Abstract
• Background and Aims The genetic structure and variability of two species of Vellozia (Velloziaceae) with restricted distribution in high-altitude quartzitic fields in south-eastern Brazil were studied. Vellozia epidendroides is short, grows on pebbly or sandy soil, and is pollinated by bees. Vellozia leptopetala is arborescent, grows on rock outcrops, and is pollinated by bees and hummingbirds. Both are self-incompatible and have a short, massive flowering strategy. The study aimed to associate differences in their genetic diversity and structure with their microhabitat distribution and pollination ecology.
• Methods Leaves from 106 and 139 plants of V. epidendroides and V. leptopetala, respectively, were collected from five patches of each species and prepared for electrophoretic analyses.
• Key Results Five enzyme systems could be reliably scored for both species. Vellozia epidendroides showed 100 % of the loci polymorphic for almost all patches. The average number of alleles per locus ranged between 2·2 and 2·4 among patches. The Wright's fixation index (F) for this species was 0·226. A significant θp value indicates that there is a reasonable genetic divergence among patches. Vellozia leptopetala presented 47·5 % of polymorphic loci. All levels of P, A, Ap and of heterozygosities were lower than those of V. epidendroides. Vellozia leptopetala showed high inbreeding within patches.
• Conclusions The relatively high values of genetic diversity indices found for V. epidendroides may be associated with its large and widespread populations. On the other hand, the low values of genetic diversity found for V. leptopetala may be related to physical isolation on outcrops and intensive foraging by territorial hummingbirds, which may hinder gene flow among patches, aggravated by the very restricted seed dispersal characteristic of the genus, that facilitates sibling mating. It is important to stress the need to preserve the specific habitats of these species of Vellozia, in particular those of V. leptopetala that has lower genetic diversity and is restricted to rock outcrop environments.
Keywords: Brazil, endemic species, genetic diversity, isozyme, rupestrian fields, Serra do Cipó, tropical plant, Vellozia epidendroides, Vellozia leptopetala
INTRODUCTION
Velloziaceae is a small family of approximately 250 tropical species distributed in four genera (Mello-Silva, 1995). Its members are characteristic landscape elements in arid biomes, in particular high-altitude fields and outcrops of South America, Africa and Madagascar (Alves and Kolbek, 1994; Ibisch et al., 1995; Porembski and Barthlott, 2000). They are perennial, desiccation-tolerant and well adapted to fire (Alves, 1994; Ibisch et al., 1995; Porembski and Barthlott, 1995) to the point of sometimes being referred to as resurrection plants (Gaff, 1987; Ibisch et al., 1995, 2001).
The majority of species are found in the ‘campo rupestre’ vegetation (high-altitude rupestrian fields) of south-eastern Brazil (Joly, 1998), associated with a quartzitic formation known as Espinhaço Range. Vellozia in particular is well represented in these environments (about 140 species, Mello-Silva, 1996), with many endemic species restricted to portions of the Espinhaço Range.
A peculiar phenological feature in many Vellozia species is that the flowering season is characteristically short (cornucopia blooming sensu Gentry, 1974), usually a few weeks for a whole population. This strategy is useful to attract pollinators, or to avoid herbivore damage through predator satiation. On the other hand, and given the high level of self-incompatibility in the genus (Sazima, 1978; Oliveira et al., 1991), a large floral display is usually detrimental for seed set, because it increases the amount of self-pollination (Rademaker and de Jong, 1998; Ohashi and Yahara, 1999; Vrieling et al., 1999). Another mechanism that contributes to reduced pollen export and therefore gene flow in Vellozia is cross-pollination among siblings, a consequence of gravity-assisted dispersal of the tiny seeds (but see Ibisch et al., 2001).
Restricted gene flow may affect the genetic structure of a population, by reducing neighbourhood size and area (Levin and Kerster, 1974; Crawford, 1984). A low number of out-crossers may favour genetic drift, increasing the chances of reduced genetic variation within populations. This reduction is due to allele loss after some generations, besides reducing heterozigosity (Wright, 1943). The lack of genetic variability in a population may, for example, reduce its resistance to parasites and predators, and its potential for adaptation to changing environmental conditions (Lewontin, 1974).
Species with a restricted geographical distribution tend to have low genetic diversity (e.g. Loveless and Hamrick, 1984; Godt et al., 1996). However, some rare species may show higher genetic variability than widespread ones (Smith and Pham, 1966; Gitzendanner and Soltis, 2000; Kang et al., 2000). In addition, phylogenetic history is another very important component determining levels of genetic variability (Felsenstein, 1985; Karron, 1987, 1997). Gitzendanner and Soltis (2000) found high degrees of correlation within genera for all measures of genetic diversity. These authors suggest comparing levels of genetic variation among congeneric species with different ranges of geographical distribution to evaluate levels of genetic variability in rare plants.
Levels of genetic diversity may also depend on population size, pollination dynamics and plant density in the area (Barrett and Kohn, 1991; Ellstram and Elam, 1993; Franceschinelli and Bawa, 2000). For example, rare species with large populations may show high levels of genetic diversity (Ellstram and Elam, 1993). However, endemic species with a particular microhabitat requirement would be likely to have a disjoint distribution and thus be more prone to suffer the effects of founder members and selective pressures.
We aimed to compare the genetic structure and diversity of populations of Vellozia epidendroides and V. leptopetala that occur in high-altitude fields of the Espinhaço Range, using allozyme markers. These species were chosen because, although they share some important reproductive biology characteristics that lead to restricted gene flow, their level of genetic isolation may differ on account of their different microhabitat requirements and pollination dynamics. Botanical studies of rupestrian Espinhaço Range Velloziaceae have traditionally focused on their anatomy and taxonomy, with a few exceptions (Sazima, 1978; Sazima and Sazima, 1990; Landau et al., 1998). This is the first attempt to examine the genetic diversity and structure of two species of Vellozia, and to associate these results with their reproductive strategy and habitat distribution.
MATERIALS AND METHODS
Study area and plant species
The study took place in rupestrian fields of Serra do Cipó, at the southern end of the Espinhaço Range, south-easthern Brazil (19°12′–19°20′S, 43°30′–43°40′W). The region is characterized by quartzitic mountains with altitudes varying between 1000–1400 m, reaching 1800 m in certain areas (Menezes and Giulietti, 1986). The climate in the region is marked by wet, mild summers and a dry winter season, which can last from 3 to 5 months. The mean annual rainfall is 1500 mm (Nimer, 1989). Rupestrian fields replace the cerrado (savanna) vegetation from around 900–1000 m (Giulietti and Pirani, 1988).
Vellozia leptopetala Goeth. et Henr. has an arborescent pseudostem 0·5–1·5 m high. Individuals grow exclusively on rock outcrops, which are distinct, isolated landscape features. The flowering period is short and associated with the beginning of the rainy season, usually between October and December. Large plants may bear up to 200 newly opened flowers when in full bloom. Vellozia epidendroides Mart. ex Schult. & Schult. has a short pseudostem (30–40 cm high) and occurs in dense and sometimes very large mats in open fields. Its flowering season is less synchronized than that of V. leptopetala, generally in April and May.
Populations of both species are distributed throughout Serra do Cipó (see Fig. 1); however, V. epidendroides extends approximately 200 km north beyond that region. The floral display of both species is quite conspicuous in the rupestrian landscape. Flowers are large, white, bisexual and short-lived (1–3 d). Pollen, exposed and abundant, is easily gathered by several bee species, both solitary and social. Vellozia leptopetala also produces a small amount of nectar that attracts hummingbirds (Sazima and Sazima, 1990). Hand self- and cross-pollination experiments showed that both species are self-incompatible; their main pollinators are leaf-cutting bees and, in V. leptopetala, also the territorial hummingbird Augastes scutatus (C.M. Jacobi, unpubl. res.). Seeds are very small (1·1–1·5 mm in diameter), spherical, and show no particular dispersal mechanism.
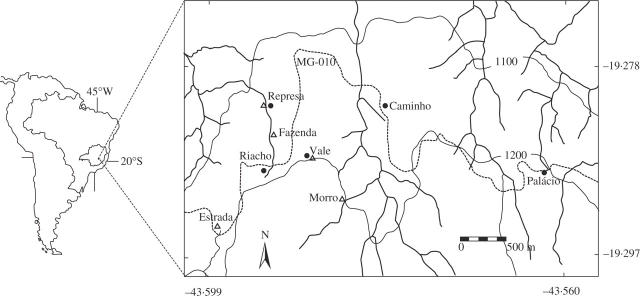
Fig. 1.
Location of the studied patches for Vellozia epidendroides (circles) and V. leptopetala (triangles). The dotted line is interstate road MG-010. Georeference: André Hirsch, Zoology Dept., ICB/UFMG (source: ESRI, 2001; IBGE, 1998).
The study site was thoroughly surveyed for patches of both species. Although outcrops were abundant, many of them were not populated by V. leptopetala. However, the spatial scale of sampling did not differ much for the studied species (Table 1).
Table 1.
Geographic distances (m) among the five patches of (a) Vellozia epidendroides and (b) V. leptopetala
| Palacio | Caminho | Riacho | Vale | Represa | |
|---|---|---|---|---|---|
| (a) V. epidendroides | |||||
| Palacio | – | ||||
| Caminho | 1,858 | – | |||
| Riacho | 3,036 | 1,492 | – | ||
| Vale | 1,571 | 995 | 506 | – | |
| Represa | 3,099 | 1,302 | 711 | 706 | – |
| Estrada | Fazenda | Morro | Vale | Represa | |
|---|---|---|---|---|---|
| (b) V. leptopetala | |||||
| Estrada | – | ||||
| Fazenda | 1,153 | – | |||
| Morro | 1,373 | 1,006 | – | ||
| Vale | 1,253 | 474 | 543 | – | |
| Represa | 1,410 | 347 | 1,315 | 776 | – |
Allozyme analyses
Material for electrophoretic analyses, consisting of young leaves from five different patches of each species, was collected in the Serra do Cipó region at altitudes between 1115 and 1210 m (Fig. 1), totalling 106 individuals of V. epidendroides and 139 individuals of V. leptopetala. Because both species have clonal growth, care was taken to sample only individuals that were physically distinct. This reduced the chances of resampling the same individual, but at the risk of omitting genuinely distinct genets, particularly in the case of V. epidendroides. However, the material collected most likely represents 70–90 % of the individuals in each patch of this species, and 90 % of V. leptopetala. The location of each patch was determined with GPS. Individuals were marked with numbers in the field and leaf samples were wrapped in aluminium foil and refrigerated in ice for transportation. Samples were stored at −80 °C until enzyme extraction.
A small piece of each leaf was ground in liquid nitrogen and the powdered tissue was mixed with extraction buffer #1 of Alfenas et al. (1998) and absorbed onto filter paper wicks. Allozyme electrophoresis was carried out on horizontal 13 % starch-gels. Seventeen enzyme systems were screened with three electrophoretic buffers: morpholine citrate, lithium borate (Alfenas et al., 1998) and histidine (O'Malley et al., 1980).
Five enzyme systems showed simple banding patterns and could be reliably scored for both species. For V. epidendroides, the systems used were UGPP (two loci, UGPP1 and UGPP2), PGM, 6PG and AAT; for V. leptopetala, UGPP (two loci, UGPP1 and UGPP2), 6PG, GDH and AAT. Uridine diphosphoglucose pyrophosphorylase, IDH, PGM and 6PG were resolved on a morpholine citrate buffer system at pH 6·5 (Alfenas et al., 1998). Aspartate aminotransferase and GDH were assayed on a lithium borate buffer at pH 8·3 (Soltis et al., 1983). Locus banding patterns were consistent with typical subunit structures. Different loci and alleles for a given system were designated sequentially, with the lowest number corresponding to the most anodally migrating locus or allele.
Data analyses
Standard measures of allozyme diversity were calculated: the proportion of polymorphic loci (P0·99), mean number of alleles per locus (A) and per polymorphic locus (Ap), observed heterozygosity (Ho) and expected heterozygosity (He = 1 – Σpi2). An estimate of inbreeding levels for each population was obtained using Wright's fixation index (F = 1 – Ho/He; Wright, 1922). Chi-square tests were used to check if frequencies of homozygotes and heterozygotes per population deviated from those expected under Hardy–Weinberg equilibrium. Nei's genetic distances (Nei, 1978) among populations were calculated. All these parameters were calculated using BIOSYS-2 (original version by Swofford and Selander, 1989; modified by Black, 1997).
Wright's (1943) F-statistics (f, θp and F) were used to measure hierarchical population structure and were calculated by the methods of Weir and Cockerham (1984). Jackknifing and bootstrapping were used for combining information over alleles and loci, and for estimating sample variances and confidence intervals, using the FSTAT program version 2.9.3.2 (Goudet, 2002).
Dendrograms were constructed to show relationships among patches, and Mantel tests were used to compare pairwise genetic distances against the geographical distances between patches. Both of these analyses were based on Nei's genetic distance (Nei, 1978) and performed with the FSTAT program version 2.9.3.2 (Goudet, 2002). An indirect estimate of gene flow was made based on the equation: Nm = (1 – θp)/4·θp, where Nm is the number of migrants per generation (Wright, 1931).
RESULTS
Vellozia epidendroides
Significant deviations from Hardy–Weinberg expectations were detected for PGM in almost every patch (Represa, Riacho, Vale and Caminho), for 6PG in Represa, and UGPP1 in Riacho (Table 2). This indicates that crosses are not occurring randomly in the studied populations.
Table 2.
Test for significant deviations from Hardy–Weinberg expectations verified through chi-square test within (a) Vellozia epidendroides and (b) V. leptopetala patches
| Represa |
Palacio |
Riacho |
Vale |
Caminho |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loci | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P |
| (a) Vellozia epidendroides | |||||||||||||||
| UGPP-1 | – | – | – | 3·487 | 1 | 0·062 | 6·858 | 1 | 0·009 | 0·831 | 1 | 0·362 | 3·227 | 1 | 0·072 |
| UGPP-2 | 0·003 | 1 | 0·959 | 2·143 | 3 | 0·543 | 0·262 | 1 | 0·609 | 1·588 | 1 | 0·208 | 2·272 | 3 | 0·518 |
| 6PG-1 | 5·158 | 1 | 0·023 | 0·130 | 1 | 0·719 | 0·654 | 1 | 0·419 | 0·503 | 1 | 0·478 | 0·379 | 1 | 0·538 |
| PGM-1 | 9·143 | 1 | 0·002 | 0·152 | 1 | 0·696 | 4·800 | 1 | 0·028 | 13·091 | 1 | 0·000 | 8·807 | 1 | 0·003 |
| AAT-1 | 0·043 | 3 | 0·998 | 1·448 | 3 | 0·694 | 1·514 | 3 | 0·679 | 0·043 | 3 | 0·998 | 0·479 | 3 | 0·924 |
| Fazenda |
Vale |
Represa |
Morro |
Estrada |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loci | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P | χ2 | d.f. | P |
| (b) V. leptopetala | |||||||||||||||
| UGPP-1 | 3·359 | 1 | 0·067 | 10·867 | 1 | 0·001 | 6·364 | 1 | 0·012 | 20·413 | 1 | 0·000 | 6·296 | 1 | 0·012 |
| UGPP-2 | – | – | – | 17·171 | 1 | 0·000 | 15·180 | 1 | 0·000 | – | – | – | – | – | – |
| 6PG-1 | 0·694 | 1 | 0·405 | 0·013 | 1 | 0·909 | 0·135 | 1 | 0·713 | 2·779 | 1 | 0·096 | 2·042 | 1 | 0·153 |
The percentage of polymorphic loci was 100 % for almost all patches, except for one that showed 80 % of polymorphic loci (Table 3). The average number of alleles per loci ranged between 2·2 and 2·4 for the studied patches. The Wright's fixation index was high for four patches and the average value among patches was 0·226.
Table 3.
Genetic diversity parameters for the five patches of Vellozia epidendroides
| Diversity indices | Represa | Palacio | Riacho | Vale | Caminho | Mean | Total |
|---|---|---|---|---|---|---|---|
| Average sample size (N) | 12·8 (0·2) | 21·8 (0·8) | 31·6 (1·3) | 11 (1·1) | 17·8 (0·8) | 19 | 95 |
| Expected heterozygosity (He) | 0·27 (0·088) | 0·413 (0·058) | 0·476 (0·017) | 0·384 (0·075) | 0·476 (0·021) | 0·404 | 0·464 |
| Observed heterozygosity (Ho) | 0·177 (0·088) | 0·42 (0·076) | 0·378 (0·034) | 0·251 (0·081) | 0·343 (0·053) | 0·314 | 0·341 |
| Average number of alleles per locus (A) | 2·00 (0·32) | 2·40 (0·24) | 2·20 (0·2) | 2·20 (0·3) | 2·40 (0·24) | 2·24 | 2·4 |
| Average number of alleles /polymorphic loci (Ap) | 2·25 | 2·4 | 2·2 | 2·2 | 2·4 | 2·29 | 2·4 |
| Percentage of polymorphic loci (P) (0·95 and 0·99) | 80 | 100 | 100 | 100 | 100 | 96 | 100 |
| Wright's F | 0·355 | −0·020 | 0·209 | 0·353 | 0·285 | 0·226 | 0·266 |
Sample size (N) refers to the number of individual plants. Standard deviations are in parenthesis.
Considering the statistical analysis for each locus (Table 4), UGPP and PGM showed large differences between expected and observed heterozygosity and consequently high fixation indices. On the other hand, AAT showed a very low difference between expected and observed values, and had a correspondingly low value for the fixation index (−0·003). Considering all loci, a high fixation estimate (0·266), and a high difference between expected and observed heterozigosities were observed.
Table 4.
Genetic diversity parameter estimates of each locus in Vellozia epidendroides and V. leptopetalas
|
V. epidendroides |
V. leptopetalas |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Loci | N | P | A | Ap | He | Ho | F | Loci | N | P | A | Ap | He | Ho | F |
| UGPP1 | 105 | 1·0 | 2·0 | 2·0 | 0·453 | 0·229 | 0·496 | UGPP1 | 132 | 1·0 | 2·0 | 2·0 | 0·479 | 0·197 | 0·590 |
| UGPP2 | 86 | 1·0 | 3·0 | 3·0 | 0·510 | 0·454 | 0·112 | UGPP2 | 139 | 1·0 | 2·0 | 2·0 | 0·158 | 0·043 | 0·728 |
| 6PG | 94 | 1·0 | 2·0 | 2·0 | 0·503 | 0·415 | 0·175 | 6PG | 129 | 1·0 | 2·0 | 2·0 | 0·486 | 0·403 | 0·171 |
| PGM | 94 | 1·0 | 2·0 | 2·0 | 0·502 | 0·255 | 0·493 | GDH | 139 | 0 | 1·0 | * | 0 | 0 | 0 |
| AAT | 96 | 1·0 | 3·0 | 3·0 | 0·353 | 0·354 | −0·003 | AAT | 139 | 0 | 1·0 | * | 0 | 0 | 0 |
| All | 95 | 1·0 | 2·4 | 2·4 | 0·464 | 0·341 | 0·266 | All | 135·6 | 0·6 | 1·6 | 2·0 | 0·225 | 0·129 | 0·439 |
See Table 3 for parameter definitions.
The significant θp value (0·101) indicates that there is a reasonable genetic divergence among patches. According to Hartl (1988), θp values of 0·05–0·15 indicate moderate genetic differentiation. Gene flow calculated among these populations was 2·23 migrants per generation. The fixation index within each patch (f) is high, as it is for each of their individuals (F; Table 5).
Table 5.
Estimates of Wright's F-statistics (f, F and θp) described for each polymorphic locus and for all patches of Vellozia epidendroides and V. leptopetala
|
V. epidendroides |
V. leptopetala |
|||||
|---|---|---|---|---|---|---|
| Loci | F | f | θp | F | f | θp |
| UGPP1 | 0·516 | 0·402 | 0·195 | 0·582 | 0·600 | 0·037 |
| UGPP2 | 0·116 | 0·062 | 0·058 | 0·627 | 0·236 | 0·703 |
| 6PG | 0·174 | 0·139 | 0·037 | 0·176 | 0·168 | −0·010 |
| PGM | 0·480 | 0·413 | 0·099 | − | − | − |
| AAT | −0·011 | −0·032 | 0·020 | − | − | − |
| Total | 0·285 | 0·202 | 0·101 | 0·390 | 0·412 | 0·028 |
| s.e. | 0·101 | 0·086 | 0·034 | 0·207 | 0·211 | 0·036 |
| P (t-test) | 0·01 | 0·05 | 0·05 | NS | NS | NS |
f = mean fixation index of individuals relative to their population; θp = populations co-ancestrality coefficient; F = mean overall inbreeding coefficient of an individual.
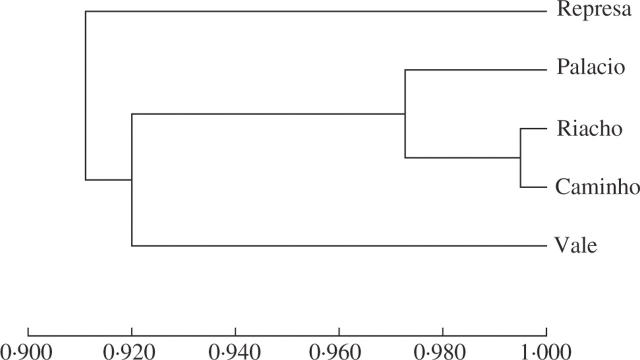
The analysis of the genetic distances among patches of V. epidendroides (Fig. 2) shows a high genetic proximity (identity index = 0·990) between Riacho and Caminho. Riacho and Represa are the most distant genetically (0·836). Geographic distances among patches did not correspond to genetic distances. Accordingly, the Mantel test showed a low, non-significant inverse correlation (r = −0·29, P = 0·42).
Fig. 2.
Dendrogram constructed according to values of Nei's similarity indices found for patches of Vellozia epidendroides from Serra do Cipó, Minas Gerais State, Brazil.
Vellozia leptopetala
None of the five patches presented polymorphism in all loci, and the mean value was 0·48 among patches and 0·6 for the species (Table 6). This low polymorphism is the result of two or more monomorphic loci in all the analyses (Table 4). All levels of P, A, Ap and of heterozygosities were lower than those of V. epidendroides. The levels of expected and observed heterozygosities were low in all patches, particularly Morro, resulting in a high Wright's fixation index.
Table 6.
Genetic diversity parameters for the five patches of Vellozia leptopetala
| Diversity indices | Fazenda | Vale | Represa | Morro | Estrada | Mean | Total |
|---|---|---|---|---|---|---|---|
| Average sample size (N) | 9·8 (0·2) | 22·2 (0·6) | 34·2 (0·8) | 36·8 (0·2) | 32·6 (1·2) | 27·12 | 135·6 |
| Expected heterozygosity (He) | 0·184 (0·114) | 0·227 (0·106) | 0·259 (0·107) | 0·201 (0·123) | 0·193 (0·118) | 0·213 | 0·225 |
| Observed heterozygosity (Ho) | 0·104 (0·065) | 0·136 (0·094) | 0·165 (0·085) | 0·099 (0·071) | 0·126 (0·078) | 0·126 | 0·129 |
| Average number of alleles per locus (A) | 1·40 (0·24) | 1·60 (0·24) | 1·60 (0·24) | 1·40 (0·24) | 1·40 (0·24) | 1·48 | 1·6 |
| Average number of alleles per polymorphic loci (Ap) | 2·0 | 2·0 | 2·0 | 2·0 | 2·0 | 2·0 | 2·0 |
| Percentage of polymorphic loci (P) (0·95 and 0·99) | 0·40 | 0·60 | 0·60 | 0·40 | 0·40 | 0·48 | 0·6 |
| Wright's F | 0·444 | 0·409 | 0·365 | 0·509 | 0·350 | 0·412 | 0·439 |
Sample size (N) refers to the number of individual plants. Standard deviations are in parenthesis.
Significant deviations from Hardy–Weinberg expectations were detected in UGPP1 for all patches and UGPP2 for patches Morro and Estrada (Table 2). Considering that two out of five analysed loci were monomorphic, the significant deviation from the Hardy–Weinberg expectations detected in UGPP loci may indicate that crossings are not occurring randomly in the studied patches.
Results of Wright's F-statistic for V. leptopetala are presented in Table 5. There is high inbreeding within the patches, particularly because of the excess of homozygotes showed by the two bands of UGPP. There is no significant genetic divergence among the patches, with θp = 0·028. The number of migrants Nm was 8·68 migrants per generation.
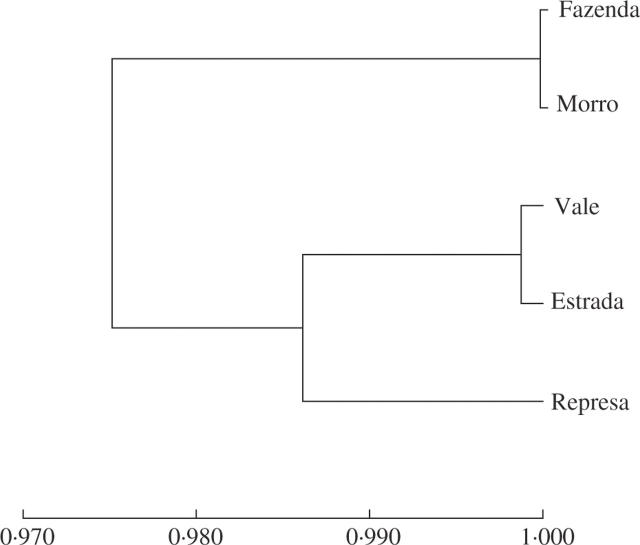
Figure 3 illustrates the proximity relations among the different patches. There is no clear relationship between the genetic distances among patches and their geographic distances, as was also seen in V. epidendroides. Here, the Mantel test also showed a low, non-significant inverse correlation (r = −0·35, P = 0·32).
Fig. 3.
Dendrogram constructed according to values of Nei's similarity indices found for patches of Vellozia leptopetala from Serra do Cipó, Minas Gerais State, Brazil.
DISCUSSION
The genetic diversity of the studied species was shown to be different. The allelic diversity of V. epidendroides was not low (A = 2·4) compared with values of other tropical plants. Hamrick and Godt (1989), in a review of 653 genetic diversity studies using allozymes, found the following values of allelic diversity (number of alleles per locus): 2·19 for woody long-lived species, 2·29 for widely distributed species, 1·81 for tropical species, and 1·99 for allogamous animal-pollinated species. The diversity of V. leptopetala (A = 1·6) was comparatively lower, and similar to values found for endemic species (A = 1·8), according to Hamrick and Godt (1989).
Hamrick and Murawski (1991) compared levels of allozyme diversity of 16 uncommon neotropical tree species with 16 common ones, and observed significantly higher values in this last group. The common species showed 61 % of polymorphic loci and a mean of 3·17 alleles per polymorphic locus. The rare species presented 42 % of polymorphic loci and a mean of 2·14 alleles per polymorphic locus. Vellozia epidendroides showed 100 % of polymorphic loci with a mean of 2·4 alleles per polymorphic locus, while V. leptopetala had 60 % of polymorphic loci and 2·0 alleles. These last values are close to the typical ones found for rare and/or endemic species (Godt et al., 1996, 2004; Mateus-Andrés and Segarra-Moragues, 2003).
The estimated rates of expected and observed heterozygosity were much higher in V. epidendroides (0·464 and 0·341, respectively) than V. leptopetala (0·225 and 0·129, respectively) and the Wright's fixation index was higher for the last species (0·439 versus 0·266). The expected heterozygosity rate found for V. epidendroides is similar to other long-lived, non-endemic tropical species (Hamrick and Godt, 1989; Moraes, 1992; Santos, 1994; Darrigo et al., 2002; Ribeiro, 2002; Vasconcelos, 2002; Carmo, 2005). On the other hand, values of expected and observed heterozygosity found for V. leptopetala were low and usually associated with endemic species (Godt et al., 1996, 2004; Mateus-Andrés and Segarra-Moragues, 2003).
High genetic diversity has been found in endemic species (Smith and Pham, 1966; Kang et al., 2000), including two from Serra do Cipó whose genetic variability is maintained by mechanisms of their reproductive strategies (Borba et al., 2001; Gomes et al., 2004). Overall, however, most endemic species have shown a significant lower percentage of polymorphic loci, mean number of alleles per locus and observed heterozygosity than their widespread congeners (Gitzendanner and Soltis, 2000). Similar results were found in our work. Vellozia epidendroides, with a wider geographical range, larger populations and less restricted habitat requirements had higher levels of genetic diversity than V. leptopetala, which has a reduced geographical range and grows exclusively on physically isolated rock outcrops. All those factors may contribute to gene flow within a small neighbourhood in V. leptopetala.
Wright's fixation index for V. leptopetala is one of the highest found among perennial plant species, suggesting that it is undergoing strong inbreeding. This inbreeding is probably biparental, since the species is self-incompatible. Possibly, the fact that Vellozia seeds have a very restricted dispersal promotes the establishment of kin seeds close to their parents, which facilitates sibling mating. Furthermore, the high fixation index and the non-random mating within patches could be associated with intensive foraging by the territorial hummingbird Augastes scutatus, one of its main pollinators.
However, indirect estimates of the number of migrants among patches—calculated by means of the θp index—were higher for V. leptopetala (Nm = 8·68) than for V. epidendroides (Nm = 2·23). Indirect estimates of gene flow reflect historical rates of flow that have occurred over many generations (Loveless, 1992). In our work, individuals could have come from different reproductive cycles and establishment; that is, from supra-annual variations in seed set, with different selective pressures on progeny and seedlings. Besides, it is possible that the high number of migrants Nm and low values of θp in V. leptopetala are also a consequence of the low genetic diversity found for this species in general, making it difficult to distinguish its populations.
Given the longevity of many Velloziaceae (Alves, 1994), the lack of correlation between genetic and geographic distance in our study could be attributed to the previous existence of a wider range than nowadays (Giulietti and Pirani, 1988). In any case, the genetic distances between the patches of both species are very small (especially for V. epidendroides) and would not be easy to detect geographically. Considering their restricted distribution and their microhabitat preference, it is important to stress the need to preserve the specific habitats of these species of Vellozia, in particular those of V. leptopetala. The protection of campo rupestre vegetation may prevent further increases in its inbreeding rates and its extinction due to lack of genetic diversity.
Acknowledgments
We thank Dr G.W. Fernandes for granting access to the research area and the director and staff of Serra do Cipó National Park for logistic support. We are indebted to Giuliana M. P. Vasconcelos, Roselaini M. do Carmo and Virginia Tenório for their assistance in the laboratory, and to André Hirsch for the map. The comments of Tim Holtsford and one anonymous referee greatly improved this final version. This research was supported by FAPEMIG (CRA 1590/98).
LITERATURE CITED
- Alfenas AC, Peters I, Brune W, Passador, GC. 1998. Eletroforese de proteínas e isoenzimas de fungos e essências florestais. Viçosa: Imprensa Universitária—Universidade Federal de Viçosa.
- Alves RJV. 1994. Morphological age determination and longevity in some Vellozia populations in Brazil. Folia Geobotanica et Phytotaxonomica 29: 55–59. [Google Scholar]
- Alves RJV, Kolbek J. 1994. Plant species endemism in savanna vegetation on table mountains (Campo Rupestre) in Brazil. Vegetatio 113: 125–139. [Google Scholar]
- Barrett SCH, Kohn JR. 1991. Genetic and evolutionary consequences of small populations size in plants: implications for conservation. In: Falk DA, Holsinger RKE, eds. Genetic and conservation of rare plants. New York: Oxford University Press, 3–30.
- Black WC. 1997. BIOSYS-2: a computer program for the analysis of allelic variation in population genetics and biochemical systematics, release 1.7. Champaign, IL: Illinois History Survey.
- Borba EL, Felix JM, Solferini VN, Semir J. 2001. Fly-pollinated Pleurothalis (Orchidaceae) species have high genetic variability: evidence from isozyme markers. American Journal of Botany 88: 419–428. [PubMed] [Google Scholar]
- Carmo RM. 2005. Biologia reprodutiva de Cabralea canjerana subsp. canjerana e a influência do tamanho do fragmento florestal no sucesso reprodutivo e diversidade genética. PhD Thesis, Universidade Federal de Minas Gerais, Brazil.
- Crawford TJ. 1984. What is a population? In: Shorrocks B, ed. Evolutionary ecology. Oxford: Blackwell Scientific Publications, 135–173.
- Darrigo MR, Franceschinelli EV, Buzato S. 2002. Estrutura genética de Hippeastrum damazianum (Amarillydaceae) e sua inter-relação com a variação na polinização e fecundidade das populações. Masters Dissertation, Universidade de São Paulo, Brazil.
- Ellstram NC, Elam DR. 1993. Population genetic consequences of small population size: implications for plant conservation. Annual Review of Ecology and Systematics 24: 217–242. [Google Scholar]
- ESRI. 2001. ArcView GIS v. 8·1. Environmental Systems Research Institute, Redlands, CA. http://www.esri.com/data/index.html
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125: 1–15. [Google Scholar]
- Franceschinelli EV, Bawa KS. 2000. The effect of ecological factors on the mating system of a South American shrub species (Helicteres brevispira). Heredity 84: 116–123. [DOI] [PubMed] [Google Scholar]
- Gaff DF. 1987. Desiccation tolerant plants in South America. Oecologia 74: 133–136. [DOI] [PubMed] [Google Scholar]
- Gentry AH. 1974. Flowering phenology and diversity in tropical Bignoniaceae. Biotropica 6: 64–68. [Google Scholar]
- Gitzendanner MA, Soltis PS. 2000. Patterns of genetic variation in rare and widespread plant congeners. American Journal of Botany 87: 783–792. [PubMed] [Google Scholar]
- Giulietti AM, Pirani JR. 1988. Patterns of geographic distribution of some plant species from Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Vanzolini PE, Heyer WR, eds. Proceedings of a Workshop on Neotropical Distribution of Patterns. Rio de Janeiro: Academia Brasileira de Ciências, 39–69.
- Gomes V, Collevatti RG, Silveira FAO, Fernandes GW. 2004. The distribution of genetic variability in Baccharis concinna (Asteraceae), an endemic, dioecious and threatened shrub of rupestrian fields of Brazil. Conservation Genetics 5: 157–165. [Google Scholar]
- Godt MJ, Johnson BR, Hamrick JL. 1996. Genetic diversity and population size in four rare southern Appalachian plant species. Conservation Biology 10: 796–805. [Google Scholar]
- Godt MJW, Walker J, Hamrick JL. 2004. Allozyme diversity in Macbridea alba (Lamiaceae), an endemic Florida mint. Journal of Heredity 95: 244–249. [DOI] [PubMed] [Google Scholar]
- Goudet J. 2002. FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3.2). Available from http://www2.unil.ch/popgen/softwares/fstat.htm
- Hamrick JL, Godt MJ. 1989. Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir, BS, eds. Plant population genetics, breeding and genetic resources. Sunderland, MA: Sinauer Associates Inc., 45–162.
- Hamrick JL, Murawski DA. 1991. Levels of allozyme diversity in populations of uncommon neotropical tree species. Journal of Tropical Ecology 7: 385–399. [Google Scholar]
- Hartl DL. 1988. A primer of population genetics. Sunderland, MA: Sinauer Associates Inc.
- IBGE. 1998. Dados Gerais do Brasil, Informações Estatísticas e Geocientíficas. Rio de Janeiro: Fundação Instituto Brasileiro de Geografia e Estatística, http://www.ibge.gov.br
- Ibisch PL, Rauer G, Rudolph D, Barthlott W. 1995. Floristic, biogeographical and vegetational aspects of Precambrian rock outcrops (inselbergs) in Eastern Bolivia. Flora 190: 299–314. [Google Scholar]
- Ibisch PL, Nowicki C, Vasquez R, Koch K. 2001. Taxonomy and biology of Andean Velloziaceae: Vellozia andina sp. nov. and notes on Barbaceniopsis (including Barbaceniopsis castillonii comb. nov.). Systematic Botany 26: 5–16. [Google Scholar]
- Joly AB. 1998. Botânica: introdução à taxonomia vegetal. São Paulo: Companhia Editora Nacional.
- Kang U, Chang CS, Kim YS. 2000. Genetic structure and conservation of the rare endemic Abeliophyllum distichum Nakai (Oleaceae) in Korea. Journal of Plant Research 113: 127–138. [Google Scholar]
- Karron JD. 1987. A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evolutionary Ecology 1: 47–58. [Google Scholar]
- Karron JD. 1997. Genetic consequences of different patterns of distribution and abundance. In: Kunin WE, Gaston KJ, eds. The biology of rarity. London: Chapman and Hall, 174–189.
- Landau EC, Gonçalves-Alvim SJ, Fagundes M, Fernandes GW. 1998. Riqueza e abundância de herbívoros em flores de Vellozia nivea (Velloziaceae). Acta Botanica Brasilica 12: 403–409. [Google Scholar]
- Levin D, Kerster HW. 1974. Gene flow in seed plants. Evolutionary Biology 7: 139–220. [Google Scholar]
- Lewontin RC. 1974. The genetic basis for evolutionary change. New York: Columbia University Press.
- Loveless MD. 1992. Isozyme variation in tropical trees: patterns of genetic organization. New Forestry 6: 67–94. [Google Scholar]
- Loveless MD, Hamrick JL. 1984. Ecological determinants of genetic structure in plant populations. Annual Review of Ecology and Systematics 15: 65–95. [Google Scholar]
- Mateus-Andrés I, Segarra-Moragues JG. 2003. Patterns of genetic diversity in related taxa of Antirrihium L. assessed using allozymes. Biological Journal of the Linnean Society 79: 299–307. [Google Scholar]
- Mello-Silva R. 1995. Aspectos taxonômicos, biogeográficos, morfológicos e biológicos das Velloziaceae de Grão-Mogol, Minas Gerais, Brasil. Boletim de Botânica, Universidade de São Paulo 14: 49–79. [Google Scholar]
- Mello-Silva R. 1996. Two new species of Vellozia (Velloziaceae) from Minas Gerais, Brazil. Botanical journal of the Linnean Society 120: 257–263. [Google Scholar]
- Menezes NL, Giulietti AM. 1986. Serra do Cipó—Paraíso dos Botânicos. Ciência Hoje. 25: 38–44. [Google Scholar]
- Moraes MLT. 1992. Variabilidade genética por isoenzimas e caracteres quantitativos em duas populações naturais de Myracroduon urundeuva F.F. & M.F. Allemão—ANACARDIACEAE (Syn: Astronium urundeuva FR Allemão Engle). PhD Thesis, Universidade de São Paulo, Brazil.
- Nei M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimer E. 1989. Climatologia do Brasil. Rio de Janeiro: IBGE.
- Ohashi K, Yahara T. 1999. How long to stay on, and how often to visit a flowering plant? A model for foraging strategy when floral displays vary in size. Oikos 86: 386–392. [Google Scholar]
- Oliveira PE, Gibbs PE, Bianchi M. 1991. Pollination and breeding system of Vellozia squamata (Liliales, Velloziaceae)—a species of the Brazilian cerrados. Botanica Acta 104: 392–398. [Google Scholar]
- O'Malley DM, Wheeler NC, Guries RP. 1980. A Manual for starch gel electrophoresis. Madison: University of Wisconsin Press.
- Porembski S, Barthlott W. 1995. On the occurrence of a velamen radicum in Cyperaceae and Velloziaceae. Nordic Journal of Botany 15: 625–629. [Google Scholar]
- Porembski S, Barthlott W. 2000. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecology 151: 19–28. [Google Scholar]
- Rademaker MCJ, de Jong TJ. 1998. Effects of flower number on estimated pollen transfer in natural populations of three hermaphroditic species: an experiment with fluorescent dye. Journal of Evolutionary Biology 11: 623–641. [Google Scholar]
- Ribeiro RA. 2002. Efeitos da fragmentação de habitats na estrutura genética de Dalbergia nigra (jacarandá-da-Bahia): uma espécie ameaçada da Mata Atlântica. MSc Dissertation, Universidade Federal de Minas Gerais, Brazil.
- Santos EMG. 1994. Ecologia da polinização, fluxo de pólen e taxa de cruzamento em Bauhinia forficata Link. (Caesalpinaceae). MSc. Dissertation, Universidade de São Paulo, Brazil.
- Sazima M. 1978. Biologia floral de espécies de Velloziaceae na Serra do Cipó, Minas Gerais. PhD Thesis, Universidade de São Paulo, Brazil.
- Sazima M, Sazima I. 1990. Hummingbird pollination in two species of Vellozia (Liliiflorae, Velloziaceae) in Southeastern Brazil. Botanica Acta 103: 83–86. [Google Scholar]
- Smith JF, Pham TV. 1966. Genetic diversity of narrow endemic Allium aseae (Alliaceae). American Journal of Botany 83: 717–726. [Google Scholar]
- Soltis DE, Haufler CH, Darrow DC, Gastony GJ. 1983. Starch gel electrophoresis of ferns: a compilation of grinding buffers, gel and electrode buffer, and staining schedules. American Fern Journal 73: 9–27. [Google Scholar]
- Swofford DL, Selander RB. 1989. Biosys-1. A computer program for the analysis of genetic variation in population genetic and biochemical systematics, version 1.7. Champaign, IL: Illinois Natural History Survey.
- Vasconcelos GMP. 2002. Diversidade genética de Myrciaria floribundae (West ex Willdenow) Berg (Cambui) em paisagem fragmentada da serra da Mantiqueira, MG. MSc. Dissertation, Universidade de São Paulo, Brazil.
- Vrieling K, Samitou-Laprade P, Cuguen J, van Dijk H, de Jong TJ, Klinkhamer PGL, 1999. Direct and indirect estimates of the selfing rate in small and large individuals of the bumblebee pollinated Cynoglossum officinale L (Boraginaceae). Ecology Letters 2: 331–337. [DOI] [PubMed] [Google Scholar]
- Weir BS, Cockerham CC. 1984. Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370. [DOI] [PubMed] [Google Scholar]
- Wright S. 1922. Coefficients of inbreeding and relationship. American Naturalist 56: 330–338. [Google Scholar]
- Wright S. 1931. Evolution in Mendelian populations. Genetics 22: 24–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. 1943. Isolation by distance. Genetics 28: 114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]