) flowers, usually with red markings, mostly in the families Amaryllidaceae, Iridaceae and Orchidaceae, that are pollinated by one of three Prosoeca species (Nemestrinidae), Prosoeca ganglbauri, P. robusta and P. longipennis. Several other plant families exploit this pollination system, including Acanthaceae, Geraniaceae and Scrophulariaceae. Among the Iridaceae are several Gladiolus species (including G. engysiphon, G. ferrugineus, G. microcarpus and G. mortonius), Hesperantha grandiflora, H. scopulosa and H. woodii, Nivenia stenosiphon, Tritoniopsis revoluta, Watsonia occulta and W. wilmsii.
Type 4. The Stenobasipteron pollination system. A fourth system has recently been recognized as distinct from the Prosoeca ganglbauri system (Potgieter and Edwards, 2005). The main pollinator, S. wiedemannii, is restricted to forest and bush habitats in eastern southern Africa. Plant species using this fly (often in combination with some Prosoeca species), include several summer-flowering, pale blue-flowered Lamiaceae, mainly Plectranthus species, some Streptocarpus species (Gesneriaceae), Gladiolus macneilii and two Hesperantha species in the Iridaceae (Goldblatt and Manning, 1999; Goldblatt et al., 2004b).
The four long-proboscid fly pollination systems outlined above share no plant or pollinator species and operate either in different parts of southern Africa or in different habitats, and at different times of the year. It appears therefore that they have evolved independently of one another and so are best regarded as quite separate pollination systems.
A striking aspect of all the long-proboscid fly systems is the apparent limit to the number of guild species that may co-occur locally. This appears to relate to the number of discrete pollen deposition sites on the pollinating insect. Pollen of each species of the guild is deposited precisely on one of six potential placement sites that have been identified on the insect, depending on the length and orientation of the stamens in the flower visited: the lower part of the face, the frons and the vertex of the head, the dorsum of the thorax, and the ventral surface of the thorax and abdomen (Fig. 3). Long-proboscid flies are fairly large insects and pollen deposits are ample, often colouring the entire thorax or head of an individual. Thus, sites of pollen deposition are readily identified with the naked eye. Typically just one plant species uses a particular pollen placement site at any locality and time, with the upper limit to the number of guild members in any one locality constrained by the availability of pollen placement sites. This has been interpreted as evidence of the negative effects of stigma clogging by foreign pollen, presumably because long-proboscid flies are not flower-constant (Manning and Goldblatt, 1996, 1997).
Large butterfly flowers (Fig. 5).
Fig. 5.

Flowers pollinated by large butterflies, with vertical sections of some flowers, and the satyrid butterfly Aeropetes tulbaghia at same scale. (A) Hesperantha coccinea. (B) Tritoniopsis lesliei, lateral and dorsal views. (C) Crocosmia aurea. (D) Freesia laxa. (E) Gladiolus saundersii. (F) G. stefaniae. (G) G. nerineoides; a brush flower in contrast to the previous two flag flowers of the same genus. Scale bar = 10 mm.
Restricted to just a few species in the genera Crocosmia, Freesia, Hesperantha, Gladiolus and Tritoniopsis (Table 1), flowers adapted for pollination by large papilionid or satyrid butterflies typically have a red to orange, rarely yellow or purple perianth, sometimes with white splashes on the lower tepals, and a relatively long, slender perianth tube (Goldblatt and Manning, 2002). Flowers are either flag or brush types (fide Faegri and van der Pijl, 1971). In the flag type, the flower is large, with spreading tepals, a somewhat enlarged, erect dorsal tepal, and the stamens are exserted. In the brush type, several smaller flowers are open at the same time, contributing to the display, as in Gladiolus nerineoides (Fig. 5G), in which the relatively small flowers are borne in a crowded horizontal spike (Goldblatt and Manning, 2002). In Crocosmia aurea, the flowers are pendent (Fig. 5C). We regard Freesia grandiflora and F. laxa (Fig. 5D) as having butterfly-adapted flowers although there are no published observations for either species.
Flowers are unscented and contain relatively large quantities of nectar, 4·5–12 mL, of relatively low concentration, 15·4–23·5 % sucrose equivalents, with an exceptional 26·1 % in Gladiolus carmineus and 24·7 % in G. saundersii (Table 2). Although nectar sugar chemistry may be of the ancestral sucrose-rich to sucrose-dominant type typical of Crocoideae, as in Tritoniopsis (Manning andGoldblatt, 2005), a shift to hexose-rich nectar is evident in Hesperantha coccinea and some Gladiolus species (Goldblatt and Manning, 2002; Goldblatt et al., 2004b) (Table 3).
Large butterfly visitors are primarily Aeropetes tulbaghia (Satyridae), which is on the wing in the southern summer, mainly January through to March. This species is responsible for the pollination of a very distinct guild of plant species in the southern African winter-rainfall zone (the Cape region), mainly Amaryllidaceae, Iridaceae and red-flowered Orchidaceae (Johnson and Bond, 1994). Other large butterflies captured or noted visiting African Iridaceae include Papilio nireus (on Crocosmia aurea and Hesperantha coccinea) and P. demodocus (on H. coccinea). The summer is peak flowering time in eastern southern Africa, an area of summer rainfall and cold dry winters, whereas the winter-rainfall zone in the south-west of the subcontinent is hot and dry in summer and relatively few plant species are then in bloom. Butterfly-pollinated Gladiolus and Tritoniopsis species from the winter-rainfall region are, in consequence, restricted to locally moist habitats (Goldblatt and Manning, 2002). Despite this, the winter-rainfall zone has the greatest number of Iridaceae adapted to pollination by large butterflies. The two species of Tritoniopsis pollinated by Aeropetes (Fig. 5B), the only common butterfly pollinator there, T. burchellii and T. triticea, are also pollinated by sunbirds and exhibit what appears to be a truly bimodal system (Manning and Goldblatt, 2005). Among the 165 species of Gladiolus in southern Africa, seven are evidently pollinated solely by Aeropetes tulbaghia (Goldblatt and Manning, 2002).
Moth flowers (Fig. 6).
Fig. 6.
Flowers pollinated by moths: (A–D), sphinxmoth flowers; (E–H) settling moth flowers. (A) Lapeirousia odoratissima. (B) Gladiolus longicollis subsp. praelongitubus. (C) Gladiolus hyalinus. (D) Tritoniopsis nervosa with the moth Hyles lineata. (E) Gladiolus emiliae. (F) Hesperantha falcata. (G) Hesperantha radiata. (H) Freesia viridis. Scale bar = 10 mm.
Of modest importance in the family, moth pollination is nevertheless significant in two genera, Gladiolus (Goldblatt and Manning, 2002) and Hesperantha (Goldblatt et al., 2004b), and is inferred for several species of Lapeirousia and the monospecific tropical African Savannosiphon (Table 1). In Hesperantha some 24 species are pollinated by settling moths in a range of families, notably Drepanogynidae, Geometridae and Noctuidae. The flowers are moderately sized with pollen tube generally 5–12 mm long. In Gladiolus, 11 species in southern Africa and a further 14 in tropical Africa have flowers adapted for moth pollination, but in this genus flowers may be moderate to large in size with tubes often exceeding 100 mm (Fig. 6B), and the pollinators are either moderate-sized settling moths in the Noctuidae or hovering hawkmoths (Sphingidae). Moth-pollinated Iridaceae typically have either pale or dull-coloured flowers with slender tubes and short or included stamens. They are often partially or completely closed during the day but expand in the late afternoon or at night, and then produce either a spicy or a ‘white-floral’ fragrance. In Gladiolus the flowers of some species remain open during the day but are often fragrant only at night (Goldblatt and Manning, 2002). In Hesperantha all moth flowers remain closed until late afternoon or after sunset. Moth flowers also occur in three species each of Babiana and Lapeirousia and one each of Freesia, Tritoniopsis and Xenoscapa. Lapeirousia odoratissima (Fig. 6A), L. schimperi and Savannosiphon have exceptionally long perianth tubes, 100–140 mm long, indicating hawkmoth pollination.
A characteristic of many moth-pollinated Iridaceae is their inconspicuous appearance during the day. In species such as Gladiolus emiliae, G. guthriei and G. hyalinus (Fig. 6C and E) the perianth is mottled with brown or grey, and in Hesperantha the lower surfaces of the tepals (which are exposed when the flowers are closed during the day) are dull maroon or reddish brown. The dull coloration is almost certainly a form of camouflage, as was postulated by Johnson (1995) for the dull coloured flowers of moth-pollinated Disa (Monadenia) ophyridea (Orchidaceae). Camouflage presumably serves to reduce robbing of floral rewards by illegitimate floral visitors as well as reducing predation of flowers by herbivorous insects and mammals. Among moth-pollinated species, Gladiolus liliaceus is unique for its reversible change in perianth pigmentation. The flowers of this species are straw-coloured to brown and unscented during the day but become pale bluish and fragrant at sunset, reversing these changes in the morning (Goldblatt and Manning, 2002).
In Hesperantha species pollinated by settling moths, nectar volumes are modest, 0·4–1·2 mL, but have high concentrations, mostly 35–45 % sucrose equivalents (Table 2). By contrast, hawkmoth-pollinated Gladiolus species with longer tubes produce higher volumes, 5–12 mL, of more dilute nectar, 20–36 % sucrose equivalents. Hawkmoth-pollinated Lapeirousia and Tritoniopsis species produce similarly dilute nectar.
Sunbird flowers (Fig. 7).
Fig. 7.

Lateral views of flowers pollinated by sunbirds, with vertical sections of some flowers, and the sunbird Nectarinia famosa at the same scale. (A) Gladiolus watsonius (section Homoglossum). (B) G. abyssinicus (section Ophiolyza). (C) G. cunonius (section Hebea). (D) Tritoniopsis caffra. (E) T. burchellii. (F) Witsenia maura (radially symmetric flower). Note the different floral form in the different species of Gladiolus. Scale bar = 10 mm.
One of the more important pollination strategies in African Iridaceae, sunbird pollination is developed in some 64 species of Nivenioideae and Crocoideae (Goldblatt et al., 1999) (Table 1). Birds consume large quantities of nectar, and most bird-pollinated species produce 10–20 mL nectar per flower. Some, however, produce considerably more, with volumes exceeding 50 mL in some Watsonia species and over 100 mL in W. vanderspuyiae (Goldblatt et al., 1999). Bird-pollinated flowers usually have an elongate perianth tube that is typically about 2 mm in diameter in the lower half and abruptly expanded in the middle into a wider cylindrical upper part 4–5 mm in diameter (Fig. 7A–E). Nectar, which is held within the lower portion of the tube, typically measures 12–20 % sucrose equivalents (Table 2), but several Gladiolus species, mostly winter/spring-blooming species, have more concentrated nectar, 30–35 % sucrose equivalents (Goldblatt et al., 1999).
In crocoid genera, sunbird flowers are zygomorphic and coloured dark red to bright scarlet or even orange, and generally lack floral markings or have the lower tepals marked with black. Associated with this pollination system are well-exserted anthers, often borne on stiff filaments, a similarly well-exserted style, and a trend toward the reduction of the lower tepals, associated with hyperdevelopment of a horizontal dorsal tepal. The trend reaches its maximum expression in bird-pollinated species of Gladiolus section Hebea in which the lower tepals are less than half the size of the dorsal; in G. cunonius they are more or less vestigial and in G. saccatus even the upper lateral tepals are reduced to scales. In the eastern tropical African G. dichrous, large red floral bracts play the predominant role in attraction and the white to pale pink flowers are largely concealed. The nivenioid genera Klattia and Witsenia (Fig. 7F) have radially symmetrical flowers with subequal tepals. In Witsenia, the tepals are erect and conceal the anthers, whereas in Klattia the anthers are well exserted.
Floral odours are always absent in bird-pollinated Iridaceae and the perianth tube is typically over 25 mm long (sometimes exceeding 50 mm). There is often a congruence between the length of the bill of the preferred pollinator (range 18–35 mm) and the length of the perianth tube that is accessible to the bill (either the entire tube in those species with a similar diameter throughout, or the wider part of the tube in species where the tube is narrowed below). In Klattia, several flowers are aggregated in a head enclosed by enlarged, coloured, bract-like leaves and these, plus the true bracts of the flowers, retain the nectar produced by individual flowers in a collective pool that overflows the relatively short perianth tubes. The large, firm floral bracts in these and some other bird-pollinated species, such as Gladiolus abbreviatus and G. dalenii, are also likely to serve a protective function, deterring nectar-thieves from piercing the side of the floral tubes.
The most common sunbird pollinators are malachite and orange-breasted sunbirds (Nectarinia famosa and N. violacea) but lesser double-collared and dusky sunbirds are important floral visitors in coastal habitats in western southern Africa and other species are implicated elsewhere in southern and tropical Africa. Genera in which sunbird pollination is important are Gladiolus (some 29 species), which has species pollinated by sunbirds throughout Africa, the largely Cape Chasmanthe (all three species), Crocosmia (three or possibly four of the eight species), and the southern African Watsonia, in which more than half the 50 species are adapted for sunbird pollination (Table 1). Sunbirds rarely hover while feeding, perching instead on the flowering stem or spike as they take nectar. For this reason, the flowering stem in bird-pollinated species is sturdy, and often considerably thicker than in related insect-pollinated species. A striking adaptation characterizes Babiana ringens, one of just three species of this genus of ca. 86 species that are pollinated by sunbirds. The stout, suberect main axis is sterile and serves as a perch while secondary branches bear several to many flowers (Anderson et al., 2005).
The flowers of Tritoniopsis burchellii and T. triticea appear to be pollinated successfully by both sunbirds and the satyrid butterfly Aeropetes, which is strongly attracted to red flowers. These species exhibit an uncommon bimodal pollination system in which pollinators of two different categories share the same flower (Manning and Goldblatt, 2005).
Hopliine beetle flowers (Fig. 8).
Fig. 8.
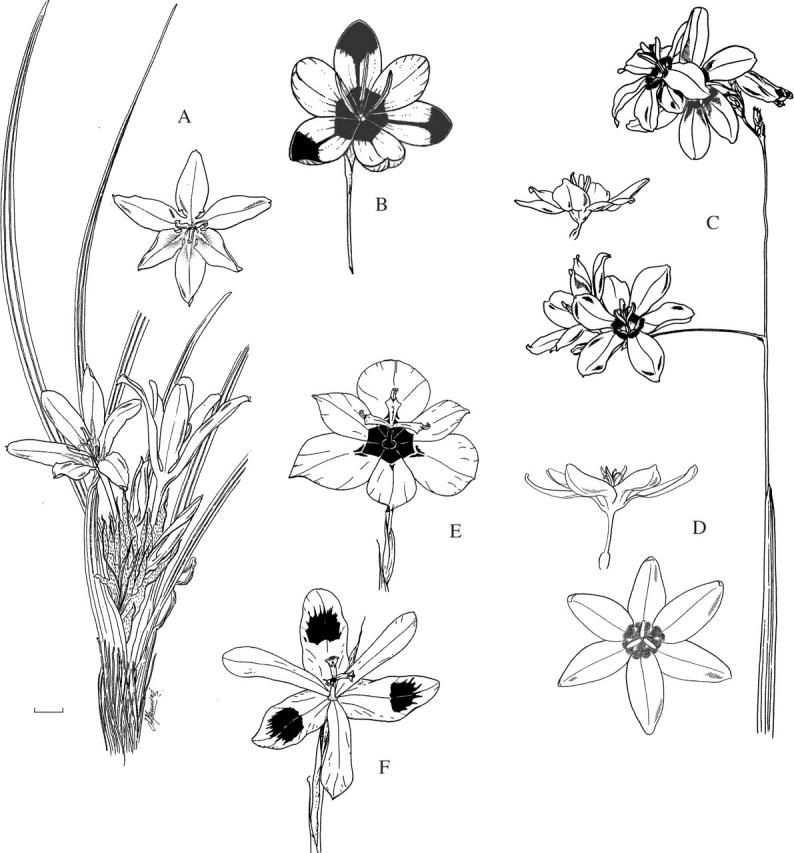
Flowers pollinated by hopliine beetles. (A) Babiana papyracea; whole plant and single flower—note the narrow perianth tube that serves only to raise the flower above the ground. (B) Hesperantha vaginata, with prominent ‘beetle marks’ in centre of flower and at tips of outer tepals. (C) Ixia superba; flowering branch and lateral view of single flower. (D) Ixia maculata; dorsal and lateral views. (E) Moraea insolens. (F) Moraea elegans. Scale bar = 10 mm.
Hopliine or monkey beetles (Scarabaeidae: Hopliini) are an important class of pollinators (Table 1) for the family, but mainly in the southern African winter-rainfall zone. They are not known to be a notable pollinator elsewhere in Africa, even in the adjacent, summer-rainfall parts of southern Africa (Goldblatt et al., 1998a), although other families of beetles are significant elsewhere. Cetoniid beetles, for example, are important pollinators of Apocynaceae: Asclepiadoideae in the grasslands of the summer-rainfall parts of southern Africa (Ollerton et al., 2003). Hopliines, which are active mainly in spring and early summer, are often present in large numbers. Overlooked as significant pollinators for many years, they are now understood to be the sole pollinators of many species, particularly those of lowland habitats, and secondary pollinators of many others (Steiner, 1998; Goldblatt et al., 1998a, 2000a). Their significance is such that they have driven selection for particular flower types in several genera of Iridaceae, with the result that hopliine-pollinated flowers are often very different from their closest relatives in pigmentation pattern, tepal size and orientation, including a shift in floral symmetry. Beetle flowers are typically radially symmetrical, salver- or shallow bowl-shaped, and relatively large, and are thus able to accommodate two or more beetles on the tepals or in the floral cup.
Hopliines use flowers as sites for assembly, competitive behaviour and copulation. They also sometimes consume pollen. The enlarged anthers, which often contain increased amounts of pollen, may compensate for losses due to consumption of grains by hopliines. They may also function as attractants. Unlike other pollinators of African Iridaceae, hopliines spend considerable time at one flower, sometimes resting there overnight. Their activity on a flower is seemingly undirected as they crawl over the perianth, contacting pollen-bearing anthers and stigmatic surfaces.
Beetle flowers are also brightly coloured and often have contrasting dark markings in the centre (Figs 8B–F and 9),the so-called ‘beetle marks’. Frequently associated with these perianth features are enlarged and dark-coloured anthers, and a shorter style, sometimes with expanded stigmatic surfaces. In genera and species that have a perianth tube, the tube is usually thick-walled with a narrow interior that is completely occupied by the style. The mouth of the tube may also be occluded by the enlarged bases of the filaments. Nectar secretion is also usually suppressed. Hopliine pollination is most frequent in Ixia and Romulea (Crocoideae; Goldblatt et al., 2000a, 2002a) and Moraea (Iridoideae; Goldblatt et al., 2005), genera that are ancestrally actinomorphic, but occurs in some species of seven more genera (Table 1), some of which are primitively zygomorphic. Exclusive hopliine pollination is inferred to occur in 20 species of Ixia (38 % of the genus) and eight of Romulea (11 %).
Fig. 9.

Pollination systems in Iridaceae. Beetle pollination. Anisonyx ursus on Moraea villosa (top row, left); Clania macgregorii on Romulea monadelpha (top row, right). Short-proboscid fly pollination. Muscid fly on Moraea ochroleuca (middle row, left); Muscid fly on Ferraria densepunctulata (middle row, right). Generalist pollination. Belenois aurata (Pieridae) on Micranthus junceus (bottom row, left); Cynthia cardui (Nymphalidae) on Nivenia parviflora (bottom row, centre). Polistes sp. (Vespidae) on Moraea inconspicua (bottom row, right).
Contrasting pigmentation patterns are almost invariably present among Iridaceae in this pollination system. Most common are darker markings in the centre of the perianth, but background colour is variable, including pink, red, blue, violet, yellow, orange or white. Dark pigmentation may extend to the stamens and style (in Ixia monadelpha, I. superba and Sparaxis elegans), or may be restricted to the anthers, for example in red- or purple-flowered Babiana villosa or white- or blue-flowered B. stricta in which the anther connective is expanded, thus providing a greater area of dark colour. Two species of Aristea exhibit particularly unusual modifications of the perianth. In A. biflora transparent windows of tissue on the lower tepal margins appear dark from above, and in A. lugens the outer tepals are reduced in size, erect and entirely dark brown or black, in contrast to the outspread white or pale blue inner tepals. The importance of ‘beetle marks’ has been questioned by Johnson and Midgely (2001), who found no statistical difference in number of visits to marked and unmarked model flowers, although they recorded a preference for orange over red, yellow or blue flowers. Their result is refuted by a study by I. Nänni (pers. comm.), which showed the reverse. Model flowers with darkened centres were strongly favoured by hopliines, approximately twice as many of which were captured on models marked with dark centres versus uniformly coloured models. The different result is difficult to explain, but is most likely a consequence of the study sites chosen.
Several species apparently adapted to pollination by hopliines also secrete nectar and/or have scented flowers, and receive visits from insects of other pollinator groups. Discussed in more detail under the heading specialized pollination systems, these include Gladiolus meliusculus, coastal populations of Babiana melanops, Moraea comptonii and M. elegans (also visited by large bees, including Apis mellifera, and Andrena sp.), and Ixia tenuifolia and Sparaxis elegans (also visited by the tabanid Philoliche atricornis).
Wasp and short-proboscid fly pollination
Wasp pollination, only recently discovered in the African Iridaceae, is restricted to Ferraria and possibly one or more species of Moraea (Bernhardt and Goldblatt, 2006). In F. divaricata and F. variabilis, dull-coloured and weakly scented flowers have a deep floral cup containing surprisingly large quantities of nectar, often exceeding 12 mL and a maximum of 36 mL in one population of F. variabilis. The nectar is also remarkable for its consistently low sugar concentration. These nectars have minimum values of about 3 % and maximum values of about 10 % sucrose equivalents and were recorded in ten different populations of these two species. These exceptionally dilute nectars are particularly remarkable as wasp flowers more often have concentrated nectar (e.g. Ollerton et al., 2003). The only recorded visitors to the flowers of these Ferraria species are Delta and Allepipona species (Eumenidae), and Jugurtia species (Masaridae). As the wasps climb over the tepal limbs into the floral bowl the dorsal part of the thorax brushes against the anthers and stigmatic surfaces and wasps emerge bearing visible loads of the distinctive orange pollen of these species. The reason for the wasp visits is uncertain, but may simply be to ingest water, which is not readily available in the semi-arid habitats favoured by these plants. Polistes and Tricarinadynerus species (Vespidae) have been captured while apparently foraging for nectar on Moraea inconspicua (Fig. 2), which has small, brown and buff tepals (Goldblatt et al., 2005a). The common theme in these wasp flowers is dull coloration and faint, somewhat unpleasant, but not distinctive odour.
Pollination by dung, flesh and game flies (Calliphoridae, Sarcophagidae and Muscidae), reported only in five species of Ferraria and two of Moraea (Table 1), is reminiscent of classic sapromyiophily (Faegri and van der Pijl, 1971). The flowers are usually dull-coloured, in Ferraria species with mottled patterning (Fig. 9), and often unpleasant rotting or fermenting odours. On warm days flowers of this type swarm with fly visitors, always seen with liberal dorsal dustings of the characteristic bright orange pollen produced by these species (Bernhardt and Goldblatt, 2006; Goldblatt et al., 2005a). Unlike in classic sapromyiophily flies do not lay eggs in these flowers and instead are rewarded with concentrated nectar, typically over 50 % sucrose equivalents.
Flowers of Moraea ochroleuca (Fig. 9) are, by contrast, yellow, sometimes with an orange cup, and have a light fruity, fermenting odour. Short-proboscid flies, especially Calliphoridae, are invariable visitors to these flowers, also sometimes visited opportunistically by native Apis mellifera.
A little-known pollination strategy is that by March flies, Bibio species (Bibionidae), recorded only in the monospecific genus Melasphaerula (Crocoideae) (Goldblatt et al., 2005b). The atypically small, zygomorphic, cream flowers with purple-brown median streaks on the lower tepals and an unusual slightly sour, aminoid (or musky) odour attract few visitors. The bibionid fly Bibio rostrata is the only insect captured on flowers of the species, apparently foraging for nectar. As the small flies, ca. 5 mm long, enter the flowers, extending a proboscis, ca. 1 mm long, into the short perianth tube, the dorsum of the thorax brushes against the anthers or stigmas, depending on whether the flowers are in male or female phase. Flies typically carry small loads of Melasphaerula pollen that is visible to the naked eye. The tiny flowers are among the smallest in the Iridaceae. The syndrome exhibited by Melasphaerula has been reported in southern Africa in the orchid Disa obtusa, which has similarly coloured and scented flowers and is pollinated by Bibio turneri (Johnson and Steiner, 1994).
REWARDS: NECTAR, POLLEN, FLORAL OILS
Nectar volume and concentration
Nectar, either produced in septal nectaries located within the radial walls of the ovary, or from small to large surface nectaries on the proximal portions of some or all the tepals (Goldblatt, 1990; Rudall et al., 2003), is the most common reward to animal visitors. In flowers in which nectar is the primary or only reward, flower structure is such that visitors foraging for nectar will passively contact pollen-bearing anthers or receptive stigmatic surfaces. Nectar volume and concentration are remarkably varied (Table 2), but correlate to some extent with pollinator size or level of activity. Large volumes are produced when pollinators are birds or large, swift insects, and small volumes are produced when pollinators are bees or settling moths. More than 100 mL of nectar has been reported, for instance, in sunbird-pollinated Watsonia vanderspuyiae whereas long-tongued (apid) bee-pollinated flowers offer between 0·5 and 2 mL of nectar. Fly- and sphinxmoth-pollinated flowers have the next highest volumes.
Nectar concentration is as variable as volume (Table 2). Flowers adapted for bee pollination have nectar ranging from 21 to over 50 % sucrose equivalents, although most often between 25 and 35 % (e.g. Goldblatt et al., 1998b). Flowers pollinated by settling moths generally have high nectar sugar concentrations, 30–45 %. By contrast, in butterfly-pollinated species nectar concentration is low, with a range of 15–26 % recorded across four genera. Flowers pollinated by long-proboscid flies typically have nectar of intermediate concentration, usually 20–30 %, but the Hesperantha species of eastern southern Africa have unexpectedly low nectar concentrations, 13·7–19·3 % (Goldblatt et al., 2004b). Butterfly-pollinated flowers exhibit the lowest nectar concentrations, with the exception of the few wasp-pollinated species of Ferraria (Iridoideae), in which nectar is typically of very low concentration, as little as 3–10 % in F. divaricata and F. variabilis (our unpubl. data), the only recorded visitors to which are eumenid and masarine wasps (Bernhardt and Goldblatt, 2006).
Two diverging patterns of nectar concentration characterize bird-pollinated flowers; concentrations are relatively low in most species, typically 12–20 % with a minimum 10 % recorded in Chasmanthe bicolor. By contrast, among winter- and early spring-flowering Gladiolus species, bird flowers produce nectar of 28–33·4 % concentration. Higher nutritional requirements when sunbirds are breeding in the winter months has been proposed as an explanation for the exceptionally high nectar concentrations at this time (Goldblatt et al., 1999).
Although the provision of nectar is usual in sub-Saharan African Iridaceae, most Aristea species produce no nectar, and are effectively pollen flowers (Goldblatt and Manning, 1997b). Only one derived species of Aristea, A. spiralis, produces nectar, this from perigonal nectaries, evidently a specialization in the genus (Rudall et al., 2003). Several species of other genera may also be primarily pollen flowers, either producing limited quantities of nectar or lacking nectar completely, a feature associated primarily with pollination by hopliine beetles. Species of Ixia subgenus Ixia all have flowers with the perianth tube closed at the apex by the filament bases and lack nectar (Goldblatt et al., 2000a), as do several of the so-called peacock moraeas, Moraea villosa and its close allies (Goldblatt et al., 2005a). Absence of nectar is also associated with vibratile (buzz) pollination, exhibited in a few Ixia species (Goldblatt et al., 2000a) of section Dichone, and also with pollination by deception, as in long-tubed Hesperantha pubinervia and H. scopulosa (Goldblatt et al., 2004b).
Nectar chemistry
Nectar sugar chemistry shows two contrasting patterns with an apparent phylogenetic bias (Table 3). Nectar produced by species of Crocoideae and Nivenioideae is typically sucrose-rich or sucrose-dominant (in the terminology of Baker and Baker, 1983). The noted preference of passerine birds, including sunbirds (Baker and Baker, 1983, 1990), for hexose (glucose plus fructose)-rich to hexose-dominant nectars is reflected in the occasional shift to nectar with elevated hexose sugar levels, for example in three bird-pollinated species of Gladiolus section Hebea, G. cunonius, G. saccatus and G. splendens, which have hexose-rich or hexose-dominant nectar (sucrose : hexose ratio 0–0·49). The three species of Chasmanthe, all with flowers adapted for bird pollination, also have hexose-rich to hexose-dominant nectar. By contrast, bird-pollinated Gladiolus species of other taxonomic sections, and bird-pollinated species of Babiana, Crocosmia, Tritoniopsis and Watsonia, have sucrose-rich nectar, with the exception of W. angusta, which has hexose-rich nectar, and W. meriana, which produces nectar borderline between sucrose- and hexose-rich.
In the Nivenioid genus Nivenia both long-tongued bee- and long-proboscid fly-pollinated species have sucrose-rich nectar (sucrose : hexose ratio 0·5–0·99) but the species of the two bird-pollinated genera of the subfamily, Klattia and Witsenia, have hexose-dominant nectar (Goldblatt et al., 1993). The single nectar-producing species of Aristea, A. spiralis, has sucrose-rich nectar, produced from perigonal nectaries.
Butterflies are reported to prefer nectar with high levels of sucrose (Baker and Baker, 1990), and although this nectar type is characteristic of some butterfly-pollinated Gladiolus species (Goldblatt and Manning, 2002), two species, G. insolens and G. nerineoides, have hexose-rich nectar. Similarly, butterfly-pollinated Hesperantha coccinea has hexose-rich nectar in contrast to the sucrose-rich or sucrose-dominant nectar of four other species of the genus examined, all pollinated by moths or long-proboscid flies. Such changes in nectar sugar chemistry are uncommon in Crocoideae and must be pollinator driven, as they evidently are in the case of the sunbird-pollinated Nivenioideae. It is noteworthy in this connection that the species of Tritoniopsis pollinated by either birds or butterflies alone, or by both, all have sucrose-dominant nectar (Manning and Goldblatt, 2005).
Nectar sugars of Iridoideae are less well sampled, but nectars are hexose-rich to hexose-dominant irrespective of pollinator (Table 3). Thus, the apparent preference of bees and long-proboscid flies for sucrose-rich to sucrose-dominant nectar, exhibited in all genera of Crocoideae, is not evident in Iridoideae. Moraea and Ferraria species pollinated by bees, flies or wasps have the same nectar sugar profile as bird-pollinated Klattia and Witsenia (Nivenioideae), even though nectar is produced from perigonal nectaries in Moraea and from septal nectaries in Klattia and Witsenia. The differences in nectar sugar chemistry in the subfamilies is consistent with Percival's (1961) observation that nectar in tubular flowers, as in Crocoideae and Nivenioideae, tends to be sucrose-rich or sucrose-dominant, whereas open or bowl-shaped flowers more often have nectar with higher proportions of hexose sugars.
Nectar of only a handful of the Iridaceae has been examined for amino acid concentration. The data, provided by the late Irene Baker and not previously published, show that amino acid concentrations are low on the histidine scale (0·098 µmol mL−1), typically 2–4 in several bird-pollinated Watsonia and Klattia species, and 3 or 7 respectively in the long-proboscid fly-pollinated Watsonia dubia and Nivenia binata. The possible presence of amino acids in wasp-pollinated Ferraria species has yet to be investigated.
Nectar sugar profiles in the African Iridaceae therefore show two patterns, one reflecting phylogenetic history and the other a response to pollinator-driven selection, notably in the bird-pollinated genera Chasmanthe, Klattia and Witsenia, as well as within selected species of bird-pollinated Gladiolus, and some butterfly-pollinated Gladiolus and Hesperantha coccinea. The shift from ancestral sucrose-rich nectar in insect-pollinated Nivenioideae to hexose-dominant nectar in bird-pollinated genera is the clearest example of selection for nectar sugar type. A similar but weaker trend is evident in some butterfly-pollinated Crocoideae. In the few Iridoideae sampled, the apparent ancestral, hexose-dominant nectar sugar ratio is maintained irrespective of pollinator, despite the reputed preference by most bees and wasps for nectar in which sucrose predominates. These observations are mirrored by nectar sugar ratios in several other African clades. For example, in Asphodelaceae: Alooideae, the predominantly sunbird-pollinated genera Aloe and Kniphofia have uniformly hexose-dominant nectar whereas Gasteria, also sunbird pollinated, and Haworthia, pollinated by solitary bees, show the opposite pattern (Van Wyk et al., 1995). In Erica (Ericaceae), similar patterns prevail: some bird-pollinated clades have sucrose-dominant nectars and others hexose-dominant nectar; most insect-pollinated species of the genus have hexose-dominant nectar but a smaller proportion show sucrose-dominance (Barnes et al., 1996). Patterns of nectar variation in African Iridaceae thus conform to the patterns in these two families.
Pollen
In Iridaceae pollen is actively collected only by Apis mellifera workers or female bees of several other families and is widely assumed to be used for provisioning nests for larvae. Species that provide pollen as the primary reward usually have conventionally coloured, yellow pollen, which is usually prominently displayed on exserted anthers borne in radially symmetric flowers. This type of floral presentation is ancestral in Aristea and Romulea and is present in most species of these genera. Nectar may be present in limited quantities in these pollen flowers, often only in trace amounts (Romulea) or is absent entirely (Aristea).
Several genera with ancestrally bilabiate flowers have also developed this type of flower, e.g. Gladiolus quadrangulus, G. stellatus, several Sparaxis species and Tritonia dubia. Likewise, the meranthia of some Moraea species are suppressed by the loss of distinction between inner and outer tepals, reduction of the style branches, and the development of a prominent staminal column around a central style. Radial symmetry in these species is thus seen to be derived as a result of a shift in pollination system.
Deterrents to deliberate pollen collection in flowers with alternative rewards include concealment of the anthers and possibly also unconventional coloration of pollen. For example, several Gladiolus and Tritonia species pollinated by long-proboscid flies have purple or brown pollen (Goldblatt and Manning, 1999, 2000a), whereas some hopliine-pollinated Babiana and Romulea species have brown or blackish anthers and pollen. In these examples the dark colour may be a form of camouflage. Alternatively, the coloured pollen may be involved in pollinator-attraction. Concealment of the anthers is more common. In many Moraea species the anthers are concealed by the outer tepal claw, which is arched to lie close to the opposed style branch above the level of the anthers, whereas in many Crocoideae the prominent dorsal tepal arches over the stamens. The role of these mechanisms in protecting pollen from moisture damage remains to be assessed.
Non-volatile floral oils
Although frequently secreted among New World genera of Iridaceae, non-volatile floral oils are only recorded from African Iridaceae in Tritoniopsis parviflora, where they are secreted from epithelial cells at the base of the tepals and in the throat of the short-tubed flower (Manning and Goldblatt, 2002). The flowers are strongly scented, with the dominant component being 3, 5-dimethoxy toluene, a scent also characteristic of floral oil-secreting southern Africa genera of Orchidaceae, such as Pterygodium and Corycium (Manning and Goldblatt, 2005). Oil secretion in Tritoniopsis contrasts with that in the Iridoideae of the New World where it is always from glandular hairs (elaiophores) either on the perianth, typically the claws of the inner tepals (Molseed, 1970; Vogel, 1974), or on the filament column (Vogel, 1971; Cocucci and Vogel, 2001). The chemical nature of the floral oil or oils in Tritoniopsis has not been determined.
COMPATIBILITY: OUTCROSSING VERSUS INBREEDING
Outcrossing is the normal mode of reproduction in the large, usually brightly coloured, hermaphrodite flowers of African Iridaceae, and is promoted by a combination of physical and physiological mechanisms. Flowers are weakly to strongly protandrous, with pollen being released some some hours or 1–3 d before stigma surfaces become available for pollen deposition. Among the several genera that have been critically studied, male and female phases of anthesis are most pronounced in Gladiolus and Tritoniopsis, in which the stigmatic surfaces are available for pollen deposition 2 or 3 d after flowers open, by which time all pollen is normally removed from a flower by pollinator activity (Goldblatt et al., 1998b; Goldblatt and Manning, 1999; Manning and Goldblatt, 2005). Stigmas are of the dry type and the incompatibility system is gametophytic and late acting where known (Heslop-Harrison, 1977; Heslop-Harrison and Shivanna, 1977). Studies in the Eurasian genus Crocus (Crocoideae) and Iris tuberosa (Iridoideae) show that incompatibility is expressed in the ovary. The ovarian grooves have enlarged epidermal cells that produce a floccular secretion that evidently provides discriminatory activity to incoming pollen tubes (Chichiriccó, 1996; Grilli Caiola and Brandizzi, 1997). In some examples, self-incompatibility was expressed within the ovule where embryos abort early in their development, an indication of post- as well as prezygotic incompatibility. Our unpublished data for African Moraea species likewise showed that self-incompatibility was expressed within the ovary, often in the ovule, and not in the style.
Spatial separation of anthers and receptive stigmatic surfaces (herkogamy) is common. Prime examples are the Iris-type flowers of Dietes and Moraea, in which the stigmatic lobes are typically held well above the anthers, but herkogamy is frequent in Crocoideae as well. In many members of the subfamily the style branches exceed the anthers when they become receptive, usually then extending forward or downward as, for example, in Gladiolus (Fig. 1B) and Tritoniopsis (Goldblatt and Manning, 1998a; Manning and Goldblatt, 2005). In Tritoniopsis the receptive phase of the stigmatic surfaces is accompanied by recurving of the filaments away from the style branches so that the anthers are moved even further from possible contact between pollen and stigmatic areas. In older flowers of other genera, however, style branches may recurve and contact the anthers, and selfing may then occur (in self-compatible species) if cross-pollination has not already been effected.
Compatibility relations have been little studied in the family but strong self-incompatibility characterizes most Moraea species, with a few striking exceptions (Goldblatt, 1981; Goldblatt et al., 2005a). There are no data for other sub-Saharan African Iridoideae (Bobartia, Dietes, Ferraria). Self-incompatibility also characterizes the crocoid genus Gladiolus (Goldblatt et al., 1998b). Among other Crocoideae, Hesperantha, Lapeirousia and Sparaxis have both incompatible and self-compatible species (Goldblatt et al., 1995, Goldblatt 2000b, 2004b) but Ixia, Romulea and Watsonia evidently show weak self-incompatibility in all species examined for the feature, and some flowers on a spike will self if not cross-pollinated (e.g. Horn, 1962; de Vos, 1972). In the monotypic Melasphaerula some capsules (often with lower than normal numbers of viable seeds) are produced on each spike by selfing in plants from which insects are excluded (Goldblatt et al., 2005b), a pattern consistent with that found in Ixia, Romulea and Watsonia.
In self-incompatible species, e.g. those of Gladiolus and Moraea, self-incompatibility prevents geitonogamous self-pollination whereas protandry and spatial separation of anthers and stigmas serve to limit stigma clogging by self-pollen.
Several species with specialized pollination systems show marked facultative autogamy, evidently a failsafe adaptation. Thus, among long-proboscid fly-pollinated Lapeirousia species, L. silenoides is self-incompatible, but in the absence of pollen transfer from another individual, both L. jacquinii and L. oreogena will produce normal capsules with full complements of viable seeds (Goldblatt et al., 1995). Babiana species do not normally produce seeds without cross-pollination but long-proboscid fly-pollinated B. tubiflora is autogamous (Goldblatt and Manning, in press) as is the bird-pollinated B. ringens (Anderson et al., 2005). Mixed incompatibility also characterizes the genus Sparaxis. Among the long-proboscid fly-pollinated species, S. variegata is self-incompatible whereas S. metelerkampiae is autogamous; among the bee-pollinated species, S. caryophyllacea and S. galeata, both of which have large, strongly scented flowers, are self-incompatible, but the smaller-flowered and unscented S. parviflora and S. villosa are facultatively autogamous (Goldblatt, 1992).
In the predominantly distylous genus Nivenia, Goldblatt and Bernhardt (1990) failed to demonstrate an associated self-incompatibility system. That result has been confirmed independently in N. binata by M. Grantham (pers. comm.). Nevertheless, the six distylous species are evidently obligate outcrossers as pin and thrum flowers cannot self due to spatial considerations. In pin flowers the style branches are held distant from the short stamens while the reverse holds in thrum flowers. Evidently, the spatial separation of pollen and stigmatic surfaces, combined with deposition of pollen on different parts of the pollinators, long-proboscid flies or anthophorine bees, maintains outcrossing by placing pollen on the stigmatic surfaces of flowers with complementary style lengths.
Lastly, certain Moraea species such as M. albiflora, M. stagnalis and M. vegeta, with small, inconspicuous flowers (at least as compared with their immediate relatives) are also autogamous (Goldblatt et al., 2005a). Likewise, the complex heterozygote species M. demissa, M. flavescens and M. pallida are autogamous (Goldblatt, 1981). Chromosome rings produced at meiosis are assumed to maintain favourable gene linkages and ensure the maintenance of structural heterozygosity. Associated with complex heterozygosity, the most common diploid chromosome number in M. demissa and M. flavescens is 2n = 9 (basic number for the clade is 2n = 12). Nevertheless, these species do receive pollinator visits and may best be regarded as facultatively autogamous. Complex heterozygosity has otherwise only been reported in the Iridaceae in the South American Gelasine elongata (Kenton and Rudall, 1987).
GENERAL CONSIDERATIONS
Pollination system and floral symmetry
Floral symmetry is an important component of pollination systems (Giurfa et al., 1999) and is particularly significant in Iridaceae. The spectrum of pollination systems among ancestrally (and thus wholly or predominantly) actinomorphic taxa and those that are ancestrally (and wholly or predominantly) zygomorphic is very different (Table 4). In the family as a whole, pollination by pollen-collecting female bees, scarab beetles and short-tongued flies is overrepresented among actinomorphic genera, whereas pollination by nectar-collecting bees, long-proboscid flies, birds, butterflies and moths, which are all long-tongued visitors, is underrepresented. These differences are partially due to the absence of a perianth tube among the predominantly actinomorphic genera of Iridoideae, which militates against the development of systems using pollinators with extended mouthparts. Within the two tubular-flowered subfamilies, Nivenioideae and Crocoideae, there are some significant differences in the pollinator spectrum between taxa with actinomorphic versus zygomorphic flowers. Although pollination by various bees is the most frequent system in both actinomorphic and zygomorphic genera, pollination by nectar-feeding bees is more than twice as common among zygomorphic genera (47·9 vs. 21·6 %), whereas pollination by pollen-collecting bees is almost absent but is well-developed in actinomorphic genera (32·5 %) (Table 4). A predominance of beetle pollination is also evident in the actinomorphic genera, either alone or in combination with visits by bees (total 20·2 %). By contrast, pollination by birds is rare among the actinomorphic taxa but is well developed among zygomorphic genera (14·5 %). This may relate to the fact that bird pollination in southern Africa is carried out by perching birds that probe the flowers from a fixed position in relation to the floral axis. This is in contrast to the New World hummingbirds, which can hover and thus approach flowers independently of the orientation to the floral axis. The notable differences in the pollinator profile between actinomorphic and zygomorphic genera of Iridaceae are directly linked to the structural syndromes of each of the pollination systems and do not reflect anomalies within the family.
Table 4.
Pollinator spectrum and floral symmetry in Iridaceae
| Species with each pollination system (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genera | Apid nectar | Apid pollen | Apid buzz | Long-p fly | Scarab beetle | Scarab/bee | Moth | Butterfly | Bird | Short-p fly | Wasp | Generalist |
| All genera | 41 | 15 | 0.8 | 12 | 6.3 | 6 | 6.3 | 1.6 | 7.6 | 0.9 | 0.3 | 2.3 |
| Actinomorphic: all subfamilies | 35 | 27 | 1.6 | 7.1 | 9.5 | 10.7 | 5 | 0.2 | 0.6 | 1.6 | 0.6 | 0.6 |
| Actinomorphic: Crocoideae + Nivenioideae | 21.6 | 32.5 | 2.7 | 12 | 11.3 | 8.9 | 8.6 | 0.3 | 1.4 | 0 | 0 | 1 |
| Zygomorphic: Crocoideae + Nivenioideae | 47.9 | 1 | 0 | 17.7 | 3.1 | 0.6 | 7.5 | 3.1 | 14.5 | 0.2 | 0 | 4.1 |
The genera Aristea, Duthiastrum, Geissorhiza, Hesperantha, Ixia, Klattia, Nivenia, Pillansia, Romulea and Savannosiphon were scored as ancestrally (and wholly or predominantly) actinomorphic. The remaining genera were treated as ancestrally (and wholly or predominantly) zygomorphic. Abbreviations as in Table 1.
Another aspect of floral symmetry that deserves further investigation is its effect on the diversification of pollination systems. An increase in the number of species per genus in Iridaceae is closely associated with an increase in the number of pollination systems that are exploited, and larger genera are generally more diverse in their pollination systems that are exploited (Fig. 10A). This relationship is evident in both actinomorphic (or polysymmetric) genera and in those that are ancestrally zygomorphic (or monosymmetric), but there are significant differences between the two symmetries in the rate at which pollination systems are added, as well as the total number of systems that are exploited (Fig 8B). The rate at which different pollination systems are added is significantly greater among zygmorphic genera, with a maximum of seven different pollination systems recorded, whereas among actinomorphic genera the maximum number of pollination systems is constrained at six per genus. Clearly, therefore, the evolution of the monosymmetric flower in Crocoideae should be viewed as a key innovation in terms of increasing the diversity of specialized pollination systems that are exploited.
Fig. 10.
Relationship between number of species and number of pollination systems per genus in ancestrally actinomorphic (open circles, broken curve) and ancestrally zygomorphic (closed circles, solid curve) genera of African Iridaceae. (A) Untransformed data; (B) logarithmically transformed data, with the two curves tested for between-group effects using the general linear model (F = 92·135, P < 0·001). A, Aristea; B, Babiana; Ch, Chasmanthe; Cr, Crocosmia; D, Duthiastrum; Fe, Ferraria; Fr, Freesia; Ge, Geissorhiza; Gl, Gladiolus; H, Hesperantha; I, Ixia; K, Klattia; L, Lapeirousia; Me, Melaspherula; Mi, Micranthus; Mo, Moraea; N, Nivenia; P, Pillansia; Ra, Radinosiphon; Ro, Romulea; Sa, Savannosiphon; Sp, Sparaxis; Th, Thereianthus; Tr, Tritonia; Ts, Tritoniopsis; Wa, Watsonia; Wi, Witsenia; X, Xenoscapa.
Specialized pollination systems
Fully 94 % of species of African Iridaceae studied have what can be considered specialized pollination systems, in which pollinators belong to just one pollinator class, and are often just a few species of a single insect family, tribe or even genus. If our informed inferences of pollinators for those not so far studied are correct, then 95 % of species have a specialized system (Table 4). Visits by what might be termed inappropriate visitors (nectar or pollen thieves) are rare, and few actually accomplish effective pollination. The marked morphological convergence in flower form among many unrelated species has been interpreted as convincing evidence for the existence of modal optima in the pollination strategies developed in Crocoideae, and for the prevalence of specialized pollination systems in the family (Manning and Goldblatt, 2005), conclusions that have been graphically corroborated by Johnson and Steiner (2003). Among 131 species of southern African Iridaceae included in their analysis, the number of pollinators per species ranged from one to ten, with a mean of one species of pollinator per plant species, while pollinator orders ranged from one to three, with a mean of one per plant species. The apparent predominance of plant species that rely on a single pollinator species may, however, prove to be an artefact of biased sampling of the long-proboscid fly and Aeropetes butterfly systems. More extensive sampling of other systems is likely to show that relatively few species are dependent on just one pollinator species. For example, plant species using large-bodied, long-tongued apid bees for pollen transfer, which is the predominant system in the family, may use the native Apis mellifera and one or more species of Anthophora or Amegilla. Likewise, species using sunbirds for their pollination depend on two or three local sunbirds for pollen transfer.
At a local level, however, a single pollinator species is often the norm. For example, along the west coast of South Africa, the only long-proboscid fly active in October and November is Moegistorhynchus longirostris, and local populations of several species depend on this single species for their pollination (Manning and Goldblatt, 1997). Similarly, in the western interior of southern Africa the only two long-proboscid fly species active from July to September are the allopatric Prosoeca peringueyi and an unnamed Prosoeca species, and some 35 species of Geraniaceae, Iridaceae and Scrophulariaceae depend on just one of these two flies for their pollination. These pollinators are truly keystone species, using the operational definition that their effective disappearance from a system results (directly or indirectly) in the virtual disappearance of several other species. The butterfly Aeropetes is likewise a keystone species for a small guild of Amaryllidaceae and Iridaceae that use only this large butterfly for their pollination in the mountains of the Cape region of South Africa.
A bimodal pollination system using sunbirds and large butterflies has recently been documented in the genus Tritoniopsis (Manning and Goldblatt, 2005), and bimodal systems using pollinators of two pollinator classes are likely to be more common in the family than the literature currently suggests. They have, for instance, been noted in four other genera of Crocoideae, Hesperantha, Ixia, Romulea and Sparaxis (Goldblatt et al., 2000a, b, 2002a, 2004b). Mixed, or more specifically, bimodal systems are morphologically intermediate between two syndromes and share two sets of pollinators adapted to different specialized pollination systems. They differ from generalist systems, which rely on a range of different pollen vectors, and in which the floral syndromes are not obviously intermediate between two or more specialized systems.
Structurally and functionally the bimodal systems in Tritoniopsis are specialized systems that combine the distinct adaptive features of two different systems. They also function as independent specialist systems when being utilized by individuals of the two different pollination classes, providing the same benefits of dedicated pollen transfer as other specialized systems. The two classes of visitors may thus be considered to be complementary. These systems differ from other specialist systems only in extending their appeal to more than one functionally analogous group of pollinators. The selective pressures driving the evolution of these systems are apparently those that normally favour the development of more generalized systems, but in this case have resulted in a modified specialist system instead.
Tritoniopsis burchellii and T. triticea are visited equally effectively by sunbirds and the butterfly Aeropetes tulbaghia, and T. toximontana is pollinated by bees and long-proboscid flies. Ixia superba has typical hopliine-adapted flowers but they also have scent and nectar, and are visited by Apis mellifera as well as two hopliine species (Goldblatt and Manning, 2004). Ixia tenuifolia has what appears to be a typical hopliine flower, but the narrow tube contains nectar, which attracts the tabanid Philoliche atricornis (Goldblatt et al., 1998a). Sparaxis elegans also receives visits from hopliines and P. atricornis, the latter visiting flowers to forage for nectar. Moraea comptonii and M. elegans have large salver-shaped flowers and prominent dark markings, typical of hopliine-adapted flowers. Both species also offer nectar and have strong, pleasant fruity odours, typical of bee-pollinated flowers. Both species are visited by hopliines and apid bees. Using pollinators from just two specialized pollinator groups, these bimodal systems seem to be quite distinct from generalist systems, which use a wider range of pollinators that often vary from year to year.
Generalist pollinated flowers apear to be quite rare in Iridaceae (Table 1) and comprise less than 3 % of species examined. Examples include the tropical African Lapeirousia erythrantha, and its close allies, L. montana and Nivenia parviflora (Fig. 2), pollinating visitors to which include small flies, a range of long- and short-tongued bees, and Lepidoptera, and in the case of N. parviflora also hopliine beetles (Goldblatt et al., 1995). Other examples include Sparaxis bulbifera and S. grandiflora subsp. acutiloba (Goldblatt et al., 2000b). Such flowers are atypical of the African Iridaceae in having a short tube (or nectar present in the top of the tube), multiple flowers open or dense populations that provide display, and prominent anthers and pollen.
The African Iridaceae, thus, are a particularly striking example of a plant family in which pollination syndromes are highly predictive of the most effective (or only) pollinator. Specialized pollination systems are most likely to develop under conditions when appropriate pollinators are predictably present (Stebbins, 1970), whereas generalization is favoured when pollinator availability is unpredictable (Waser et al., 1996). Several factors determine the relative prevalence of these two ends of the pollination continuum, including plant life history, successional status, abundance and breeding system (Baker, 1965; Feinsinger, 1983; Schemske, 1983; Bond, 1994; Johnson and Steiner, 2000). The relatively long-lived nature of cormous Iridaceae, their propensity for vegetative reproduction and the relatively dispersed nature of flowering plants among the vegetation accord with three of the conditions that have been proposed to favour the development of specialist pollination strategies (Bond, 1994; Johnson and Bond, 1994; Waser et al., 1996). Indeed, the prevalence of specialized pollination systems in African Iridaceae is one of the major sources of evidence for Johnson and Steiner's (2000, 2003) arguments in defence of the pollination syndrome concept and the frequency of specialist pollinator systems in southern Africa.
The proportional representation of different pollination systems is relatively consistent among most of the larger, zygomorphic-flowered genera of Iridaceae in the Cape region. Typically, 50–67 % of the species are pollinated by bees, and a further 11–40 % are pollinated by long-proboscid flies, with birds, butterflies, moths and beetles accounting for the remaining species (Manning and Goldblatt, 2005). The genera Tritoniopsis and Watsonia are noteworthy exceptions. Watsonia is predominantly (70 % of the species) adapted to pollination by birds, with a proportionate decrease in the prevalence of bee pollination. Bird pollination is also overrepresented in Tritoniopsis, although not to the same degree (25 %), along with pollination by the butterfly Aeropetes. The reason for the unusual dominance of bird pollination in Watsonia is uncertain, but the skewed pollinator profile in Tritoniopsis has been ascribed to the unusual flowering time in the genus. Most Tritoniopsis species flower in summer and autumn, rather than spring, which places them outside of the period during which most insect pollinators, with the conspicuous exception of Aeropetes, are active across its range.
Many of the morphological changes associated with shifts in pollination syndrome seem relatively minor, often mainly involving floral tube length and floral pigmentation patterns. This transition in morphology is readily accompanied by shifts in nectar quality and quantity as there is already substantial overlap in ranges in nectar volume and concentration within the various pollination strategies. We are uncertain how complex shifts in floral scent types may be but scent is fairly variable within many species of Iridaceae (Goldblatt et al., 2004b), providing a basis for selection. The various mixed pollination systems that have been identified in the family illustrate how evolutionary transformations can proceed between bee and fly pollination, and between bird and butterfly pollination, while maintaining successful pollination during the transition phase (Manning and Goldblatt, 2005).
Shifts in pollination system and what they mean
Most genera of Iridaceae with ten or more species have developed more than one pollination system, indicating a degree of lability between systems within the family. This is often even more evident from phylogenetic analysis of individual genera, in which derived pollination systems are shown to have evolved repeatedly within genera in different lineages. A molecular-based phylogeny of Moraea (Goldblatt et al., 2002b) shows the radially symmetric, non-meranthiod flower, a signal of the pollen-collecting female bee pollination syndrome, to have evolved four times among the 70 species in the analysis. Likewise, the two sapromyiophilous species of Moraea are unrelated. Although only morphology-based phylogenies and classifications based on phylogenetic principles are available for other genera, these often also show multiple evolution of long-proboscid fly, bird, hopliine or lepidopteran pollination. In Gladiolus, long-proboscid fly pollination, which occurs in 28 species, probably evolved 12 times (Goldblatt et al., 2001). In Babiana, which has 17 long-proboscid fly-pollinated species, the strategy has evolved at least four times (Goldblatt and Manning, in press). Bird pollination probably arose at least six times in Gladiolus, and twice in Babiana. In Gladiolus, pollination by butterflies evolved at least three times and moth pollination six times.
Using the most conservative phylogenetic scenarios, pollination shifts are estimated to have occurred a minimum of 32 times in the 165 species of southern African Gladiolus, one shift for every five species, and at least 14 times in the 86 species of Babiana, one shift for every six species. Pollination in other genera appears less labile but even in Romulea, the four long-proboscid fly-pollinated species belong in two different subgenera. Crocosmia, with just eight species, has three discrete pollination systems, for ancestral apid bee, butterfly and sunbird.
Although it is still not possible to estimate with confidence the number of pollination shifts for all genera, the general conclusion is that pollinator shifts are frequent. The actual significance of this in terms of species diversification remains uncertain. For Lapeirousia, Goldblatt and Manning (1996) showed that in seven pairs of immediately related species (identified by morphology-based phylogenetic analysis), members of a pair either had different habitat preferences or were fully allopatric, or both, and only three pairs had different pollinators. There were no examples of species pairs with different pollinators alone that were sympatric or parapatric. The conclusion for these examples in Lapeirousia is that pollinator shifts alone are not the driving force for speciation, but rather that shifts reinforce reproductive isolation in peripheral populations.
The examples in Lapeirousia seem applicable to other genera for which we have no well-supported phylogenies, but can nevertheless identify some species pairs because of their obvious morphological specializations. In fact, we cannot identify a single example of sympatric species pairs that have different pollinators except when the species have different flowering times.
The high incidence of specialized pollination systems in southern African Orchidaceae and Iridaceae has been cited as evidence of pollinator-driven speciation in the region (Johnson and Steiner, 2000, 2003), despite the obvious anomaly that 15 of the 20 largest genera in the winter-rainfall flora (Goldblatt and Manning, 2000) are highly conservative in their floral morphology and, by inference, in their pollination strategies. These include several of the largest genera, among them Aspalathus (Fabaceae: 272 species), Agathosma (Rutaceae: 143 species), Phylica (Rhamnaceae: 133 species), Lampranthus (Aizoaceae: 124 species) and Oxalis (Oxalidaceae: 118 species). Although floral variation and pollinator divergence are undoubtedly significant in facilitating species packing and the local occurrence of multiple species of the same genera flowering at the same time, their role in driving speciation in African Iridaceae is still equivocal.
In a more general context, Johnson and Steiner (2003) suggest that the apparently greater specialization in pollination systems among southern African plants is related to the higher incidence of specialized pollinators in the region, as well as the depauperate pollinator fauna, which although low in numbers are high in functional types. It has not yet been established how frequent specialized pollination systems are among the southern African members of families other than the Orchidaceae and Iridaceae. In addition, the causes of the high diversity of different pollination systems in Iridaceae and other families requires further examination. In this context, it is significant that of the five largest genera in the flora of the southern African winter-rainfall zone (Goldblatt and Manning, 2000) that display a high incidence of specialist pollination syndromes, no fewer than four are wholly or partially geophytic: Pelargonium (Geraniaceae), Moraea and Gladiolus (Iridaceae) and Disa (Orchidaceae), with just Erica (Ericaceae) entirely shrubby. The significance of the coincidence between life-form (the geophytic habit in particular) and pollinator specialization may lie in the nature and long-term climatic stability of this region. Such climatic stability could be expected to permit the development of specialist systems, in accordance with Stebbins's (1970) observation that a plant should specialize on the most abundant or effective pollinator when pollinator availability is reliable. Mild winter temperatures and predictable rainfall have been identified as the main determinants of the wealth of geophytes that characterizes the flora of the region (Esler et al., 1999). It is therefore tempting to consider that the high degree of specialist pollination systems in temperate African Iridaceae may be linked to the same ecological factors that have favoured their radiation as geophytes.
The high diversity of pollination systems in African Iridaceae at various taxonomic levels contrasts with the situation in many other African plant genera, and even families, which are often conservative for pollination system, a phenomenon emphasized by Johnson et al. (1998) in their study of the radiation of pollination systems in the Africa genus Disa. That genus exhibits remarkable diversity in its pollination systems, and is evidently exceptional within Orchidaceae, where many large genera show single pollination systems. In some taxa at least, the apparently conservative nature of their pollination systems is an artefact of their circumscription, with the genera defined artificially by those characters that represent adaptations for a particular pollination system. For example, in Iridaceae a cycle of generic reduction followed the understanding that Gladiolus was not monophyletic when the genera Acidanthera, Anomalesia, Homoglossum, Kentrosiphon and Oenostachys were recognized. It is now understood that Acidanthera was defined by floral adaptations for moth pollination, while the remaining four were defined by adaptations for bird pollination (Goldblatt, 1996; Goldblatt and Manning, 1998). Most, if not all, genera of African Iridaceae are now believed to be monophyletic and are defined by vegetative and fruit morphology, some floral characters, and correlated chromosome numbers.
POLLINATION IN EXTRA-AFRICAN IRIDACEAE
Pollination of Iridaceae outside Africa has received scant attention from biologists. Iris (subfamily Iridoideae), with over 260 species, the largest and most widespread genus of the family, has received very little study and very little has been reported for the Old World temperate genus Crocus (Crocoideae), with over 80 species and the only non-African member of its subfamily. The tubular, radially symmetric flowers of Crocus vernus produce nectar, which is forced into the upper portion of the tube where it is accessible to bees (Knuth, 1909), but at least two other species are nectarless, C. alatavicus (our unpubl. data) and C. hyemalis (A. Dafni, pers. comm.) and thus function as pollen flowers and are unlikely to receive visits from nectarivorous insects. The floral tube then functions as a pseudopedicel, raising the flowers above the ground in this uniformly acaulescent genus. Early studies in pollination in Iris showed that species were visited by bumble bees and their foraging pattern gave rise to the meranthium concept. Each outer tepal and its opposed petaloid style crests function as a bilabiate pollination unit, and thus to a bee appears as a single gullet flower. Reward to visitors is nectar concealed within the tubular part of the perianth. Bumble bees and perhaps other bees large enough to force apart the tepal from the opposed style branch then climb down the ‘gullet’ to reach nectar held below the bases of the tepals. In so doing, they will brush against the stigma lobe, and on exiting will carry dorsal loads of passively acquired pollen (in exactly the same way as described above for the closely related Moraea). The pattern described for a few cultivated species of Iris in Germany may prove almost universal for the genus, but the North American I. fulva, flowers of which have a very long gullet and dilute nectar, is visited predominantly by hummingbirds (Emms and Arnold, 2000; Wesselingh and Arnold, 2000). Another exception in Iris is section Oncocyclus, the bizarrely coloured flowers of which lack nectar guides. The flowers also produce no nectar. Iris species of Israel and nearby are reported to be visited exclusively by male anthophorine bees that use the flowers as shelter for the night (Sapir et al., 2005).
Among the New World members of the family, all Iridoideae, Sisyrinchium (tribe Sisyrinchieae) is exceptional. Several species secrete floral oils from club-shaped oil glands (elaiophores) located on the filament column (Vogel, 1971, 1974), as described in detail by Cocucci and Vogel (2001). Similar trichome elaiophores also occur on the tepal claws in Tigridieae, including Alophia (syn. Eustylis), Cypella, Ennealophus, Herbertia, many species of Tigridia and Trimezieae, including Neomarica (Molseed, 1970; Vogel, 1974; Lee, 1994). The distribution of nectaries on the tepals varies, although in general the zone of glandular trichomes is restricted to the adaxial surfaces of the smaller inner tepals, often concentrated in pouches at the juncture of the tepal claw and limb. In Herbertia there are nectaries on the smaller inner tepals and the claws of the outer tepals. At least two genera of the tribe, Sessilanthera and Cobana, have porose anthers and on this basis are expected to be buzz pollinated. Absence of nectar is often associated with buzz pollination and information available indicates that these two genera lack nectaries and elaiophores (Molseed and Cruden, 1969; Ravenna, 1974). Eleutherine and Nemastylis also evidently lack nectaries (Molseed, 1970) but species of these genera have conventional longitudinal anther dehiscence.
Red-flowered and presumably hummingbird-pollinated species of Tigridia (once placed in a separate genus Rigidella) secrete copious amounts of sugary nectar (Cruden, 1971) from nectaries on the inner tepal claws. The nectar is held within folds of the tepal surface. Other Tigridieae may also secrete sugar nectar from perigonal nectaries, although this is inadequately documented. Molseed (1970) mentioned fly and wasp visitors in some small-flowered species of Tigridia. The flowers of this group resemble those of the African Ferraria and wasp pollination would thus not be surprising.
Apart from Sisyrinchium, genera of tribe Sisyrinchieae do not produce floral oils and, moreover, do not have conventional nectaries either. Most thus evidently function as pollen flowers. The exception is Olsynium, in which at least O. douglasii and O. junceum are known to secrete nectar (Forcone et al., 1998; Rudall et al., 2003), although at least O. philippi does not produce nectar (Cocucci and Vogel, 2001). In O. douglasii the outer surface of the hollow filament column is smooth, but has darkly staining vascularized tissue beneath the enlarged bulbous base, indicating a nectary. The inner surface of the swollen part of the filament column is convoluted and thin-walled. Nectar presumably secreted in this area then accumulates in the partly enclosed chamber formed by the narrow basal part of the column and the tubular base of the tepals. Pollination in the genus has received little attention but at least O. douglasii is visited by a range of bees, including bumble bees, Bombus spp. (Apidae), and Osmia sp. (Megachilidae), that forage for nectar and pollen (our unpubl. data).
Nothing is known of the pollination of the Australasian members of the family, including Libertia and Orthrosanthus, shared with Australasia and South America, but all except Diplarrena have radially symmetric flowers with prominent anthers and appear superficially to be bee flowers, evidently offering only pollen as a reward. The exception, Diplarrena, has zygomorphic flowers, concealed anthers and does secrete nectar (Rudall et al., 2003).
The concept of phylogenetic constraint (Bernhardt and Goldblatt, 2006) may be used to explain the apparently narrower range of pollination systems in extra-African members of the Iridaceae. With the exception of Crocus and a few other members of subfamily Crocoideae that occur in Eurasia, all non-African genera belong to subfamilies that have radially symmetric flowers, without a hollow, elongate perianth tube. This largely limits the possibility for the development of the wide range of flower types that have evolved in the various genera of Crocoideae. The diverse flowers encountered in subfamily Iridoideae, both in Africa and elsewhere, are highly variable in colour and marking, and some (Iris, Moraea) have meranthia, the units of which resemble zygomorphic flowers, but they lack a true tube that restricts access to nectar to all but a few specialized visitors. The secretion of floral oil, so prevalent in Neotropical and Mexican Iridoideae, is an innovation that accompanies sometimes extensive adaptive radiation, and has opened one unique pollination niche for the family outside Africa.
Acknowledgments
We gratefully acknowledge support for field work by grants 4816-92, 5153-93, 5408-95, 5994-97, 6704-00, 7103-01 and 7316-02 from the National Geographic Society (United States). We thank Peter Bernhardt for productive collaboration in the past, Ingrid Nänni and Lendon J. Porter for their help and companionship in the field, Colin Paterson-Jones for use of the photograph of Nectarinia, and Naseef Salaam for preparing the colour figures.
LITERATURE CITED
- Anderson B, Cole WW, Barrett SCH. 2005. Specialized bird perch aids cross-pollination. Nature 435: 41–42. [DOI] [PubMed] [Google Scholar]
- Baker HG. 1965. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, eds. The genetics of colonizing species. New York: Academic Press, 147–168.
- Baker HG, Baker I. 1983. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Scientific and Academic editions, 117–141.
- Baker HG, Baker I. 1990. The predictive value of nectar chemistry to the recognition of pollinator types. Israel Journal of Botany 39: 157–166. [Google Scholar]
- Barnes K, Nicolson SW, van Wyk B-E. 1995. Nectar sugar composition in Erica. Biochemical Systematics and Ecology 23: 419–423. [Google Scholar]
- Bernhardt P, Goldblatt P. 2000. The diversity of pollination mechanisms in the Iridaceae of southern Africa. In: Wilson K, Weston P, eds. Monocots: systematics and evolution. Melbourne: CSIRO, 301–308.
- Bernhardt P, Goldblatt P. 2006. The role of phylogenetic constraints in the evolution of pollination mechanisms in the Iridaceae of sub-Saharan Africa. In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG, eds. Monocots: comparative biology and evolution. Claremont: Rancho Santa Ana Botanic Garden, in press.
- Bond WJ. 1994. Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Philosophical Transactions of the Royal Society of London, Series B 344: 83–90. [Google Scholar]
- Chichiriccó G. 1996. Intra- and interspecific reproductive barriers in Crocus (Iridaceae). Plant Systematics and Evolution 201: 83–92. [Google Scholar]
- Cocucci AA, Vogel S. 2001. Oil-producing flowers of Sisyrinchium species (Iridaceae) and their pollinators in southern South America. Flora 196: 26–46. [Google Scholar]
- Cruden RW. 1971. The systematics of Rigidella (Iridaceae). Brittonia 23: 217–225. [Google Scholar]
- Daumann E. 1970. Das Blütennektarium der Monocotyledonen unter besonderer Berücksichtigung seiner systematischen und phylogenetischen Bedeutung. Feddes Repertorium 80: 463–590. [Google Scholar]
- Davies TJ, Barraclough TG, Savolainen V, Chase MW. 2004. Environmental causes for plant biodiversity gradients. Philosophical Transactions of the Royal Society of London, Series B 359: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos MP. 1972. The genus Romulea in South Africa. Journal of South African Botany, Suppl. 9.
- De Vos MP. 1983. The African genus Tritonia Ker-Gawler: Part 2. Sections Subcallosae and Montbretia. Journal of South African Botany 49: 347–422. [Google Scholar]
- Emms SK, Arnold ML. 2000. Site-to-site differences in pollinator visitation patterns in a Louisiana iris hybrid zone. Oikos 91: 568–578. [Google Scholar]
- Esler KJ, Rundel PW, Vorster P. 1999. Biogeogrophy of prostrate-leaved geophytes in semi-arid South Africa: hypotheses on functionality. Plant Ecology 142: 105–120. [Google Scholar]
- Feinsinger P. 1983. Coevolution and pollination. In: Futuyama DJ, Slatkin M, eds. Coeveolution. Sunderland, MA: Sinauer, 282–331.
- Faegri K, van der Pijl L. 1971. The principles of pollination ecology, 3rd edn. New York: Pergamon Press.
- Forcone A, Galetto L, Bernardello LM. 1998. Floral nectar chemical composition of some species from Patagonia. Biochememical Systematics and Ecology 25: 395–402. [Google Scholar]
- Giurfa M, Dafni A, Neal PR. 1999. Floral symmetry and its role in plant–pollinator systems. International Journal of Plant Sciences 160 (Suppl. 6): S41–S50. [DOI] [PubMed] [Google Scholar]
- Goldblatt P. 1981. Systematics and biology of Homeria (Iridaceae). Annals of the Missouri Botanical Garden 68: 413–503. [Google Scholar]
- Goldblatt P. 1990. Phylogeny and classification of Iridaceae. Annals of the Missouri Botanical Garden 77: 607–627. [Google Scholar]
- Goldblatt P. 1992. Phylogenetic analysis of the South African genus Sparaxis (including Synnotia) (Iridaceae: Ixioideae), with two new species and a review of the genus. Annals of the Missouri Botanical Garden 79: 143–159. [Google Scholar]
- Goldblatt P. 1993. The woody Iridaceae: systematics, biology and evolution of Nivenia, Klattia and Witsenia. Portland, OR: Timber Press.
- Goldblatt P. 1996. Gladiolus in tropical Africa. Portland, OR: Timber Press.
- Goldblatt P, Bernhardt P. 1990. Pollination biology of Nivenia (Iridaceae) and the presence of heterostylous self-compatibility. Israel Journal of Botany 39: 93–111. [Google Scholar]
- Goldblatt P, Bernhardt P. 1999. Pollination of Moraea species (Iridaceae) with a staminal column. Annals of the Missouri Botanical Garden 86: 47–56. [Google Scholar]
- Goldblatt P, Manning JC. 1996. Phylogeny and speciation in Lapeirousia subgenus Lapeirousia (Iridaceae subfamily Ixioideae). Annals of the Missouri Botanical Garden 83: 346–361. [Google Scholar]
- Goldblatt P, Manning JC. 1997a. New species of Aristea section Racemosae (Iridaceae) from the Cape Flora, South Africa. Novon 7: 357–365. [Google Scholar]
- Goldblatt P, Manning JC. 1997b. New species of Aristea section Pseudaristea (Iridaceae) from South Africa and notes on the taxonomy and pollination biology of the section. Novon 7: 137–144. [Google Scholar]
- Goldblatt P, Manning JC. 1998. Gladiolus in southern Africa. Cape Town: Fernwood Press.
- Goldblatt P, Manning JC. 1999. The long-proboscid fly pollination system in Gladiolus (Iridaceae). Annals of the Missouri Botanical Garden 86: 758–774. [Google Scholar]
- Goldblatt P, Manning JC. 2000a. The long-proboscid fly pollination system in southern Africa. Annals of the Missouri Botanical Garden 87: 146–170. [Google Scholar]
- Goldblatt P, Manning JC. 2000b. Cape plants: a conspectus of the vascular plants of the Cape Region of South Africa. Strelitzia 7.
- Goldblatt P, Manning JC. 2002. Evidence for moth and butterfly pollination in Gladiolus (Iridaceae: Crocoideae). Annals of the Missouri Botanical Garden 89: 110–124 [Google Scholar]
- Goldblatt P, Manning JC. 2004. New species of Ixia (Crocoideae) and Moraea (Iridoideae), and taxonomic notes on some other African Iridaceae. Novon 14: 288–298. [Google Scholar]
- Goldblatt P, Manning JC. in press. Floral biology of Babiana (Iridaceae: Crocoideae): adaptive floral radiation and pollination. Submitted to Annals of the Missouri Botanical Garden.
- Goldblatt P, Bernhardt P, Manning JC. 1989. Notes on the pollination mechanisms of Moraea inclinata and M. brevistyla (Iridaceae). Plant Systematics and Evolution 163: 201–209. [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 1995. Pollination biology of Lapeirousia subgenus Lapeirousia (Iridacaeea) in southern Africa: floral divergence and adaptation for long-tongued fly-pollination. Annals of the Missouri Botanical Garden 82: 517–534. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 1998a. Pollination of petaloid geophytes by monkey beetles (Scarabaeidae: Ruteliinae: Hopliini) in southern Africa. Annals of the Missouri Botanical Garden 85: 215–230. [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 1998b. Adaptive radiation of bee-pollinated Gladiolus species (Iridaceae) in southern Africa. Annals of the Missouri Botanical Garden 85: 492–517. [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 1999. Evidence of bird pollination in Iridaceae of southern Africa. Adansonia 21: 25–40. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 2000a. Adaptive radiation of pollination mechanisms in Ixia (Iridaceae: Crocoideae). Annals of the Missouri Botanical Garden 87: 564–577. [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 2000b. Adaptive radiation of pollination mechanisms in Sparaxis (Iridaceae: Ixioideae). Adansonia 22: 57–70. [Google Scholar]
- Goldblatt P, Manning JC, Bernhardt P. 2001. Radiation of pollination systems in Gladiolus (Iridaceae: Crocoideae) in southern Africa. Annals of the Missouri Botanical Garden 88: 713–734. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 2002a. Floral biology of Romulea (Iridaceae: Crocoideae): a progression from a generalist to a specialist pollination system. Adansonia 24: 243–262. [Google Scholar]
- Goldblatt P, Savolainen V, Porteous O, Sostaric I, Powell M, Reeves G, Manning JC, Barraclough TG, Chase MW. 2002b. Radiation in the Cape flora and the phylogeny of peacock irises Moraea (Iridaceae) based on four plastid DNA regions. Molecular Phylogeny and Evolution 25: 341–360. [DOI] [PubMed] [Google Scholar]
- Goldblatt P, Manning JC, Dunlop G. 2004a. Crocosmia and Chasmanthe: biology, classification and cultivation. Portland, OR: Timber Press.
- Goldblatt P, Nänni I, Bernhardt P, Manning JC. 2004b. Floral biology of Hesperantha (Iridaceae: Crocoideae): shifts in flower colour and timing of floral opening and closing radically change the pollination system. Annals of the Missouri Botanical Garden 91: 186–206. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 2005a. Pollination mechanisms in the African genus Moraea (Iridaceae: Iridoideae): floral divergence and adaptation for pollen vector variability. Adansonia 27: 21–46. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 2005b. The floral biology of Melasphaerula (Iridaceae: Crocoideae); is this monotypic genus pollinated by March flies (Diptera: Bibionidae)? Annals of the Missouri Botanical Garden 92: 268–274. [Google Scholar]
- Goldblatt P, Davies TJ, Savolainen V, Manning JC, van der Bank M. 2005c. Phylogeny of Iridaceae subfamily Crocoideae based on plastid DNAs. In: Columbus JT, Friar EA, Porter JM, Prince LM, Simpson MG, eds. Monocots: comparative biology and evolution 2. Claremont: Rancho Santa Ana Botanic Garden, in press.
- Goldblatt P, Manning JC, Snijman D. 2005d. Cape plants: corrections and additions to the flora. Bothalia 35: 35–46. [Google Scholar]
- Grilli Caiola M, Brandizzi F. 1997. Pollen–pistil interactions in Hermodactylus tuberosus Mill. (Iridaceae). Plant Biosystems 131: 197–205. [Google Scholar]
- Heslop-Harrison Y. 1977. The pollen–stigma interaction: pollen tube penetration in Crocus. Annals of Botany 41: 913–922. [Google Scholar]
- Heslop-Harrison Y, Shivanna KR. 1977. The receptive surface of the angiosperm stigma. Annals of Botany 41: 1233–1258. [Google Scholar]
- Horn W. 1962. Breeding research on South African plants: III. Intra- and interspecific compatibility in Ixia L., Sparaxis Ker., Watsonia Mill. and Zantedeschia Spreng. Journal of South African Botany 28: 269–277. [Google Scholar]
- Johnson SD. 1995. Moth pollination of the cryptic Cape orchid, Monadenia ophyridea. Flora 190: 105–108. [Google Scholar]
- Johnson SD, Bond WJ. 1994. Red flowers and butterfly pollination in the fynbos of South Africa. In: Arianoutsou M, Groves RH, eds. Plant–animal interactions in Mediterranean-type ecosystems. The Hague: Kluwer, 137–148.
- Johnson SD, Midgley JJ. 2001. Pollination by monkey beetles (Scarabaeidae: Hopliini): do colour and dark centers of flowers influence alighting behavior? Environmental Entomology 30: 861–868. [Google Scholar]
- Johnson SD, Steiner KE. 1994. Efficient pollination of the mass-flowering Cape orchid Disa obtusa Lindl. (Orchidaceae) by Bibio turneri Edwards (Diptera: Bibionidae). African Entomology 2: 64–66. [Google Scholar]
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. 2000. Specialized pollination systems in southern Africa. South African Journal of Botany 99: 345–348. [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. 1998. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). American Journal of Botany 85: 402–411. [PubMed] [Google Scholar]
- Kenton A, Rudall PJ. 1987. An unusual case of complex heterozygosity in Gelasine azurea (Iridaceae), and its implications for reproductive biology. Evolutionary Trends in Plants 1: 95–103. [Google Scholar]
- Knuth P. 1909. Handbook of flower pollination 3 (transl. Ainsworth Davis JR). Oxford: Clarendon Press.
- Lee J. 1994. Fatty oil production by Alophia drummondii (Iridaceae) and modified oil collection behavior of Centris (Centridini–Apidae). Masters Thesis, University of Texas, Austin.
- Manning JC, Goldblatt P. 1996. The Prosoeca peringueyi (Diptera: Nemestrinidae) pollination guild in southern Africa: long-tongued flies and their tubular flowers. Annals of the Missouri Botanical Garden 83: 67–86. [Google Scholar]
- Manning JC, Goldblatt P. 1997. The Moegistorhynchus longirostris (Diptera: Nemestrinidae) pollination guild: long-tubed flowers and a specialized long-proboscid fly pollination system in southern Africa. Plant Systematics and Evolution 206: 51–69. [Google Scholar]
- Manning JC, Goldblatt P. 2002. The pollination of Tritoniopsis parviflora (Iridaceae) by the oil-collecting bee Rediviva gigas (Hymenoptera: Melittidae): the first record of oil-secretion in African Iridaceae. South African Journal of Botany 68: 171–176. [Google Scholar]
- Manning JC, Goldblatt P. 2005. Radiation of pollination systems in the Cape genus Tritoniopsis (Iridaceae: Crocoideae) and the development of bimodal pollination strategies. International Journal of Plant Sciences 166: 459–474. [Google Scholar]
- Manning JC, Goldblatt P, Winter PDJ. 1999. Two new species of Gladiolus (Iridaceae: Ixioideae) from southern Africa and notes on long-proboscid fly pollination in the genus. Bothalia 29: 217–223. [Google Scholar]
- Manning JC, Goldblatt P, Snijman D. 2002. The colour encyclopedia of Cape bulbs. Portland, OR: Timber Press.
- Marloth R. 1898. Die Ornithophilie in der Flora Sudafrikas. Berichte Deutsche Botanische Geselschaft 19: 176–179. [Google Scholar]
- Molseed E. 1970. The genus Tigridia (Iridaceae) of Mexico and Central America. University of California Publications in Botany 54: 1–128. [Google Scholar]
- Molseed E, Cruden RW. 1969. Sessilanthera, a new genus of American Iridaceae. Brittonia 21: 191–193. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer C, Kellie S. 2003. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany 92: 807–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival MS. 1961. Types of nectar in angiosperms. New Phytologist 60: 235–281. [Google Scholar]
- Potgieter CJ, Edwards TJ, Miller RM, van Staden J. 1999. Pollination of seven Plectranthus spp. (Lamiaceae) in southern Natal, South Africa. Plant Systematics and Evolution 218: 99–112. [Google Scholar]
- Potgieter CJ, Edwards TJ. 2005. The Stenobasipteron wiedemanni (Diptera, Nemestrinidae) pollination guild in eastern southern Africa. Annals of the Missouri Botanical Garden 92: 254–267. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Timber Press.
- Ravenna P. 1974. Cobana, a new genus of central American Iridaceae. Botaniser Notiser 127: 104–109. [Google Scholar]
- Rudall PJ, Manning JC, Goldblatt P. 2003. Evolution of floral nectaries in Iridaceae. Annals of the Missouri Botanical Garden 90: 613–631. [Google Scholar]
- Schemske DW. 1983. Limits to specialization and coevolution in plant–animal mutualisms. In: Nitecki MH, ed. Coevolution. Chicago: Chicago University Press, 67–109.
- Sapir Y, Schmida A, Ne'eman G. 2005. Pollination of Oncocyclus irises (Iris: Iridaceae) by night sheltering male bees. Plant Biology 7: 417–424. [DOI] [PubMed] [Google Scholar]
- Scott Elliot G. 1890. Ornithophilous flowers in South Africa. Annals of Botany 4: 265–280. [Google Scholar]
- Scott Elliot G. 1891. Notes on the fertilisation of South African and Madagascan flowering plants. Annals of Botany 5: 333–405. [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms. 1. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Steiner KE. 1998. Beetle pollination of peacock moraeas in South Africa. Plant Systematics and Evolution 209: 47–65. [Google Scholar]
- Thomson JD, Wilson P, Valenzuela M, Malzone M. 2000. Pollen presentation and pollination syndromes, with special reference to Penstemon. Plant Species Biology 15: 11–29 [Google Scholar]
- Van Wyk B-E, Whitehead CS, Glen HF, Hardy DS, Van Jaarsveld J, Smith GF. 1993. Nectar sugar composition in the subfamily Alooideae (Asphodelaceae). Biochemical Systematics and Ecology 21: 249–253. [Google Scholar]
- Vlok JHJ. 2005. Why do some flowers close at night? Veld & Flora 2005: 76–79. [Google Scholar]
- Vogel S. 1954. Blütenbiologische Typen als Elemente der Sippengliederung. Botanische Studien 1: 1–338. [Google Scholar]
- Vogel S. 1971. Ölproduzierende Blumen, die durch ölsammelnde Bienen bestäubt werden. Naturwissenchaften 58: 58. [Google Scholar]
- Vogel S. 1974. Ölblumen und ölsammelnde Bienen. Tropische und subtropische Pflanzenwelt 7: 1–267. [Google Scholar]
- Vogel S. 1981. Bestäubungskonzepte der Monokotylen und ihr Ausdruck im System. Berichte der Deutsches Botanische Gesellschaft 94: 663–675. [Google Scholar]
- Waser NM, Chittka K, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Wesselingh RA, Arnold ML. 2000. Nectar production in Louisana Iris hybrids. International Journal of Plant Sciences 161: 245–251. [DOI] [PubMed] [Google Scholar]