Abstract
• Background and Aims Pollination by insects that spend long periods visiting many flowers on a plant may impose a higher risk of facilitated self-pollination. Orchids and asclepiads are particularly at risk as their pollen is packaged as pollinia and so can be deposited on self-stigmas en masse. Many orchids and asclepiads have adaptations to limit self-deposition of pollinia, including gradual reconfiguration of pollinaria following removal. Here an unusual mechanism—anther cap retention—that appears to prevent self-pollination in the South African orchid Eulophia foliosa is examined.
• Methods Visits to inflorescences in the field were observed and pollinators collected. Visitation rates to transplanted inflorescences were compared between a site where putative pollinators were abundant and a site where they were rare. Anther cap retention times were determined for removed pollinaria and atmospheric vapour pressure deficit was recorded concurrently. Anther cap anatomy was examined using light microscopy.
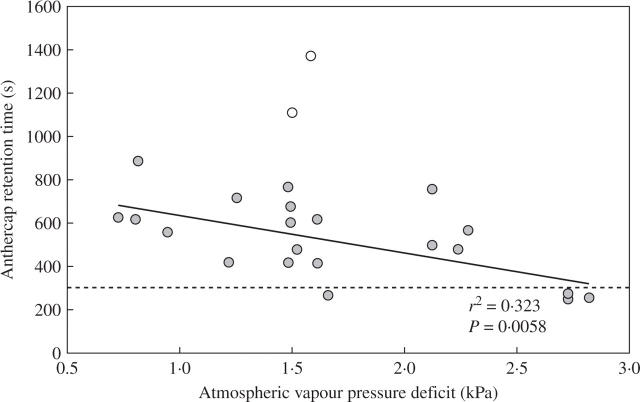
• Key Results Eulophia foliosa is pollinated almost exclusively by Cardiophorus obliquemaculatus (Elateridae) beetles, which remain on the deceptive inflorescences for on average 301 s (n = 18). The anther cap that covers the pollinarium is retained for an average of 512 s (n = 24) after pollinarium removal by beetles. In all populations measured, anther cap dimensions are greater than those of the stigmatic cavity, thus precluding the deposition of self-pollinia until after the anther cap has dropped. An anatomical investigation of this mechanism suggests that differential water loss from regions of the anther cap results in opening of the anther cap flaps. This is supported by observations that as atmospheric vapour pressure deficits increased, the duration of anther cap retention was reduced.
• Conclusions Flowers of E. foliosa are specialized for pollination by elaterid beetles. Retention of anther caps for a period exceeding average visit times by beetles to inflorescences appears to prevent facilitated self-pollination in E. foliosa effectively.
Keywords: Eulophia foliosa, Orchidaceae, Elateridae, pollinarium reconfiguration, pollinia, anther cap retention, geitonogamy, anti-selfing mechanism, beetle pollination
INTRODUCTION
In both the Orchidaceae and the Asclepiadoideae (Apocynaceae), pollen is usually packaged in units known as pollinia. In both groups the pollinia are associated with a variety of accessory structures, making up a pollinarium—the functional unit removed from the flower by the pollinators (Bookman, 1981; Johnson and Edwards, 2000). In most monandrous orchids, only a single visit is required to remove the entire complement of pollen from a flower. If the species is self-compatible then self-pollination, either through geitonogamy or facilitated autogamy, may be particularly crippling as it potentially involves all of the pollen and ovules of a flower. Selfing affects the female function of flowers resulting in inbred progeny (Darwin, 1878; Charlesworth and Charlesworth, 1987) as well as ovule discounting (Barrett, 2002). Selfing can also affect the male function of flowers, through the loss of potential to export pollen to other plants, a process known as pollen discounting (Barrett, 2002).
Many orchids and asclepiads have adaptations to reduce the likelihood of self-pollination. In orchids the absence of a reward in many species may be an important mechanism to discourage repeat visits on an inflorescence (Dressler, 1981; Johnson and Nilsson, 1999; Johnson et al., 2004). A more obvious adaptation, first recognized in orchids by Darwin (1867), is the change in orientation that pollinaria undergo following removal. In both orchids and asclepiads, freshly removed pollinaria are orientated such that if the pollinator immediately revisited the flower, or other flowers on the inflorescence, the pollinia would be incorrectly orientated to be deposited on the stigma. It is only after the accessory structures of the pollinaria, such as the caudicles or stipes in orchids (Johnson and Edwards, 2000) and translator arms in asclepiads (Bookman, 1981), change their shape after a specific interval that the pollinia are correctly orientated to strike the stigma. Orchid examples include those cited in Johnson and Edwards (2000) and Peter and Johnson (2005), while Queller (1985) describes changes in pollinarium orientation in Asclepias exaltata.
Two other less commonly documented mechanisms associated with pollinaria to avoid self-depositions of pollinia have been recognized. Borba and Semir (1999) found that pollinia of two species of Bulbophyllum have to dry and shrink over about 2 h before they can be inserted into the stigmatic cavity. This mechanism has also been reported in Trigonidium obtusum (Singer, 2002).
A second mechanism has been demonstrated in some epidendroid orchids and involves the retention of the anther cap – anther tissue that covers the pollinarium while it is still in place on the column. In most species, the anther cap immediately drops off the pollinarium following its removal by a pollinator, but in a few species the anther cap clasps the pollinia, making them unavailable for a period, before the anther cap drops off. This delay may function as a mechanism to reduce geitonogamous pollen deposition (Dressler, 1981).
van der Pijl and Dodson (1966) cite anther cap retention times of about 20 min in a number of species of Catasetum and 2–3 h in Cycnoches lehmanni. Singer and Cocucci (1999) documented anther cap retention times of up to 40 min in Pleurothallis luteola, and Borba and Semir (2001) recorded anther cap retention times of up to 30 min in Pleurothallis teres and P. ochreata. All three of these small South American orchids are pollinated by small flies (Phoridae and Chloropidae) which spend considerable time visiting individual inflorescences, apparently collecting rewards. Both studies suggest anther cap retention is a mechanism to limit geitonogamous pollen transfer by insects that spend considerable time visiting an individual plant.
Catling and Catling (1991) recorded anther cap retention times of between 8 and 110 min in Tipularia discolor, depending on the ambient relative humidity. At ambient relative humidity of 60–65 % anther caps were retained for between 30 and 40 min. At a relative humidity of 90–93 % this increased to about 100 min. The authors noted that the two flaps of the anther cap that envelope the pollinia gradually open until the anther cap no longer grasps the pollinia and so falls off. This observation coupled with the increased anther cap retention times at high relative humidity point to the changing water status of the cells of the anther cap as the primary mechanism behind the delayed dropping of the anther cap. The anther cap in T. discolor is small enough to fit into the stigmatic cavity. However, the differing microrelief of the pollinia compared with that of the anther cap effectively limits deposition in comparison with those pollinaria without anther caps.
Preliminary observations of the South African orchid Eulophia foliosa indicate that small click beetles (Elateridae) are the main visitors. Furthermore, the pollinaria were noted to have persistent anther caps. We hypothesized that anther cap retention in E. foliosa is a mechanism that functions to limit self-pollination in a plant specialized for pollination by slow-moving beetles.
Beetle pollination is under-studied, although Bernhardt (2000) reviewed specialized beetle pollination in 184 angiosperm species in 34 families. Beetle pollination is much less common in species with zygomorphic flowers. These include the orchid examples listed below, as well the remarkable Orchidantha inouei (Lowiaceae) pollinated by dung beetles (Sakai and Inoue, 1999). In South Africa, beetles may be important pollinators, with estimates of up to 34 % of plant taxa being beetle-pollinated in some ecosystems (Bernhardt, 2000). Monkey beetles (Hopliini, Rutelinae, Scarabaeidae) are important pollinators of many geophytes (Goldblatt et al., 1998). Flower chafer beetles (Cetoniinae, Scarabaeidae) are perhaps equally important in the eastern parts of South Africa (C. I. Peter, pers. obs.).
There are fewer than ten reports of beetle pollination in the Orchidaceae. Most involve systems in which beetles are part of mixed pollinator assemblages. For example, Listera ovata is visited by 283 species of insects but primarily Ichneumonid wasps, saw flies (Tenthredinidae) and beetles such as Elateridae, Cantharidae and Bruchidae (Nilsson, 1981). Another European orchid, Coeloglossum viride, is pollinated by both wasps and beetles. The latter include Elateridae and Cantharidae (Silén, 1906, cited in Proctor et al., 1996; C. I. Peter et al., unpubl. data). Gutowski (1990) noted cerambycid beetles pollinating the European orchid Dactylorhiza fuschii in the forests of north-eastern Poland, but elsewhere in Europe this species is pollinated by bumble bees and honeybees (Dafni and Woodell, 1986).
Examples of more specialized beetle pollination systems in orchids are starting to emerge from the southern hemisphere. These include the unusual Pterglossaspis ruwenzoriensis, which is found both in central Africa and in South America. The flowers are dark purple inside and arranged on relatively crowded inflorescences. The yeasty scent is probably produced from the papilose adaxial surface of the labellum. A ‘jelly-like’ nectar is secreted from the base of the column. In Argentina, this species is pollinated by the cetonid beetle Euphora lurida (Singer and Cocucci, 1997).
Steiner (1998a) reported that the deceptive South African orchid Ceratandra grandiflora is specialized for pollination by monkey beetles (Hopliini, Scarabaeidae). Unlike those of related species, the yellow flowers of C. grandiflora are crowded into capitate inflorescences, providing a large landing platform for the beetles. Steiner interpreted this as a case of generalized food deception coupled with rendezvous pollination, as beetles were often observed mating in the flowers.
The aims of this study therefore were to establish whether (1) anther cap retention can function to reduce pollinator-mediated self-pollination in Eulophia foliosa and (2) a specialized beetle pollination system occurs in this species.
MATERIALS AND METHODS
Study species
Eulophia foliosa (Lindl.) Bolus is a terrestrial orchid about 30 cm in height. The inflorescence emerges concurrently with the vegetative shoot from its base (Fig. 1A). The inflorescence bears numerous small flowers in crowded inflorescences of up to 70 flowers (average 40, Fig. 1B). Each flower is pale green with a dark maroon labellum. Two solid pollinia forming a single pollinarium are housed at the end of a relatively long, slender column beneath an enclosing anther cap. The flowers provide no discernible reward, and are faintly honey-scented to humans. The vegetative shoot bears two or three erect plicate leaves (Fig. 1A). This species grows in open grassland throughout much of the eastern part of South Africa from near sea-level to approximately 2000 m in the Drakensberg Mountains, but is absent above this altitude in the Drakensberg. The distribution extends from Grahamstown in the south northwards through eastern South Africa to Limpopo Province in the far north of South Africa (Fig. 2) and parts of Zimbabwe (Thomas, 1998).
Fig. 1.

Eulophia foliosa occurs in large populations in grasslands throughout the eastern parts of South Africa (A). The inflorescences are crowded with many apple-green flowers (B). In most populations inspected, numerous Cardiophorus obliquemaculatus (Elateridae) clickbeetles (G) were found visiting the inflorescences (C–F). Occasionally at higher altitude sites in KwaZulu-Natal we observed the much larger Atricelaphinis tigrina (Cetoniinae) visiting the inflorescences and removing the pollinaria (H). Scale bars = A, 100 mm; B, 20 mm; C–F, H, 5 mm; G, 1 mm.
Fig. 2.
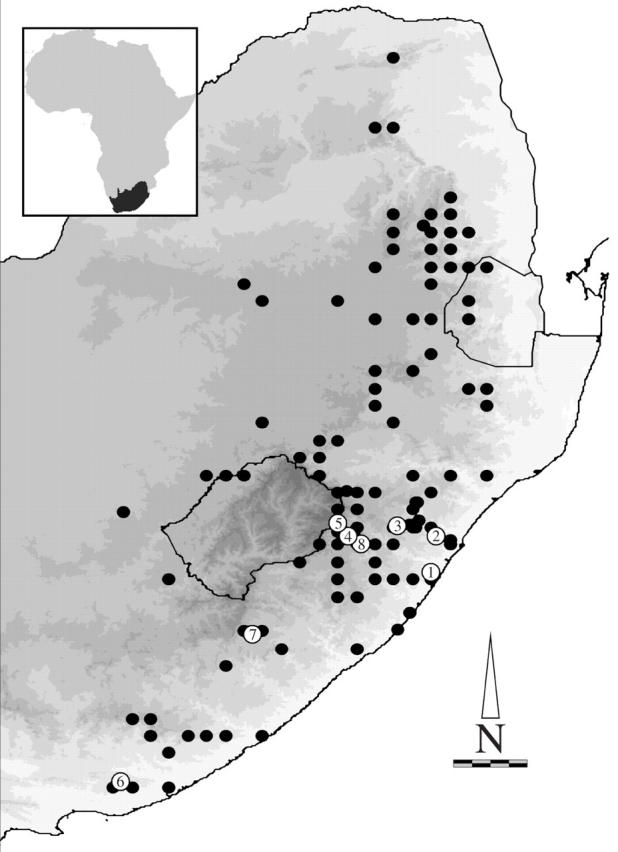
Distribution of Eulophia foliosa in South Africa. Study sites include (1) Vernon Crookes Nature Reserve, (2) Camperdown, (3) Wahroonga, (4) Himeville Nature Reserve, (5) Sani Pass (all in KwaZulu-Natal) and (6) Dassie Kranz near Grahamstown in the Eastern Cape Province. Additional observations of Cardiophorus obliquemaculatus visiting E. foliosa inflorescences were made at (7) Ugie as well as Pevensy and a site east of Underberg (both indicated by point 8). Increasing altitudes are shown in darker shades of grey. Scale bar = 100 km.
Study sites
Field work was undertaken at a number of sites in the KwaZulu-Natal and Eastern Cape provinces of South Africa, ranging from around 300 m near the coast to approx. 2000 m in the Drakensberg Mountains. Sites in KwaZulu-Natal include Vernon Crookes Nature Reserve, Camperdown, Wahroonga, Himeville Nature Reserve and Sani Pass below the South African border post (Fig. 2). In the Eastern Cape Province a large population was investigated at Dassie Kranz on the summit of the Rietberg south of Grahamstown (Fig. 2). Additional observations of visits to the inflorescences of E. foliosa were made near Pevensy, east of Underberg (KwaZulu-Natal) and near Ugie (Eastern Cape).
Pollinator observations
Insects were observed visiting the inflorescences at most of the study sites and, at a number of the sites, insects were collected bearing pollinaria (Table 1). Insects were collected directly into an ethyl acetate killing jar or first knocked into an insect net beside the inflorescence before being killed. Identified insects are lodged in the collection of the first author and the Albany Museum, Grahamstown. Visits to the inflorescences were observed at a number of sites, and in the Grahamstown population it was possible to time the duration of these visits. Behaviour of beetles on inflorescences was recorded using a digital dictaphone. The duration of the visits in seconds was determined from software used to play the recordings. Pollinators bearing pollinaria of E. foliosa were also collected visiting flowers of other species, primarily Helichrysum nudifolium (Asteraceae), in the vicinity of the orchids.
Table 1.
Pollinators and visitors to Eulophia foliosa observed at the various study sites
| Site number* | Site | Time (h)† | Visitors | Number | Number with pollinaria (%) |
|---|---|---|---|---|---|
| 1 | Vernon Crookes | 7 | Cardiophorus obliquemaculatus | 6 | 3 (50) |
| Elateridae | 1 | 0 | |||
| Pompilidae | 1 | 0 | |||
| Hopliniiae | 2 | 0 | |||
| Unidentified Coleoptera | 2 | 0 | |||
| 2 | Drummond | 9 | C. obliquemaculatus | 43 | 16 (36) |
| Cardiotarsus species | 9 | 0 | |||
| Cyrtothyrea marginalis | 1 | 0 | |||
| Curculionidae | 2 | 0 | |||
| 3 | Wahroonga | 4 | C. obliquemaculatus | 5 | 0 |
| Atricelaphinis tigrina | 2 | 2 (100) | |||
| C. marginalis | 1 | 0 | |||
| Hopliniiae | 6 | 0 | |||
| 4 | Himeville | 8 | A. tigrina | 5 | 2 (40) |
| 5 | Sani Pass | 4 | C. obliquemaculatus‡ | approx. 5 | 0 |
| 6 | Grahamstown | 15 | C. obliquemaculatus | 32 | 7 (22) |
| Pompilidae | 1 | 0 | |||
| 7 | Ugie | <1 | C. obliquemaculatus | approx. 5 | 0 |
| 8 | Pevensy | <1 | C. obliquemaculatus | 3 | 0 |
| 8 | East of Underberg | <1 | C. obliquemaculatus | 3 | 0 |
Corresponds with the site numbers given in Fig. 1.
Estimated time spent observing the inflorescences at each site.
On transplanted inflorescences.
Transplant experiment
In 2001 and 2002, despite large numbers of E. foliosa plants in flower in Himeville Nature Reserve, pollinaria removal and deposition rates were observed to be nearly zero. Intensive searches failed to find any elaterid beetles in the area. At the nearby Sani Pass site, within the range of E. foliosa but without a natural population of this orchid in the vicinity, elaterid beetles were numerous. To determine whether the lack of pollination at the Himeville site was a simple consequence of the lack of beetles, 60 inflorescences of E. foliosa were collected and 30 of these were ‘transplanted’ to Himeville Nature Reserve (pollinators rare) and 30 to the Sani Pass site (pollinators present). Inflorescences were first inspected and flowers showing signs of visitation were removed. They were then positioned at a natural height above the grass canopy with the cut ends of the scape in vials of water.
After 3 d the transplanted inflorescences were collected and pollinaria removal and deposition were determined. Data for the two sites were compared using the Mann–Whitney U-test. From these data, pollen transfer efficiency (PTE), the percentage of removed pollinia that are deposited on stigmas (cf. Johnson et al., 2004), was determined. PTE for a number of other populations, including Grahamstown, Ugie and Pevensy, was also determined.
Breeding systems
The breeding system of this species was investigated in the Grahamstown population. Pollinators were excluded from the inflorescences using black netting bags and flowers were either selfed or pollinated with pollinia from plants growing more than 20 m away. In addition, flowers were marked and left unmanipulated to test for autogamy, which is common in Eulophia (Williamson, 1984; C. I. Peter, unpubl. data). Each inflorescence had only one self-pollinated and one out-crossed flower, thus avoiding pseudoreplication. More flowers were marked as unmanipulated controls but these were also spread across many inflorescences. Seven unpredated inflorescences and five mature capsules were recovered for each of the self- and cross-pollinated treatments. A t-test was used to examine the differences between the quality of self-pollinated and cross-pollinated capsules and seeds set.
Pollinarium bending and anther cap retention
Pollinarium reconfiguration through bending is common in Eulophia. While attempting to record the bending rates of the pollinaria of E. foliosa it was observed that the anther caps were difficult to dislodge. In some cases forcefully removing the anther cap breaks the pollinia, still enclosed in the anther cap, from the stipe. The time it took for the anther cap to become loose enough to be removed by a moderate air current was therefore recorded.
Pollinaria were removed from the flower using the tip of a fingernail. Pollinaria were then held in an exposed position where they received sunlight and were exposed to approx. 15 km h−1 breeze (approx. 0·4 m s−1). Suspecting this response to be driven by changing water status of the cells of the anther cap, we recorded ambient temperature and relative humidity using automatic data loggers. From these two measurements, atmospheric vapour pressure deficit (VPD) was calculated according to the equations of Goff and Gratch (1946). VPD describes the gradient of water from the cells to the atmosphere and assumes the cells of the anther cap to be wet and freely losing water. VPD is a more relevant measurement of the gradient of water from the plant to the environment than relative humidity alone (Monteith, 1965) as plants are known to respond directly to VPD (Lösch and Tenhunen, 1981).
Positioning of the pollinaria in a constant airflow is therefore important, not only as it provides constant agitation so that an endpoint (dropping of the anther cap) can be determined, but the breeze also reduces any boundary layer conditions around the pollinaria. These times were recorded for pollinaria from fresh flowers selected from random plants in the Grahamstown population from 0800 h until midday on 19 December, 2004. In most cases only one flower was used per inflorescence. For four inflorescences, anther cap retention times were recorded for two flowers.
Anther cap anatomy
Flowers were preserved in 70 % ethanol and dehydrated in an alcohol–butanol series before being embedded in paraplast wax. Sections of 10–15 µm thickness were cut using a microtome. Mounted sections were stained with safranin and fast green and then imaged.
Anther cap and stigma dimensions
The widths and lengths of anther caps from different populations were recorded with digital callipers, as were the widths and lengths of stigmas and the width of the two pollinia from the flower. Only one flower was measured per inflorescence.
RESULTS
Pollinators
Many visitors to the inflorescences were observed at all the sites investigated (Table 1). However, only two beetle species bore E. foliosa pollinaria. By far the most numerous visitors to the inflorescences were Cardiophorus obliquemaculatus (Elateridae) and at Vernon Crookes, Drummond and Grahamstown a number of individuals bearing pollinaria were collected (Table 1). At these sites, C. obliquemaculatus were observed to enter flowers and remove pollinaria. The beetles clamber all over the inflorescence and probe the flowers wherever there is a space or depression such as those between petals. Where these attempts find the main opening of the flower, the curvature of the labellum forces the insect to assume the ‘correct’ orientation to enter the flower fully. This curvature also forces the elytra or thorax of the beetles up against viscidium of the pollinarium (Fig. 1F). The attachment of the anther cap–pollinarium unit to the column is firm, as is the viscidium attachment to the elytra or thorax of C. obliquemaculatus. As a result, the beetle may struggle for a number of minutes to remove the pollinarium from the column before it can exit the flower.
One beetle was also observed depositing pollinia on a stigma, a process that took in excess of 10 min and also entailed much struggling by the beetle. This observation is supported by a smaller number of beetles bearing visicidia, which suggests that these beetles may have deposited pollinia on stigmas.
Numerous visits of C. obliquemaculatus to inflorescences of E. foliosa were observed at the Grahamstown site. These visits ranged between 45 and 550 s (average 302 s) in duration and usually entailed the beetle probing only a few flowers per inflorescence—typically fewer than five. More than one C. obliquemaculatus beetle on an inflorescence was routinely observed, but beetles were never observed mating.
Beetles removing pollinaria were observed on five occasions at Grahamstown, twice at Drummond and once at Vernon Crookes. In all cases the beetles left the inflorescences with the anther caps still covering the pollinia and, although a few of these beetles probed other flowers following the removal of the pollinia, no beetles depositing pollinia on self-stigmas were observed.
Although a number of C. obliquemaculatus were also observed on the inflorescences at other sites (Wahroonga, Pevensy, Underberg and Ugie), no individuals bearing pollinaria were collected. This may be an artefact of limited observation time. At these sites (except Wahroonga) observations were limited to less than 1 h each (Table 1).
At Wahroonga and Himeville a few much larger Atricelaphinis tigrina (Cetoniinae) beetles visiting the inflorescences were collected. Some of these beetles removed pollinaria (Fig. 1H). No beetles were observed depositing pollinia and no beetles bearing viscidia, indicating that they may have deposited pollinia, were collected.
In addition, a number of other beetle visitors to the inflorescences were observed, including larger elaterids (Cardiotarsus species), weevils (Curculionidae) and occasionally monkey beetles (Hopliini, Scarabaeidae). A few visits to the inflorescence by two small pompilid wasps were observed (Grahamstown, Vernon Crookes) and a scoliid wasp (Hesketh Conservation Area, Pietermaritzburg, South Africa). None of these insects bore pollinaria of E. foliosa.
Transplant experiment
The mean proportion of flowers with pollinaria removed from transplanted inflorescences was significantly higher at Sani Pass, where C. obliquemaculatus were common, compared with Himeville Nature Reserve, where pollinators were rare (Z = 2·44, P = 0·015). This was despite the activity of a troop of baboons that disturbed many inflorescences apparently chewing on many of them, removing flowers and substantially reducing our sample size (Table 2). The mean proportion of flowers with pollinia deposited was low at Sani Pass (0·004) and zero at Himeville. This difference was not significant (Z = 1·12, P = 0·264).
Table 2.
Pollen transfer efficiency (PTE; cf. Johnson et al., 2004) for both transplanted plants and natural populations
| Site | No. of flowers | Flowers with pollinaria removed (%) | Flowers with pollinaria deposited (%) | PTE (%) |
|---|---|---|---|---|
| Transplanted to Sani Pass (pollinators) | 618 | 2.7 | 0.4 | 8 |
| Transplanted to Himeville (pollinators rare) | 951 | 0.4 | 0 | 0 |
| Grahamstown | 83 | 49 | 20 | 21 |
| Ugie | 60 | 48 | 7 | 7 |
| Pevensy | 37 | 35 | 19 | 27 |
| Cobham | 37 | 59 | 16 | 14 |
PTE was nil at Himeville, where none of the eight pollinia that were removed (four pollinaria from 951 flowers) were deposited on stigmas in the transplanted group. PTE in the plants transplanted to the site where pollinators were abundant was considerably higher. Three of the 38 removed pollinia (19 pollinaria) were deposited on stigmas (7·9 %), which is within the range of PTE recorded in natural populations (Table 2).
PTE at other sites ranged from 6·8 % at Ugie to 20·7 and 26·9 % for the Grahamstown and Pevensy populations, respectively (Table 2). At the Grahamstown site, 9 % of flowers on inflorescences produced fruit (n = 27 plants).
Breeding system
While fruit set was the same for both cross-pollinated and self-pollinated treatments, the quality of self-pollinated fruit in terms of capsule weight and seed weight was lower (Table 3), although this was not significant (t12 = 1·26, P = 0·23 and t12 = 1·59, P = 0·14, respectively). However, cross-pollinated capsules produced significantly more fertile seeds than self-pollinated seeds (t8 = 4·57, P = 0·002). There is no evidence of autogamy in Eulophia foliosa and pollinators are required for fruit set (Table 3).
Table 3.
Breeding system of Eulophia foliosa (see text for statistical analysis)
| Crossed | Selfed | Unmanipulated | |
|---|---|---|---|
| Fruit set, % (n) | 71 (7) | 71 (7) | 0 (37) |
| Mean capsule weight, grams (s.e.) | 0.106 (0.033) | 0.053 (0.015) | 0 |
| Mean seed weight, grams (s.e.) | 0.020 (0.009) | 0.004 (0.002) | 0 |
| Seeds with embryos, % (s.e.) | 71.5 (10.0) | 14.6 (6.63) | 0 |
Anther cap retention
Anther cap retention times ranged from 249 s during warm, dry conditions to 887 s in the cool, humid morning, with an average retention time of 512 s. This is significantly longer than the average time of 302 s the beetles spend on the inflorescences (t43 = 11·8, P = 0·000). This excludes the very long retention times in excess of 1000 s recorded for anther caps from a single plant in the population. This plant was also excluded from the regression in Fig. 3. The opening of the ‘proximal flaps’ of anther caps (arrowheads, Fig. 4) frees the anther cap from the rest of the pollinarium (Fig. 4). Once the anther cap has dropped, the exposed pollinia (Fig. 4D) are available for deposition on a subsequent visit. It is clear from Fig. 3 that this process occurs more rapidly when the water vapour gradient from the anther cap cell to the atmosphere is large during conditions of high VPD. This suggests that water loss is the primary mechanism behind dropping of the anther cap.
Fig. 3.
Anther cap retention time decreases with increasing vapour pressure deficit (VPD), which describes the gradient of water vapour from the cells to the atmosphere. VPD is calculated from ambient temperature and relative humidity. Pollinaria were orientated in a moderate breeze of approx. 0·4 m s−1 to reduce boundary layer conditions around the pollinarium. Two outliers are from one specific plant and are excluded from the regression (n = 21). Dotted line indicates the average visit times of Cardiophorus obliquemaculatus to inflorescences.
Fig. 4.
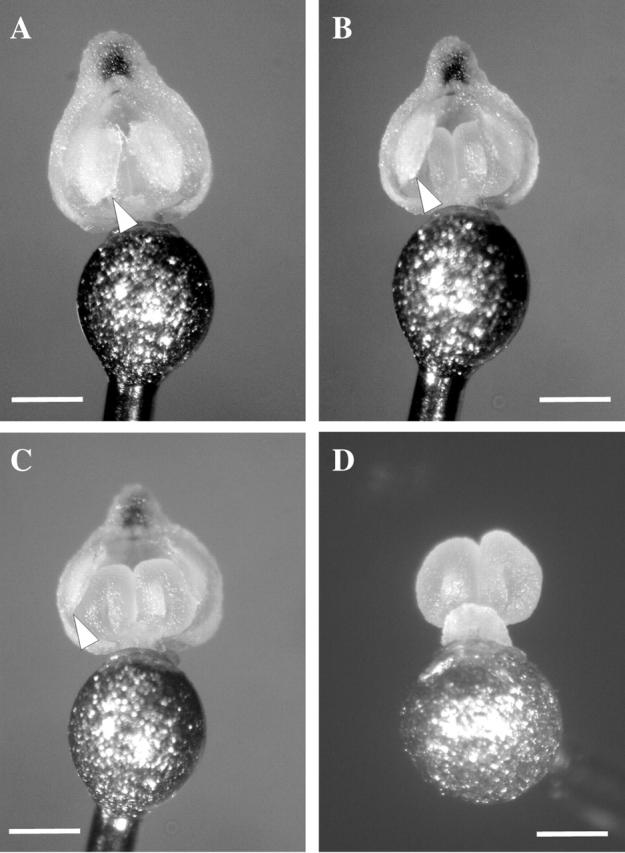
View of the proximal (rostellum side) of a pollinarium removed from the flower on the head of an insect pin. (A) Pollinarium with anther cap in place—‘flaps’ of the anther cap (arrowheads) enclose the pollinarium in the anther cap. (B) Anther cap ‘flaps’ begin to open, (C) anther cap ‘flaps’ completely open and (D) anther cap blown off 9 min after removal, exposing the pollinia for deposition. Scale bar = 0·5 mm.
Interestingly, the pollinaria also undergo a change in orientation. This occurs in many other species of Eulophia and typically involves the stipe of the pollinarium bending through up to 180° so that the pollinia are orientated in the opposite direction to that when they are first removed. In this position, the pollinia can be scraped off by the distal lip of the stigma, bringing about deposition (C. I. Peter, unpubl. data). In E. foliosa bending takes approximately 60 s but this is difficult to determine as the movement of the pollinarium is masked by the anther cap, which envelopes the pollinia. Once the anther cap has dropped the pollinia are already correctly orientated to ensure that they strike the stigma.
Anther cap anatomy
The anther cap is made up of four or five layers of cells in most places. Two regions of cells appear to play a role in the opening of the anther cap to expose the pollinia. There is a region of amorphous cells with thin cell walls, lightly stained with fast green. These cells mark the abscission layer where the anther cap attaches to the column (tn1 in Fig. 5A). Adaxial to these cells are layers of smaller cells with thicker cell walls stained by both safranin and fast green, appearing purple (tk). Following anther cap removal, both the small thick-walled cells and large thin-wall cells appear dehydrated (Fig. 5B). However, the small thick-walled cells change dimensions only slightly relative to the large thin-walled cells, which shrivel substantially. As a result of this differential shrinking (Fig. 5B, fine arrows), the anther cap flaps open outwards (Fig. 5B, blue arrows).
Fig. 5.
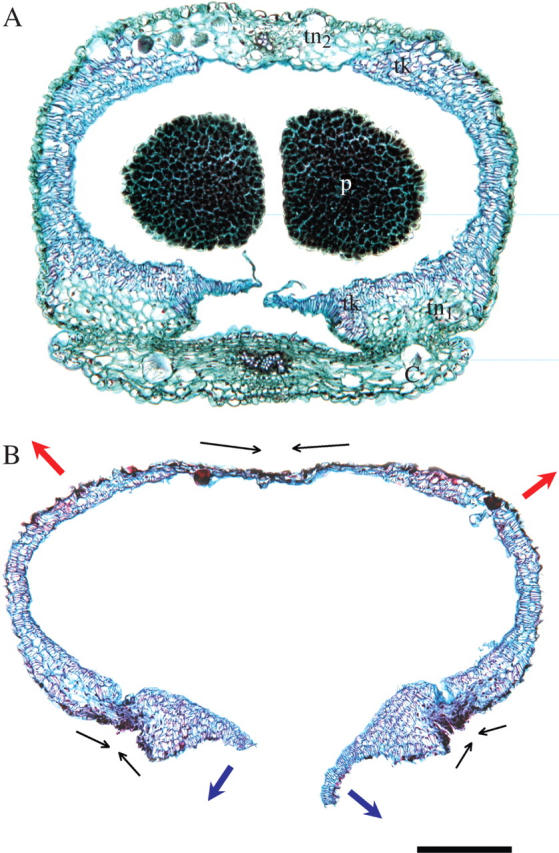
(A) Anatomy of Eulophia foliosa anther cap and pollinia (p) in cross-section while still attached to the column (c). The anther cap is made up of both thick-walled (tk) and two zones of thin-walled (tn) cells. One zone of thin-walled, lightly stained cells marks the abscission layer of the anther cap from the column (tn1); the second zone is a band of cells along the distal margin of the anther cap relative to the rest of the column (tn2). (B) Cross-section of an anther cap dropped from the pollinarium following its natural opening sequence. Arrows are as explained in the text. Scale bar = 250 µm.
A second mechanism also involves the differential shrinking of the cells along a band marking the distal edge of the anther cap relative to the column (tn2 in Fig. 5A). There is a single layer of thick-walled cells forming the adaxial layer of cells of the anther cap. Abaxial to this are poorly defined thin-walled cells (tn2), which appear to shrink and contract relative to the single layer of thick-walled cells (in the direction of the fine arrows, Fig. 5B). This shrinking increases the distance between the two flaps (Fig. 5B, red arrows), further opening the anther cap to facilitate its dropping. This flaring is evident when comparing the width of the anther cap in Fig. 4A and B.
Anther cap and stigma dimensions
Anther cap widths are consistently wider than the stigmas in all populations (Table 4), although individual flowers occasionally had stigmas wider than their anther cap. Averaged across all populations, the anther caps were 0·13 mm (11 %) wider than the stigmas. There was little variation in these measurements within flowers found on a single inflorescence (data not shown). The stigmas were on average 0·31 mm (25 %) wider than the paired pollinia (Table 4). Besides these measurements, the lengths of the anther caps were also measured. The anther caps were substantially longer than the stigmas, the average for all populations being 0·86 mm.
Table 4.
Comparison of anther cap and pollinia widths with the widths of stigmas in different populations of Eulophia foliosa
| Population | Anther cap width (mm) | Stigma width (mm) | Pollinia width (mm) | Anther cap wider than stigma by (mm) | Stigma wider than pollinia by (mm) | n |
|---|---|---|---|---|---|---|
| Grahamstown | 1.24 (0.06) a | 1.09 (0.06) | 0.70 (0.03) a | 0.15 (0.10) | 0.39 (0.07) | 5 |
| Himeville | 1.47 (0.03) b | 1.30 (0.03) | 1.04 (0.04) b | 0.17 (0.04) | 0.26 (0.02) | 5 |
| Drummond | 1.30 (0.10) ab | 1.12 (0.07) | 0.87 (0.03) ab | 0.19 (0.04) | 0.25 (0.04) | 2 |
| Pevensy | 1.36 (0.04) ab | 1.19 (0.07) | 0.91 (0.08) ab | 0.18 (0.06) | 0.28 (0.12) | 4 |
| Ugie | 1.26 (0.04) a | 1.22 (0.09) | 0.93 (0.09) ab | 0.04 (0.05) | 0.30 (0.05) | 5 |
| Average | 1.33 | 1.19 | 0.89 | 0.14 | 0.30 | n…n = 21 |
Means that are not significantly different (Tukey test) share common letters. Standard error is given in parentheses.
Over the course of the study, the stigmas of 1869 flowers were inspected as part of the PTE calculations. In only one instance was an anther cap found clogging a stigma.
DISCUSSION
The results of this study are consistent with the idea that anther cap retention in Eulophia foliosa serves a functional role to prevent facilitated self-pollination by the slow-moving beetles that are the primary pollinators of this species. In this species, pollinaria that retain their anther caps after removal are too large to be inserted into the stigmatic cavities of the flowers. This mechanism thus differs from that reported for Tipularia discolor, in which the anther cap is small enough to be inserted into the stigma, but prevents physical adherence to the stigma surface (Catling and Catling, 1991).
The possession of a mechanism to prevent facilitated self-pollination may be of particular benefit to ‘epidendroid’ orchids, as these orchids have only a few solid pollinia per flower (two in this case), which often function as a single unit. Self-insertion of these pollinia could compromise fitness because of both inbreeding depression in selfed progeny, loss of ovules that would otherwise have been cross-fertilized, and pollen discounting, the loss of pollen that would otherwise have been exported as part of the male function of a plant (Barrett, 2002). Self-pollination may be less detrimental for orchids with ‘orchidoid’-type pollinaria, which are massulate, having pollen packed into smaller sub-units (Johnson and Edwards, 2000). Even if some of these massulae are wasted on self-stigmas, there are probably many more massulae per pollinarium available for later export.
Although polliniarium reconfiguration through bending is by far the most commonly documented mechanism that prevents self-pollination in orchids and asclepiads (cf. Peter and Johnson, 2005), there are a number of reports of anther cap retention (this study; van der Pijl and Dodson, 1966; Dressler, 1981; Catling and Catling, 1991; Singer and Cocucci, 1999; Borba and Semir, 2001) as well as pollinia shrinking (Borba and Semir, 1999; Singer, 2002). Peter and Johnson (2005) compared reconfiguration mechanisms in orchids and asclepiads. They found that in species with pollinaria that reconfigure through a bending mechanism, these movements are rapid, taking less than 150 s on average. In all reported cases of anther cap retention and pollinia shrinking, reconfiguration times are considerably longer (this study; van der Pijl and Dodson, 1966; Catling and Catling, 1991; Singer and Cocucci, 1999; Borba and Semir, 2001). This suggests there is an upper time limit after which pollinarium bending can no longer precisely protect against selfing. Bending mechanisms probably entail differential water loss from small regions of tissue at the base of the stipe (Darwin, 1867) or from regions of the stipe itself (our pers. obs.). As a result, controlled water loss from small regions of tissue is only possible over the lower range of reconfiguration times. Where visit times are longer, slower pollinarium reconfigurations are only possible through alternative mechanisms that entail water loss from larger regions of tissue such as whole pollinia or large parts of the anther cap.
Little is known about the once-off reconfiguration movements of pollinaria. Darwin (1867) attributed the movement of pollinaria to the loss of water from regions of the viscidium or stipe. He also showed that in some cases the pollinarium bent back to its initial position when placed in water. The movement of pollinaria and anther cap flaps described here is distinct from nastic movements of leaves and flowers that repeatedly move (van Doorn and van Meeteren, 2003; Peter et al., 2004). In pollinaria and this example of anther cap retention, the movement is delayed and then proceeds to completion, reconfiguring the pollinia for deposition. The data presented here, both the correlation of anther cap retention times to atmospheric vapour pressure deficit and the observation of anther cap anatomy, point to water loss from specific regions of cells as the mechanism behind the opening of the anther cap to expose the pollinia. However, little is known about the anatomy of orchid anthers, and further study is needed (R. L. Dressler, pers. comm.).
Both anther cap retention and pollinium shrinking reconfiguration mechanisms are likely to be restricted to the ‘higher epidendroid’ orchids (sensu Cameron et al., 1999), with solid pollinia covered by anther caps. Species known to retain their anther caps are scattered through at least four tribes (sensu Cameron et al., 1999) in the sub-family and there is no obvious phylogenetic pattern to the occurrence of this phenomenon. It is likely that anther cap retention occurs in many more species of epidendroid orchids, particularly species with small, rewarding flowers pollinated by slow-moving insects which spend long periods on an inflorescence.
This mechanism of anther cap retention is different to that of all other Eulophia species we have investigated, where the anther cap drops off the removed pollinarium almost immediately. Anther cap retention in E. foliosa is probably a derived condition which protects this species against self-pollination by slow-moving elaterid beetles which spend considerable periods on its inflorescences. This is supported by our observation of beetles removing pollinaria over the course of long visits to inflorescences, but in all cases anther caps prevented self-deposition and promoted pollinia export. Actual rates of self-pollination in E. foliosa are difficult to measure as the pollinia cannot be labelled using stains (Peakall, 1989) or tags (Nilsson et al., 1992) without disturbing the anther cap function.
Beetle pollination appears to be fairly common in the South African flora, but most documented cases involve brightly coloured and unscented flowers that are pollinated by Hopliinid monkey beetles (Goldblatt et al., 1998; Steiner, 1998b; Bernhardt, 2000; Johnson and Midgley, 2001). The cryptic dull green scented flowers of E. foliosa (Fig. 1) are not consistent with this pollination system, instead showing similarities to flowers of orchids for which other beetles have been shown play a role in pollination (Nilsson, 1981; Singer and Cocucci, 1997). These include Listera ovata and Coeloglossum viride in the northern hemisphere, both of which, interestingly, are also visited by elaterid beetles (Nilsson, 1981; Silén 1906, cited in Proctor et al., 1996; C. I. Peter et al., unpubl. data).
Eulophia foliosa has non-rewarding flowers, unlike many other beetle-pollinated orchids, such as L. ovata, C. viride and Pteroglossaspsis ruwenzoriensis. This is in line with other species of Eulophia—of approx. 30 other species examined to date, all are deceptive and offer no reward (C. I. Peter, unpubl. data). Steiner (1998a) suggested a system of rendezvous pollination for Ceratandra grandiflora, the only other deceptive orchid known to be beetle pollinated. None of the many C. obliquemaculatus beetles observed visiting the inflorescences of E. foliosa were mating, so the use of flowers as a rendezvous site is unlikely to be important in this system.
Specialized pollination by the elaterid beetle C. obliquemaculatus seems likely in E. foliosa given the fact that this insect is the sole pollinator at many sites across the geographical range of the species (Fig. 2), and the transplant experiment also shows an association between pollination success and the presence of these beetles (Table 2). This is a third example of specialized beetle pollination as reported in two other species of southern hemisphere orchids (Singer and Cocucci, 1997; Steiner, 1998a).
The pollination biology of the large, primarily African genus Eulophia remains largely undocumented, but it is probable that specialization for beetle pollination may be found to occur in other Eulophia species with flowers that have shallow or absent spurs (C. I. Peter, unpubl. data).
CONCLUSIONS
The data presented here suggest that anther cap retention for a period that exceeds the average visit times by pollinators prevents geitonogamous pollen deposition in Eulophia foliosa, an orchid pollinated exclusively by the elaterid beetle Cardiophorus obliquemaculatus. This delayed movement is in response to the differential shrinkage of tissue of the anther cap driven by the loss of water from these cells. As such, it represents a rare example of an orchid specialized for pollination by beetles.
Acknowledgments
The NRF and Rhodes University are acknowledged for funding and KZN Wildlife is thanked for permission to work in their reserves. Kerry, Pan and Darwin provided welcome company in the field. Kerry Peter, Peter Bernhardt, Edurado Borba and an anonymous reviewer are thanked for suggested improvements to the manuscript.
LITERATURE CITED
- Barrett SCH. 2002. Sexual interference of the floral kind. Heredity 88: 154–159. [DOI] [PubMed] [Google Scholar]
- Bernhardt P. 2000. Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Plant Systematics and Evolution 222: 293–320. [Google Scholar]
- Bookman SS. 1981. The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification in terminology for the genus. American Journal of Botany 68: 675–679. [Google Scholar]
- Borba EL, Semir J. 1999. Temporal variation in pollinarium size after its removal in species of Bulbophyllum: a different mechanism preventing self-pollination in Orchidaceae. Plant Systematics and Evolution 217: 197–204. [Google Scholar]
- Borba EL, Semir J. 2001. Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Annals of Botany 88: 75–88. [Google Scholar]
- Cameron KM, Chase MW, Whitten WM, Kores PJ, Jarrell DC, Albert VA, Yukawa T, Hills HG, Goldman DH. 1999. A phylogenetic analysis of the Orchidaceae: evidence form RBCL nucleotide sequences. American Journal of Botany 86: 208–224. [PubMed] [Google Scholar]
- Catling PM, Catling VR. 1991. Anther-cap retention in Tipularia discolor. Lindleyana 6: 113–116. [Google Scholar]
- Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. [Google Scholar]
- Dafni A, Woodell SRJ. 1986. Stigmatic exudate and the pollination of Dactylorhiza fuchsii (Druce) Soo. Flora 178: 343–350. [Google Scholar]
- Darwin C. 1867. On the various contrivances by which British and foreign orchids are fertilised by insects and on the good effects of intercrossing. London: John Murray. [PMC free article] [PubMed]
- Darwin C. 1878. The effects of cross- and self-fertilization in the vegetable Kingdom, 2nd edn. London: John Murray.
- van Doorn WG, van Meeteren U. 2003. Flower opening and closure: a review. Journal of Experimental Botany 54: 1801–1812. [DOI] [PubMed] [Google Scholar]
- Dressler RL. 1981. The Orchids: natural history and classification. Cambridge, MA: Harvard University Press.
- Goff JA, Gratch S. 1946. Low-pressure properties of water from −160 F to 212 F. Transactions of the American Society of Heating and Ventilating Engineers 52: 95–121. [Google Scholar]
- Goldblatt P, Bernhardt P, Manning JC. 1998. Pollination of petaloid geophytes by monkey beetles (Scarabaeidae: Rutelinae: Hopliini) in southern Africa. Annals of the Missouri Botanical Garden 85: 215–230. [Google Scholar]
- Gutowski JM. 1990. Pollination of the orchid Dactylorhiza fuchsii by longhorn beetles in primeval forests of northeastern Poland. Biological Conservation 51: 287–297. [Google Scholar]
- Johnson SD, Edwards TJ. 2000. The structure and function of orchid pollinia. Plant Systematics and Evolution 222: 243–269. [Google Scholar]
- Johnson SD, Midgley JJ. 2001. Pollination by monkey beetles (Scarabaeidae: Hopliini): do color and dark centers of flowers influence alighting behavior? Environmental Entomology 30: 861–868. [Google Scholar]
- Johnson SD, Nilsson LA. 1999. Pollen carryover, geitonogamy and the evolution of deceptive pollination systems in orchids. Ecology 80: 2607–2619. [Google Scholar]
- Johnson SD, Peter CI, Agren J. 2004. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proceedings of the Royal Society of London Series B, Biological Sciences 271: 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösch R, Tenhunen JD. 1981. Stomatal responses to humidity—phenomenon and mechanism. In: Jarvis PG, Mansfield TA, eds. Stomatal physiology. Cambridge: Cambridge University Press, 137–161.
- Monteith JL. 1965. Evaporation and the environment. In: Fogg GE, ed. The state and movement of water in living organisms. Cambridge: Cambridge University Press.
- Nilsson LA. 1981. The pollination ecology of Listera ovata (Orchidaceae). Nordic Journal of Botany 1: 461–480. [Google Scholar]
- Nilsson LA, Rabakonandrianina E, Pettersson B. 1992. Exact tracking of pollen transfer and mating in plants. Nature 360: 666–668. [Google Scholar]
- Peakall R. 1989. A new technique for monitoring pollen flow in orchids. Oecologia 79: 361–365. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. 2005. Doing the twist: a test of Darwin's cross-pollination hypothesis for pollinium reconfiguration. Biology Letters, doi:10.1098/rsbl.2005.0385. [DOI] [PMC free article] [PubMed]
- Peter CI, Dold AP, Barker NP, Ripley BS. 2004. Pollination biology of Bergeranthus multiceps (Aizoaceae) with preliminary observations of repeated flower opening and closure. South African Journal of Science 100: 624–629. [Google Scholar]
- van der Pijl L, Dodson CH. 1966. Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press.
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. Portland, OR: Timber Press.
- Queller DC. 1985. Proximate and ultimate causes of low fruit production in Asclepias exalta. Oikos 441: 373–381. [Google Scholar]
- Sakai S, Inoue T. 1999. A new pollination system: dung-beetle pollination discovered in Orchidantha inouei (Lowiaceae, Zingiberales) in Sarawak, Malaysia. American Journal of Botany 86: 56–61. [PubMed] [Google Scholar]
- Singer RB. 2002. The pollination mechanism in Trigonidium obtusum Lindl (Orchidaceae: Maxillariinae): sexual mimicry and trap-flowers. Annals of Botany 89: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Cocucci AA. 1997. Pollination of Pteroglossaspis ruwenzoriensis (Rendle) Rolfe (Orchidaceae) by beetles in Argentina. Botanica Acta 110: 338–342. [Google Scholar]
- Singer RB, Cocucci AA. 1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14: 47–56. [Google Scholar]
- Steiner KE. 1998a. The evolution of beetle pollination in a South African orchid. American Journal of Botany 85: 1180–1193. [PubMed] [Google Scholar]
- Steiner KE. 1998b. Beetle pollination of peacock moraeas (Iridaceae) in South Africa. Plant Systematics and Evolution 209: 47–65. [Google Scholar]
- Thomas SA. 1998. A preliminary checklist of the genus Eulophia. Lindleyana 13: 170–202. [Google Scholar]
- Williamson G. 1984. Observations of a mechanism by which self-pollination may occur in Eulophia (Orchidaceae). Journal of South African Botany 50: 417–423. [Google Scholar]