Abstract
• Background and Aims It is well documented that C4 grasses have a shorter distance between longitudinal veins in the leaves than C3 grasses. In grass leaves, however, veins with different structures and functions are differentiated: large longitudinal veins, small longitudinal veins and transverse veins. Thus, the densities of the three types of vein in leaves of C3 and C4 grasses were investigated from a two-dimensional perspective.
• Methods Vein densities in cleared leaves of 15 C3 and 26 C4 grasses representing different taxonomic groups and photosynthetic subtypes were analysed.
• Key Results The C4 grasses had denser transverse veins and denser small longitudinal veins than the C3 grasses (1·9 and 2·1 times in interveinal distance), but there was no significant difference in large longitudinal veins. The total length of the three vein types per unit area in the C4 grasses was 2·1 times that in the C3 grasses. The ratio of transverse vein length to total vein length was 14·3 % in C3 grasses and 9·9 % in C4 grasses. The C3 grasses generally had greater species variation in the vascular distances than the C4 grasses. The bambusoid and panicoid C3 grasses tended to have a denser vascular system than the festucoid C3 grasses. There were no significant differences in the interveinal distances of the three vein types between C4 subtypes, although the NADP-malic enzyme grasses tended to have a shorter distance between small longitudinal veins than the NAD-malic enzyme and phosphoenolpyruvate carboxykinase grasses.
• Conclusions It seems that C4 grasses have structurally a superior photosynthate translocation and water distribution system by developing denser networks of small longitudinal and transverse veins, while keeping a constant density of large longitudinal veins. The bambusoid and panicoid C3 grasses have a vascular system that is more similar to that in C4 grasses than to that in the festucoid C3 grasses.
Keywords: C3 and C4 photosynthesis, interveinal distance, longitudinal vein, photosynthetic type, Poaceae, transverse vein
INTRODUCTION
The leaves of C4 plants have different anatomical features from those of C3 plants. Usually, C4 leaves are characterized by Kranz-type anatomy, in which the vascular bundle is surrounded by organelle-rich bundle sheath (BS) cells, and this tissue layer is further surrounded by radially arranged mesophyll (M) cells. In contrast, in C3 leaves, the M cells are well developed relative to the BS cells, which include only a few organelles (Dengler and Nelson, 1999). In C4 photosynthesis, atmospheric CO2 is initially fixed in the M cells, then decarboxylation and refixation of CO2 occur in the BS cells (Hatch, 1987).
C4 plants are divided into three C4 subtypes differing in the process of decarboxylation of C4 acids: the NADP-malic enzyme (NADP-ME), NAD-malic enzyme (NAD-ME) and phosphoenolpyruvate carboxykinase (PCK) types (Hatch, 1987). The difference in biochemical function is associated with that in structural features of leaves. In C4 grasses, in general, the NADP-ME grasses have the BS that originated from the mestome sheath, whereas both the NAD-ME and PCK grasses have the BS that originated from the parenchyma sheath (Dengler and Nelson, 1999). The BS cells of the C4 subtypes also differ in the structure, intracellular position and amount of the chloroplasts and mitochondria (Hatch, 1987; Prendergast et al., 1987; Yoshimura et al., 2004). The quantitative balance of photosynthetic tissues (Hattersley, 1984; Ohsugi and Murata, 1986; Dengler et al., 1994) and organelles (Yoshimura et al., 2004) between the M and BS cells reflects the difference in biochemical function of the photosynthetic subtypes.
In the early stage of C4 plant studies, it was reported that C4 leaves have a denser vascular system than C3 leaves (Takeda and Fukuyama, 1971). This is clearly seen in leaves of grasses, which possess parallel venation. This distinctive difference between C3 and C4 leaves is usually expressed as a difference in the interveinal distance (distance between vein centres) (Takeda and Fukuyama, 1971; Crookston and Moss, 1974). For the efficient operation of C4 photosynthesis, a short distance between the M and BS cells is a prerequisite for rapid diffusion of photosynthetic metabolites (Hatch, 1987). The proximity of veins may also be needed for the proper expression of photosynthetic enzymes in the M and BS cells (Langdale and Nelson, 1991; but see Wakayama et al., 2003).
C4 plants have higher photosynthetic rates under high irradiance and at high temperatures than C3 plants (Ehleringer and Monson, 1993). In general, greater photosynthetic rates would result in greater rates of photosynthate export, to remove recently formed photosynthate from leaves rapidly and so avoid end-product inhibition of photosynthesis (Roth-Nebelsick et al., 2001). C4 plants have been reported to show higher export rates of photosynthate than C3 plants (Hofstra and Nelson, 1969; Gallaher et al., 1975; Lush, 1976; Grodzinski et al., 1998; Leonardos and Grodzinski, 2000). In support of higher rates of translocation, some researchers have pointed to the denser vascular system (Crookston and Moss, 1974) and larger cross-sectional area of phloem (Gallaher et al., 1975) in C4 leaves. The vascular bundle is composed of two kinds of conducting tissues: the xylem and phloem. Thus, it appears that C4 leaves have a denser hydraulic network than C3 leaves.
The CO2-concentrating mechanism of the C4 pathway gives C4 plants an efficient photosynthetic mechanism under low stomatal conductance and thus a higher water use efficiency and photosynthetic ability under environments of low water availability than is the case for C3 plants (Ehleringer and Monson, 1993; Sage, 2004). It is generally accepted that C4 plants evolved from C3 plants, accompanied by modifications to anatomical and biochemical features of leaves. A change in vein density of leaves undoubtedly occurred during the evolution from C3 to C4 plants (Sage, 2004; Ueno and Sentoku, 2006).
Leaf veins show a hierarchical order and have different structures. In grass leaves, the differentiation of transverse and longitudinal veins of different sizes allows a division of labour (Yamazaki, 1960; Lush, 1976; Altus and Canny, 1982, 1985; Altus et al., 1985; Fritz et al., 1989). The large longitudinal veins run from the leaf blade into the sheath. However, most of the small longitudinal veins in the leaf blade terminate at the junction of the blade and sheath (Chonan et al., 1974; Colbert and Evert, 1982; Russell and Evert, 1985; Dannenhoffer and Evert, 1994). The large longitudinal veins serve primarily in longitudinal transport of photosynthate outside the leaf blade. The small longitudinal veins serve primarily in collecting photosynthate from nearby photosynthetic cells. The transverse veins connect the longitudinal veins, and play important roles in the lateral transport of photosynthate from the small to the large longitudinal veins (Altus and Canny, 1982).
Water moves in the three types of vein opposite to the direction of photosynthate. The network system for water movement matches the structural demands of an efficient irrigation system (Pelletier and Turcotte, 2000; Roth-Nebelsick et al., 2001). Water absorbed in the root rises through the large longitudinal veins from the leaf base to the tip of the leaf blade. In the leaf blade, water moves laterally from the large longitudinal veins via the transverse veins to the small longitudinal veins, and is distributed to the M or transpired from stomata (Altus and Canny, 1985; Altus et al., 1985; Canny, 1990).
In order to relate the vascular architecture to physiological functions of the leaves, we need to consider the localization and functional partitioning of different types of veins. To our knowledge, however, most previous studies of the vascular density of C3 and C4 grass leaves have neglected vein types: only the distance between longitudinal veins has been measured (Takeda and Fukuyama, 1971; Crookston and Moss, 1974; Kawamitsu et al., 1985; Dengler et al., 1994). Exceptionally, Oguro et al. (1985) investigated the densities of transverse veins in leaves of some Panicum species.
Here, we analysed the densities of the three types of vein in leaves of various C3 and C4 grasses from a two-dimensional perspective. This family includes all three C4 subtypes and has been investigated sufficiently for the photosynthetic types (Hattersley and Watson, 1992; GPWG, 2001). In addition, C3 grasses consist of phylogenetically different groups such as the festucoid, bambusoid and panicoid grasses, which differ in their temperature requirements for growth. Thus, this family provides an ideal subject for comparative analysis of leaf vascular systems in plants that differ in photosynthetic types and ecological characteristics.
MATERIALS AND METHODS
Plant materials
Table 1 lists the grass species examined in this study: 15 C3 species and 26 C4 species. We divided the C3 grasses into two subgroups depending on growth and flowering period: the panicoid and bambusoid C3 grasses and the festucoid C3 grasses. The bambusoid and panicoid C3 grasses grow in summer, and the flowering period is late summer to early autumn. The festucoid C3 grasses grow in spring, and the flowering period is late spring. Taxonomically, the panicoid, bambusoid and festucoid grasses belong to the subfamilies Panicoideae, Bambusoideae and Pooideae, respectively (Clayton and Renvoize, 1992). The C4 species were divided into three subgroups depending on the C4 biochemical subtypes: eight NADP-ME-type species, nine NAD-ME-type species and nine PCK-type species. All NADP-ME-type grasses, the three Panicum species in the NAD-ME-type grasses, and the four Brachiaria species, Panicum maximum and Urochloa texana in the PCK-type grasses belong to the subfamily Panicoideae. The other grasses of the NAD-ME and PCK types belong to the subfamily Chloridoideae (Clayton and Renvoize, 1992).
Table 1.
The C3 and C4 grass species examined in this study, which are divided according to photosynthetic types and phylogenetic groups
| NADP-ME | NAD-ME | PCK |
|---|---|---|
| (a) C4 species | ||
| Panicoid | Panicoid | Panicoid |
| Digitaria sanguinalis (L.) Scopoli | Panicum coloratum L. var. makarikariense Goossens | Brachiaria brizantha (Hochst. Ex A. Rich) Stapf |
| D. violascens Link | P. dichotomiflorum Michaux | B. decumbens Stapf |
| Echinochloa crus-galli P. Beauv. | P. miliaceum L. | B. humidicola (Rendle) Schweick. |
| Paspalum distichum L. | Chloridoid | B. mutica (Forsk.) Stapf |
| Setaria glauca (L.) P. Beauv. | Cynodon dactylon (L.) Persoon | Panicum maximum Jacq. |
| S. viridis (L.) P. Beauv. var minor (Thunb.) Ohwi | Eleusine coracana (L.) Gaertner | Urochloa texana (Buckley) Webster |
| Sorghum sudanense Stapf | E. indica (L.) Gaertner | Chloridoid |
| Spodiopogon cotulifer (Thunb.) Hackel | Eragrostis cilianensis (Allioni) Vignolo-Lutati | Chloris gayana Kunth |
| E. ferruginea (Thunb.) P. Beauv. | Sporobolus indicus R. Br. var. purpureo-suffusus (Ohwi) T. Koyama | |
|
Leptochloa chinensis (L.) Nees |
Zoysia tenuifolia Willd. |
|
| Bambusoid |
Panicoid |
Festucoid |
| (b) C3 species | ||
| Leersia japonica Makino | Hymenachne indica Buse | Agropyron tsukushiense (Honda) Ohwi var. transiens (Hackel) Ohwi |
| Oryza sativa L. cv. Reiho | Isachne globosa O. Kuntze | Alopecurus aequalis Sobol. var. amurensis (Komar.) Ohwi |
| Panicum bisulcatum Thunberg | Avena fatua L. | |
| Beckmannia syzigachne (Steud.) Fernald | ||
| Briza minor L. | ||
| Bromus catharticus Vahl | ||
| B. rigidus Roth | ||
| Dactylis glomerata L. | ||
| Lolium multiflorum Lam. | ||
| Poa acroleuca Steud. | ||
Seeds of 13 species (four Brachiaria species, Chloris gayana, Eleusine coracana, Oryza sativa, Panicum coloratum, P. dichotomiflorum, P. maximum, P. miliaceum, Sorghum sudanense and Urochloa texana) were sown in pots filled with fertilized field soil, and plants were raised outdoors in Tsukuba and Fukuoka, Japan, in summer. The plants were watered daily. Plants of the remaining 29 species growing naturally in the field in Fukuoka and Tsukuba were used.
Leaf samples were collected in May for the festucoid C3 grasses and in August to September for the panicoid and bumbsoid C3 grasses and all C4 grasses. No significant differences in the interveinal distances occur among different leaf positions in rice plants, except that those in the primary leaves are somewhat smaller (Yamazaki, 1963). In barley leaves, the flag leaf and the first and second leaves below it tend to have similar leaf vein densities (Hanson and Rasmusson, 1975). Therefore, we used either flag leaves or the first leaves below the flag leaves for our experiments. Leaf blades were cut and immediately fixed in a mixture of formaldehyde, acetic acid and ethanol in water (FAA). The plants collected in the field were also retained as voucher specimens for exact identification.
Preparation of cleared leaves
Cleared leaf blades were prepared by a method described in Ueno (1995). The middle portions of fixed leaf blades were boiled in 70 % ethanol for about 10–20 min. After washing in distilled water several times, they were transferred to boiling 85 % lactic acid for 20 min, and then stored in chloral hydrate-saturated ethanol before analysis. The leaf vasculature was observed without staining under a light microscope.
Quantitative data of leaf vascular systems
Leaf veins were divided into three types: large longitudinal veins, small longitudinal veins and transverse veins (Chonan et al., 1974). The two types of longitudinal vein were distinguished by diameter in paradermal view under the light microscope (Fig. 1).
Fig. 1.
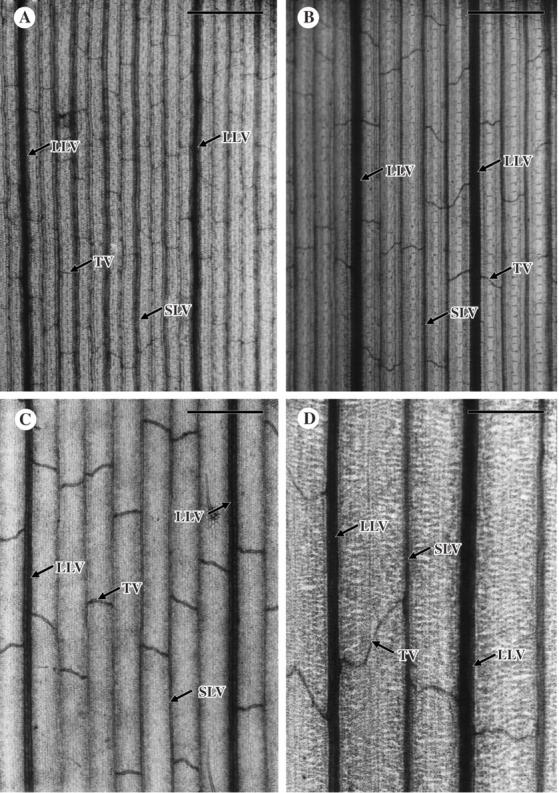
Paradermal view of cleared leaf blades of C3 and C4 grasses. (A) Digitaria sanguinalis, an NADP-ME C4 species. (B) Eleusine indica, an NAD-ME C4 species. (C) Panicum bisulcatum, a panicoid C3 species. (D) Briza minor, a festucoid C3 species. LLV, large longitudinal vein; SLV, small longitudinal vein; TV, transverse vein. The magnification of the four photomicrographs is the same. Scale bars = 250 µm.
The distances between small longitudinal veins, between large longitudinal veins and between transverse veins were represented by means of 30 measurements of middle portions of 3–6 leaf blades taken from three plants. The distance between longitudinal veins was measured between the centres of adjacent veins. The transverse veins are usually curved, unlike the parallel longitudinal veins (Fig. 1). For the distance between transverse veins, therefore, the mean of the minimum and maximum distances between adjacent transverse veins running between a pair of longitudinal veins was calculated. The distance between small longitudinal veins was multiplied by that between transverse veins for each species to indicate areolar area, which represents the minimum area of photosynthetic tissue surrounded by veins.
The number of transverse veins per unit leaf area was measured on photomicrographs (70×) obtained from the middle portions of the 3–6 leaf blades. The lengths of longitudinal and transverse veins per unit leaf area were measured with a curvimeter on the same photomicrographs. The total vein length per unit leaf area and the ratio of transverse vein length to total vein length were calculated using these values.
Statistical analysis
We tested the significance at P < 0·05 of any differences in mean values generated for each species between the C4 and C3 groups and between the five subgroups (the NADP-ME, the NAD-ME, the PCK, the C3 bambusoids and panicoids, and the C3 festucoids) using one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test (statistical software R 2.1.0, R Foundation of Statistical Computing, 2005). To test phylogenetic differences in the C4 grasses, we also analysed any differences between the C4 panicoids, the C4 chloridioids, the C3 bambusoids and panicoids, and the C3 festucoids using the same statistical test.
RESULTS
General features of leaf vascular system in grasses
In the paradermal view of cleared leaf blades, longitudinal veins run at regular intervals in parallel (Fig. 1). The size difference and the existence of strands of hypodermal sclerenchyma allowed the large longitudinal veins to be distinguished from the small longitudinal veins. The transverse veins connected two adjacent longitudinal veins, irrespective of size. The pattern of connection varied even within a leaf: at a variety of angles and from straight to curved.
Interveinal distances of longitudinal and transverse veins
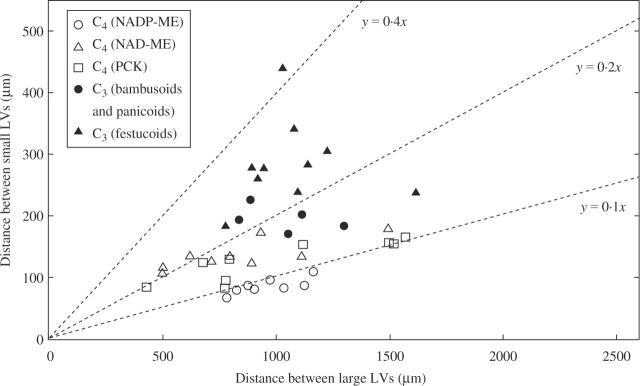
Figure 2 shows the relationship between large longitudinal veins and small longitudinal veins in the C3 and C4 grasses. The C4 grasses had a significantly shorter distance between small longitudinal veins than the C3 grasses: the mean value of the C3 grasses was 2·2 times that of the C4 grasses (Table 2; P < 0·05). Within the C4 grasses, the distance between small longitudinal veins in the NADP-ME grasses was shorter than those in the NAD-ME and PCK grasses (Table 2), although the difference was not significant. Within the C3 grasses, there was a significant difference between the bambusoid and panicoid C3 grasses and the festucoid C3 grasses (Table 2). There was also a significant difference between the festucoid C3 grasses and the three C4 groups. When the C4 grasses were compared between the phylogenetic groups, there was no significant difference between the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 2.
Relationship of the distance between large longitudinal veins and that between small longitudinal veins in leaf blades of C3 and C4 grasses. Slopes of dotted lines show the ratio of the distance between small longitudinal veins (y) to that between large longitudinal veins (x). LV, longitudinal vein.
Table 2.
Means of various measures of vascular density in the leaf blades of grasses of different groups and subgroups
| Group and subgroup | No. of species | Distance between small LVs (A) (µm) | Distance between large LVs (µm) | Distance between TVs (B) (µm) | Areolar area (A × B) (×10−3 mm2) | Total vein length per unit leaf area (mm mm−2) | Ratio of TV length to total vein length (%) | No. of TV per unit leaf area (mm−2) |
|---|---|---|---|---|---|---|---|---|
| C4 | 26 | 118·5 ± 6·4a | 938·4 ± 62·2a | 765·6 ± 29·8a | 91·5 ± 6·5a | †10·6 ± 0·6a | †9·9 ± 0·6a | 9·8 ± 0·7a |
| C3 | 15 | 255·5 ± 18·5b | 1059·6 ± 54·5a | 1460·0 ± 154·5b | 395·3 ± 56·1b | 5·1 ± 0·3b | 14·3 ± 1·1b | 3·0 ± 0·6b |
| NADP-ME | 8 | 86·3 ± 4·3a | 960·5 ± 48·8a | 761·6 ± 54·1a | 64·3 ± 2·5a | 14·3 ± 0·7a | 7·2 ± 1·4a | 13·0 ± 2·6a |
| NAD-ME | 9 | 138·2 ± 7·9ab | 840·2 ± 106·0a | 725·9 ± 30·6a | 100·8 ± 7·7a | 8·9 ± 0·4b | 10·6 ± 2·4ab | 8·0 ± 1·7b |
| PCK | 9 | 127·3 ± 11·0a | 1017·0 ± 140·8a | 808·8 ± 66·0a | 105·1 ± 14·4a | ‡8·7 ± 0·8bc | ‡11·8 ± 1·4ab | 8·8 ± 1·2b |
| C3 bambusoids and panicoids | 5 | 195·0 ± 9·4b | 1037·2 ± 82·7a | 883·6 ± 168·5a | 176·5 ± 42·3a | 6·1 ± 0·3cd | 15·0 ± 1·9b | 5·6 ± 0·9b |
| C3 festucoids | 10 | 285·8 ± 21·8c | 1070·8 ± 73·3a | 1748·0 ± 147·1b | 504·7 ± 54·3b | 4·5 ± 0·3d | 14·0 ± 1·4b | 1·8 ± 0·3c |
| C4 panicoids | 17 | 115·4 ± 8·6a | 1049·8 ± 75·7ab | 782·2 ± 42·6a | 90·6 ± 9·1a | 11·2 ± 0·9ab | 9·8 ± 0·9ab | 10·2 ± 0·9ab |
| C4 chloridoids | 9 | 124·3 ± 9·4a | 728·0 ± 69·8ac | 734·1 ± 31·1a | 92·0 ± 8·8a | ‡9·2 ± 0·6abc | ‡10·2 ± 0·9abc | 9·1 ± 0·9abc |
| C3 bambusoids and panicoids | 5 | 195·0 ± 9·4b | 1037·2 ± 82·7abc | 883·6 ± 168·5a | 176·5 ± 42·3a | 6·1 ± 0·3bcd | 15·0 ± 1·9ac | 5·6 ± 0·9bcd |
| C3 festucoids | 10 | 285·8 ± 21·8c | 1070·8 ± 73·3ab | 1748·0 ± 147·1b | 504·7 ± 54·3b | 4·5 ± 0·3cd | 14·0 ± 1·4ac | 1·8 ± 0·3cd |
Values are given as mean ± s.e.
n = 25 species;
n = 8 species.
Values followed by the same letter are not significantly different at P < 0·05.
LV, longitudinal vein; TV, transverse vein.
With respect to the distance between large longitudinal veins (Fig. 2), the mean value of the C3 grasses was 1·1 times that of the C4 grasses, but this difference was not significant (Table 2). Likewise, there were no significant differences between the five subgroups (Table 2). No correlation was found between the density of large longitudinal veins and that of small longitudinal veins in the C3 grasses (r = 0·045, NS). Low positive correlations were found between the two densities in all grasses examined (r = 0·319, P < 0·05) and in the C4 grasses (r = 0·526, P < 0·01). On the other hand, there were high positive correlations between the two densities in the NADP-ME (r = 0·773, P < 0·05), NAD-ME (r = 0·794, P < 0·05) and PCK (r = 0·864, P < 0·01) grasses. The ratio of the distance between small longitudinal veins to that between large longitudinal veins tended to be lower in C4 grasses than in C3 grasses (Fig. 2). Between the panicoid and chloridoid C4 grasses, there was a tendency that chloridoid C4 grasses had a somewhat shorter distance between large longitudinal veins than the panicoid C4 grasses (Table 2).
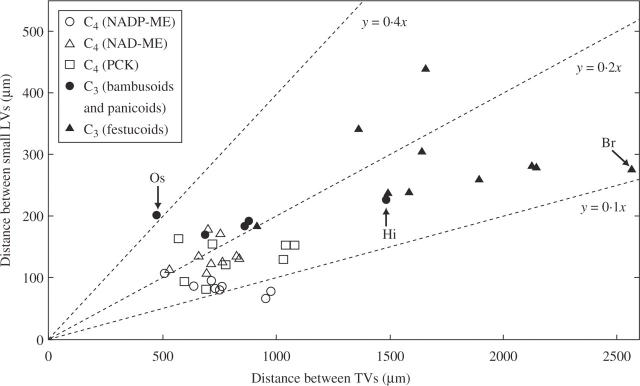
Figure 3 shows the relationship between transverse veins and small longitudinal veins in the C3 and C4 grasses. There was a significant difference in the distance between transverse veins between the C3 and C4 grasses: the mean value of the C3 grasses was 1·9 times that of the C4 grasses (Table 2; P < 0·05). The only significant difference between subgroups was that between the festucoid C3 grasses and the other four subgroups as a whole (Table 2); the mean value was 2·0 times that of the bambusoid and panicoid C3 grasses (Table 2). The values of all the bambusoid and panicoid C3 grasses except Hymenachne indica (1491 µm) were comparable with those of C4 grasses (Fig. 3). The bambusoid C3 grass O. sativa showed the shortest distance between transverse veins (481 µm) among all grass species examined (Fig. 3). The festucoid C3 grass Bromus rigidus showed the greatest distance (2574 µm; Fig. 3). There was a positive correlation between the distance between small longitudinal veins and that between transverse veins in all grasses (Fig. 3; r = 0·744, P < 0·05). There was no significant difference in the distance between transverse veins in the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 3.
Relationship of the distance between transverse veins and that between small longitudinal veins in leaf blades of C3 and C4 grasses. Slopes of dotted lines show the ratio of the distance between small longitudinal veins (y) to that between transverse veins (x). LV, longitudinal vein; TV, transverse vein; Br, Bromus rigidus; Hi, Hymenachne indica; Os, Oryza sativa.
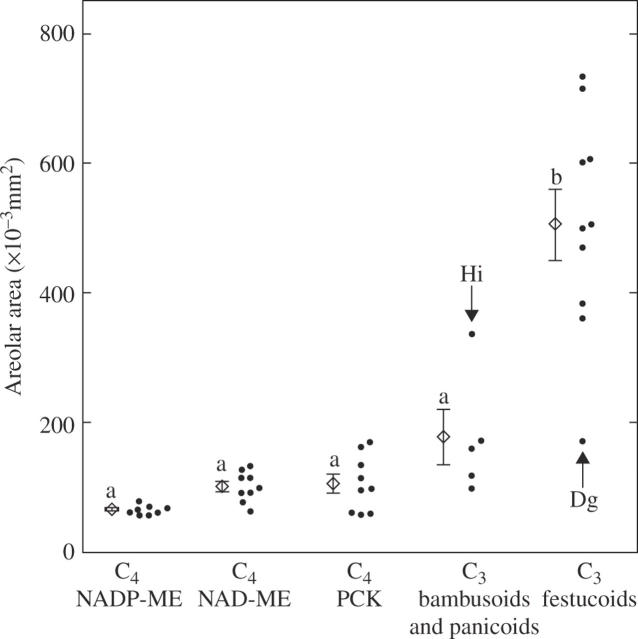
The mean value of the areolar area (the minimum area of photosynthetic tissue surrounded by veins) of the C3 grasses was 4·3 times that of the C4 grasses (Table 2; P < 0·05). In the five subgroups, the festucoid C3 grasses had a significantly higher value than the other four subgroups (Table 2). Exceptionally, the festucoid C3 grass Dactylis glomerata showed a low value (170 × 10−3 mm2), whereas the panicoid C3 grass Hymenachne indica showed a high value (337 × 10−3 mm2; Fig. 4). Although there was no statistical difference between the other four subgroups, the mean value was lowest in the NADP-ME grasses and highest in the bambusoid and panicoid C3 grasses (Table 2). There was no significant difference in the mean value between the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 4.
Comparison of the areolar area (the minimum photosynthetic tissue area surrounded by veins) in leaf blades of C3 and C4 grasses. The values were calculated from the distances between small longitudinal veins and the distances between transverse veins. The mean and s.e. are shown for the respective subgroups. Values followed by the same lower case letter are not significantly different at P < 0·05. Dg, Dactylis glomerata; Hi, Hymenachne indica.
Vein densities per unit leaf area
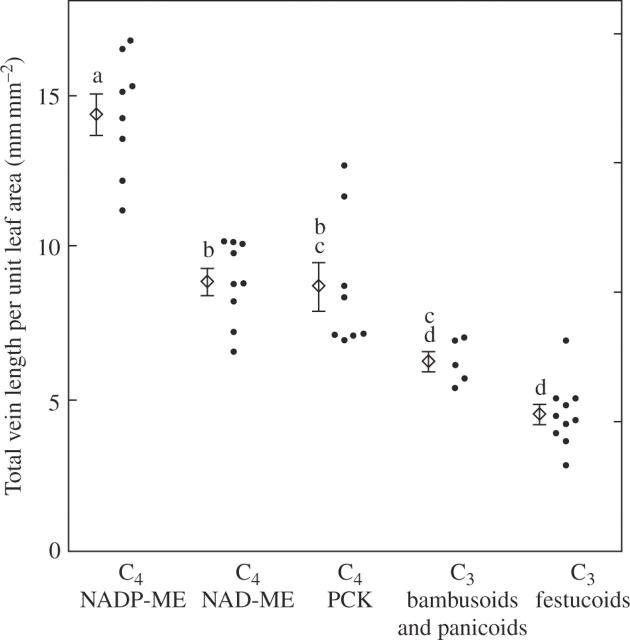
Several measures of leaf vein densities were calculated (Table 2). The mean value of total vein length per unit leaf area in C4 grasses was 2·1 times that in C3 grasses (Table 2). In the five subgroups, it was significantly higher in the NADP-ME grasses than in the other four subgroups (Table 2; Fig. 5). There was no significant difference between the NAD-ME and PCK grasses, between the PCK and the bambusoid and panicoid C3 grasses, or between the bambusoid and panicoid C3 and the festucoid C3 grasses (Table 2). There was no significant difference in the mean value between the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 5.
Comparison of total vein length per unit leaf area in leaf blades of C3 and C4 grasses. The mean and s.e. are shown for the respective subgroups. Values followed by the same lower case letter are not significantly different at P < 0.05.
The ratio of transverse vein length to total vein length was 1·4 times higher in the C3 grasses than in the C4 grasses (Table 2). There was no significant difference in the mean values between the three C4 subgroups, although the mean value in the NADP-ME grasses was significantly lower than those in the two C3 subgroups (Table 2; Fig. 6). There was no significant difference in the mean value between the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 6.
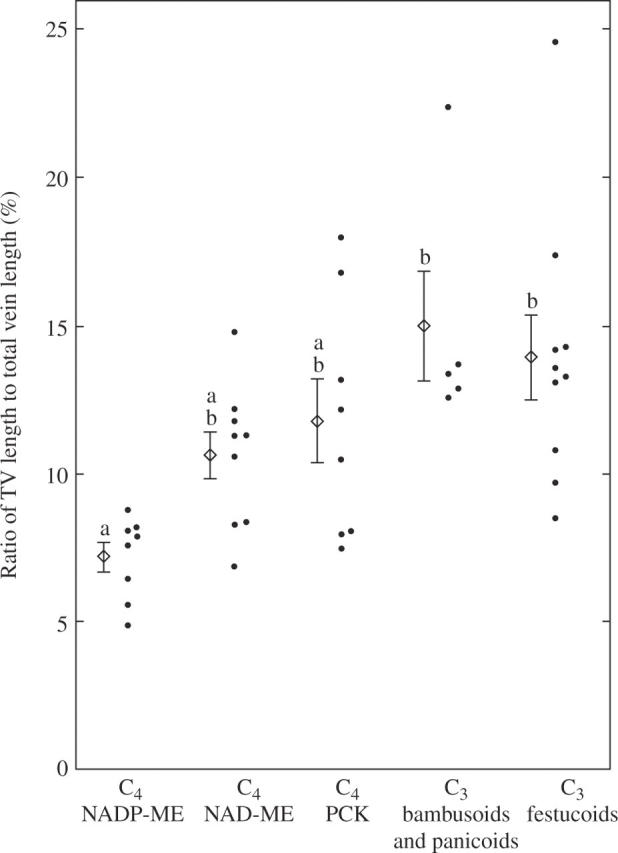
Comparison of the ratio of transverse vein length to total vein length per unit leaf area in leaf blades of C3 and C4 grasses. The mean and s.e. are shown for the respective subgroups. Values followed by the same lower case letter are not significantly different at P < 0·05.
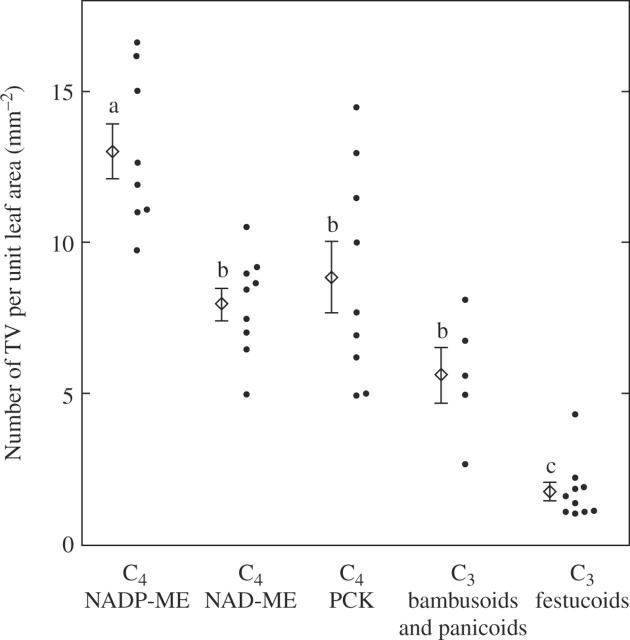
The number of transverse veins per unit leaf area in the C4 grasses was 3·3 times that in the C3 grasses (Table 2). The mean value of the NADP-ME grasses was significantly higher than the other five subgroups, and that of the festucoid C3 grasses was significantly the lowest (Table 2; Fig. 7). There was no large difference in the mean value between the panicoid C4 and the chloridoid C4 grasses (Table 2).
Fig. 7.
Comparison of the number of transverse veins per unit leaf area in leaf blades of C3 and C4 grasses. The mean and s.e. are shown for the respective subgroups. Values followed by the same lower case letter are not significantly different at P < 0·05.
DISCUSSION
Functional implications of differences of leaf vascular systems between C3 and C4 grasses
Our study confirmed that C4 grasses have a denser system of small longitudinal veins than C3 grasses, as reported in previous work (e.g. Takeda and Fukuyama, 1971; Crookston and Moss, 1974; Kawamitsu et al., 1985; Dengler et al., 1994). Our study is the first to demonstrate that the statistically significant differences observed between C3 and C4 grasses are due to the differences in small longitudinal veins, not large longitudinal veins. Furthermore, this study demonstrates that the leaves of C4 grasses tend to develop transverse veins more densely than do those of C3 grasses, although some C3 grasses also had a short distance between transverse veins. Oguro et al. (1985) reported that in some Panicum species, C4 species tend to have shorter distance between transverse veins than C3 species.
The large longitudinal veins contain a greater cross-sectional area of phloem than the small longitudinal veins (Altus and Canny, 1982; Colbert and Evert, 1982; Russell and Evert, 1985). The small longitudinal vein has about the same phloem area throughout the length of the blade, whereas the phloem area of a large longitudinal vein increases from the tip to the base (Altus and Canny, 1982). The transverse veins, which have a single sieve tube (Kuo et al., 1972; Chonan et al., 1985; Tiba and Frean, 1989), connect the longitudinal veins. The transverse veins and small longitudinal veins play a vital role in lateral transport of photosynthate from the small to the large longitudinal veins (Altus and Canny, 1982). Thus, denser development of transverse veins will probably be effective for movement of photosynthate from the small to the large longitudinal veins. Our study demonstrates that C4 grasses have acquired a superior photosynthate translocation system by developing denser networks of small longitudinal and transverse veins, while keeping a constant density of large longitudinal veins.
This feature is also shown by the small areolar area. If we attempt to correlate structural features of leaves to photosynthate translocation, the leaf thickness should also be considered, because the volume of photosynthetic tissue would be more strongly involved in translocation than the areolar area. Dengler et al. (1994) reported that the leaves of C4 grasses are significantly thinner than those of C3 grasses, if C3 bambusoid grasses, which have the thinnest leaves, are not included. Within C4 grasses, there were no significant differences in the leaf thickness between NADP-ME, NAD-ME and PCK grasses, but NAD-ME C4 grasses with PCK-like leaf anatomy have thinner leaves than other C4 subtypes (Dengler et al., 1994). In the NAD-ME C4 grasses examined in our study, only P. dichotomiflorum has this PCK-like anatomy (Ohsugi and Murata, 1986). Thus, the festucoid C3 grasses would have greater photosynthetic tissue volume than the C4 grasses, because they have thick leaves with high areolar area.
It should also be noted that biochemical mechanisms such as sugar transport (Williams et al., 2000), plasmodesmatal distribution in the BS cells (Botha, 1992) and the architecture of the conducting tissue are involved in the process of photosynthate translocation. It has been reported that C4 species usually show preferential localization of sucrose phosphate synthase in the M cells, although there is a wide variation in the relative abundance between the M and BS cells among C4 species (Lunn and Furbank, 1999). Thus, trioses produced in the BS cells must move first to the M cells before being converted to sucrose. Newly formed sucrose must then be returned to the BS cells and loaded to the phloem, suggesting a more complicated pathway of photosynthates in C4 species as compared with that in C3 species.
Reflecting the different conducting roles, the large longitudinal veins contain vessels of larger diameter than the small longitudinal veins (Kuo et al., 1974). The diameter of the largest vessel in the large longitudinal veins decreases with distance along the blade toward the tip, but that in the small longitudinal veins does not change (Altus et al., 1985). The transverse veins possess only one vessel of similar diameter at all places in the leaf blade (Altus et al., 1985). It appears that the leaves of C4 grasses have structurally a better water distribution system than those of festucoid C3 grasses by developing denser networks of the small longitudinal and transverse veins.
Leaf vascular system in C4 grasses
Kawamitsu et al. (1985) reported that the C4 grasses with BS cells that originated from mestome sheath cells (NADP-ME type) have a shorter interveinal distance in leaves than those with BS cells that originated from parenchyma sheath cells (NAD-ME and PCK types). They found no difference in the interveinal distance between the NAD-ME and PCK grasses. Other workers have reported the following order for the interveinal distance: NADP-ME < PCK < NAD-ME in Panicum C4 grasses (Ohsugi and Murata, 1986) and NADP-ME = PCK ≤ NAD-ME in more divergent C4 grasses (Dengler et al., 1994). We found no significant differences in the distance between small longitudinal veins between the three C4 subgroups, although the mean value of the NADP-ME grasses was lower than those of the NAD-ME and PCK grasses. Thus, our data roughly correspond to the results of Kawamitsu et al. (1985), although the previous study did not distinguish the two types of longitudinal vein. It is unclear whether the features of the vascular system of C4 subgroups are associated with some biochemical functions of photosynthesis, although it has been suggested that the interveinal distance could affect photosynthesis in C4 grasses, resulting in differences in photon capture (quantum yield) (Ehleringer et al., 1997; Ogle, 2003). Fisher and Evert (1982) reported a vein density of 7·97 mm mm−2 for Amaranthus retroflexus, an NAD-ME C4 dicot having a reticulated vascular system. This value is comparable with those of the NAD-ME C4 grasses we examined.
When the measures of vascular density of the C4 grasses were compared between the panicoid C4 and the chloridoid C4 grasses, there were no significant differences, except that the chloridoid C4 grasses had a somewhat shorter distance between large longitudinal veins than the panicoid C4 grasses. Thus, it seems that a similar change in the leaf vascular system occurred in parallel between the two phylogenetic lines, although further studies would be required for the density of large longitudinal veins.
Leaf vascular system in C3 grasses
Considerable differences in leaf vascular systems within the C3 grasses were revealed. The bambusoid and panicoid C3 grasses generally had a shorter distance between small longitudinal veins than the festucoid C3 grasses, but there were no differences in the distance between large longitudinal veins. The distance between transverse veins was generally shorter in the bambusoid and panicoid C3 grasses than in the festucoid C3 grasses. Thus, the bambusoid and panicoid C3 grasses have leaves with a denser vascular system than the festucoid C3 grasses. The two C3 subgroups differ in the seasonal growth pattern: the former subgroup grows in summer, as do C4 grasses, but the latter subgroup grows in spring. All the C3 grasses we examined grow in sunny habitats; however, the bambusoid and panicoid C3 grasses grow in wet habitats, whereas the festucoid C3 grasses grow in mesic habitats. It remains unknown whether the gas exchange and water physiology of leaves differ between the two C3 subgroups. Interestingly, the festucoid C3 grasses tend to have larger stomata in the leaf blade than the bambusoid and panicoid C3 grasses, but a lower density of stomata (Ashida and Sugino, 1984; Kawamitsu et al., 1996). For instance, O. sativa leaves show the highest density of stomata among grass species, but the stomatal size is very small (Kawamitsu et al., 1996). Such C3 grasses, even though they grow in wet places, may have a high evaporative demand in order to lower the leaf temperature in the heat of the day. It has also been reported that even in paddy fields with enough water, rice plants are often subject to water stress at midday on a fine day because of intense transpiration over water absorption from roots, which is caused by a high vapour pressure deficit, accompanied by a decrease in leaf water potential and stomatal closure (Ishihara and Hirasawa, 1978; Ishihara and Saitoh, 1987). It would be interesting to study whether the two C3 subgroups have developed different strategies in the vascular and stomatal architectures for water movement and transpiration in leaves.
Ecological and evolutionary implications of leaf vascular systems in C3 and C4 grasses
The vein density of leaves is influenced by various environmental factors. One of the factors that lead to higher vein densities is reduction of soil water availability. Likewise, high temperature induces a similar response in leaves (Uhl and Mosbrugger, 1999; Roth-Nebelsick et al., 2001). These environmental conditions are generally advantageous to the performance of C4 plants, because C4 photosynthesis is more efficient than C3 photosynthesis under environments that promote photorespiration (Ehleringer and Monson, 1993). However, it seems that a change in atmospheric CO2 concentration has no significant effect on the vein density of leaves in both the short and long term (Uhl and Mosbrugger, 1999), unlike the response of stomatal density, which declines as the CO2 concentration increases (Woodward, 1987), although more data are needed to understand the effect of CO2 concentration on the vein density (Roth-Nebelsick et al., 2001). An increase in the vein density of leaves might be an anatomical pre-conditioning to the evolution of C4 plants from C3 plants (Sage, 2004). Increasing vein density may initially have little effect on the performance of an effective CO2 concentration mechanism, but may enhance the water status of leaves in hot environments (Sage, 2004). Kocacinar and Sage (2003) have found that C4 dicots have a stem xylem structure and hydraulic function differing from that of C3 dicots, reflecting their greater water use efficiency and lower water requirements. It is unknown whether C4 grasses also have such characteristics.
Recent molecular phylogenetic studies on the grasses have demonstrated that C4 photosynthesis originated multiple times among several closely related subfamilies (Kellogg, 2001). The earliest divergent branches in the grasses are the C3 bambusoids and C3 festucoids. The remainder of the family is in a large clade (the PACC clade) with a mix of C3 and C4 members. It includes the panicoids, the chloridoids, a lineage with Aristida and Stipagrostis, and a lineage with Eriachne (Sinha and Kellogg, 1996; Kellogg, 2001). Our study indicates that the festucoids have a sparse leaf vascular system, whereas a dense vascular system occurs in the two lineages, the panicoids and chloridoids, together with evolution of C4 photosynthesis. A recent molecular phylogenetic study on the Panicoideae has demonstrated that C3 photosynthesis is the ancestral condition in this subfamily and that C4 photosynthesis arose at least eight times (Giussani et al., 2001). It is interesting to note that the panicoid C3 grasses have a vascular system that is more similar to that in C4 grasses than to that in the festucoid C3 grasses. The bambusoid grasses we examined also had a relatively dense leaf vascular system, which is comparable with that of the panicoid C3 grasses. The acquisition of this structural characteristic may partly be related to similar ecological features in these two groups. However, C4 grasses with a denser leaf vascular system evolved within the panicoids but not within the bambusoids of old origin. A more extensive study would be required to understand the evolution of leaf vascular systems within the grass family, especially with respect to the bambusoids, the C3 panicoids and the remaining two C4 lineages including Aristida and Eriachne.
Acknowledgments
We thank Dr Y. Kawamoto (University of the Ryukyus) for his kind gift of seeds of Brachiaria species.
LITERATURE CITED
- Altus DP, Canny MJ. 1982. Loading of assimilates in wheat leaves. I. The specialization of vein types for separate activities. Australian Journal of Plant Physiology 9: 571–581. [Google Scholar]
- Altus DP, Canny MJ. 1985. Water pathways in wheat leaves. I. The division of fluxes between different vein types. Australian Journal of Plant Physiology 12: 173–181. [Google Scholar]
- Altus DP, Canny MJ, Blackman DR. 1985. Water pathways in wheat leaves. II. Water conducting capacities and vessel diameters of different vein types, and the behaviour of the integrated network. Australian Journal of Plant Physiology 12: 183–199. [Google Scholar]
- Ashida K, Sugino M. 1984. Studies on the stomata in the leaf blades of gramineous weeds. Weed Research (Japan) 29: 138–146. [Google Scholar]
- Botha CEJ. 1992. Plasmodesmatal distribution, structure and frequency in relation to assimilation in C3 and C4 grasses in southern Africa. Planta 187: 348–358. [DOI] [PubMed] [Google Scholar]
- Canny MJ. 1990. What becomes of the transpiration stream? New Phytologist 114: 341–368. [DOI] [PubMed] [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. 1974. Morphology of vascular bundles of leaves in gramineous crops. I. Observations on vascular bundles of leaf blades, sheaths and internodes in rice plants. Proceedings of the Crop Science Society of Japan 43: 425–432. [Google Scholar]
- Chonan N, Kawahara H, Matsuda T. 1985. Ultrastructure of transverse veins in relation to phloem loading in the rice leaf. Japanese Journal of Crop Science 54: 160–169. [Google Scholar]
- Clayton WD, Renvoize SA. 1992. A system of classification for the grasses. In: Chapman GP, ed. Grass evolution and domestication. New York: Cambridge University Press, 338–353.
- Colbert JT, Evert RF. 1982. Leaf vasculature in sugarcane (Saccharum officinarum L.). Planta 156: 136–151. [DOI] [PubMed] [Google Scholar]
- Crookston RK, Moss DN. 1974. Interveinal distance for carbohydrate transport in leaves of C3 and C4 grasses. Crop Science 14: 123–125. [Google Scholar]
- Dannenhoffer JM, Evert RF. 1994. Development of the vascular system in the leaf of barley (Hordeum vulgare L.). International Journal of Plant Sciences 155: 143–157. [Google Scholar]
- Dengler NG, Nelson T. 1999. Leaf structure and development in C4 plants. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 133–172.
- Dengler NG, Dengler RE, Donnelly PM, Hattersley PW. 1994. Quantitative leaf anatomy of C3 and C4 grasses (Poaceae): bundle sheath and mesophyll surface area relationships. Annals of Botany 73: 241–255. [Google Scholar]
- Ehleringer JR, Monson RK. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24: 411–439. [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. [DOI] [PubMed] [Google Scholar]
- Fisher DG, Evert RF. 1982. Studies on the leaf of Amaranthus retroflexus (Amaranthaceae): morphology and anatomy. American Journal of Botany 69: 1133–1147. [Google Scholar]
- Fritz E, Evert RF, Nasse H. 1989. Loading and transport of assimilates in different maize leaf bundles. Digital image analysis of 14C-microautoradiographs. Planta 178: 1–9. [DOI] [PubMed] [Google Scholar]
- Gallaher RN, Ashley DA, Brown RH. 1975. 14C-photosynthate translocation in C3 and C4 plants as related to leaf anatomy. Crop Science 15: 55–59. [Google Scholar]
- Giussani LM, Cota-Sanchez JH, Zuloaga FO, Kellogg EA. 2001. A molecular phylogeny of the grass subfamily Panicoideae (Poaceae) shows multiple origins of C4 photosynthesis. American Journal of Botany 88: 1993–2012. [PubMed] [Google Scholar]
- GPWG (Grass Phylogeny Working Group) 2001. Phylogeny and subfamilial classification of the grasses (Poaceae). Annals of the Missouri Botanical Garden 88: 373–457. [Google Scholar]
- Grodzinski B, Jiao J, Leonardos ED. 1998. Estimating photosynthesis and concurrent export rates in C3 and C4 species at ambient and elevated CO2. Plant Physiology 117: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JC, Rasmusson DC. 1975. Leaf vein frequency in barley. Crop Science 15: 248–251. [Google Scholar]
- Hatch MD. 1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895: 81–106. [Google Scholar]
- Hattersley PW. 1984. Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll:bundle sheath area ratios. Annals of Botany 53: 163–179. [Google Scholar]
- Hattersley PW, Watson L. 1992. Diversification of photosynthesis. In: Chapman GP, ed. Grass evolution and domestication. New York: Cambridge University Press, 38–116.
- Hofstra G, Nelson CD. 1969. A comparative study of translocation of assimilated 14C from leaves of different species. Planta 88: 103–112. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Hirasawa T. 1978. Relationship between leaf and xylem water potentials in rice plants. Plant and Cell Physiology 19: 1289–1294. [Google Scholar]
- Ishihara K, Saitoh K. 1987. Diurnal courses of photosynthesis, transpiration, and diffusive conductance in the single-leaf of the rice plants grown in the paddy field under submerged condition. Japanese Journal of Crop Science 56: 8–17. [Google Scholar]
- Kawamitsu Y, Hakoyama S, Agata W, Takeda T. 1985. Leaf interveinal distances corresponding to anatomical types in grasses. Plant and Cell Physiology 26: 589–593. [Google Scholar]
- Kawamitsu Y, Agata W, Hiyane S, Murayama S, Nose A, Shinjyo C. 1996. Relation between leaf gas exchange rate and stomata. I. Stomatal frequency and guard cell length in C3 and C4 grass species. Japanese Journal of Crop Science 65: 626–633. [Google Scholar]
- Kellogg EA. 2001. Evolutionary history of the grasses. Plant Physiology 125: 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocacinar F, Sage RF. 2003. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant, Cell and Environment 26: 2015–2026. [Google Scholar]
- Kuo J, O'Brien TP, Zee S-Y. 1972. The transverse veins of the wheat leaf. Australian Journal of Biological Science 25: 721–737. [Google Scholar]
- Kuo J, O'Brien TP, Canny MJ. 1974. Pit-field distribution, plasmodesmatal frequency, and assimilate flux in the mestome sheath of wheat leaves. Planta 121: 97–118. [DOI] [PubMed] [Google Scholar]
- Langdale JA, Nelson T. 1991. Spatial regulation of photosynthetic development in C4 plants. Trends in Genetics 7: 191–196. [DOI] [PubMed] [Google Scholar]
- Leonardos ED, Grodzinski B. 2000. Photosynthesis, immediate export and carbon partitioning in source leaves of C3, C3–C4 intermediate and C4 Panicum and Flaveria species at ambient and elevated CO2 levels. Plant, Cell and Environment 23: 839–851. [Google Scholar]
- Lunn JE, Furbank RT. 1999. Sucrose biosynthesis in C4 plants. New Phytologist 143: 221–237. [Google Scholar]
- Lush WM. 1976. Leaf structure and translocation of dry matter in a C3 and a C4 grass. Planta 130: 235–244. [DOI] [PubMed] [Google Scholar]
- Ogle K. 2003. Implications of interveinal distance for quantum yield in C4 grasses: a modeling and meta-analysis. Oecologia 136: 532–542. [DOI] [PubMed] [Google Scholar]
- Oguro H, Hinata K, Tsunoda S. 1985. Comparative anatomy and morphology of leaves between C3 and C4 species in Panicum. Annals of Botany 55: 859–869. [Google Scholar]
- Ohsugi R, Murata T. 1986. Variations in the leaf anatomy among some C4 Panicum species. Annals of Botany 58: 443–453. [Google Scholar]
- Pelletier JD, Turcotte DL. 2000. Shapes of river networks and leaves: are they statistically similar? Philosophical Transactions of the Royal Society B: Biological Sciences 355: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast HVD, Hattersley PW, Stone NE. 1987. New structural/biochemical associations in leaf blades of C4 grasses (Poaceae). Australian Journal of Plant Physiology 14: 403–420. [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. 2001. Evolution and function of leaf venation architecture: a review. Annals of Botany 87: 553–566. [Google Scholar]
- Russell SH, Evert RF. 1985. Leaf vasculature in maize (Zea mays L.). Planta 164: 448–458. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161: 341–370. [DOI] [PubMed] [Google Scholar]
- Sinha NR. Kellogg EA. 1996. Parallelism and diversity in multiple origins of C4 photosynthesis in the grass family. American Journal of Botany 83: 1458–1470. [Google Scholar]
- Takeda T, Fukuyama M. 1971. Studies on the photosynthesis of the Gramineae. 1. Differences in photosynthesis among subfamilies and their relations with the systematics of the Gramineae. Proceedings of the Crop Science Society of Japan 40: 12–20. [Google Scholar]
- Tiba SD, Frean ML. 1989. A comparative study of the structure–function relationship of cross veins in leaves of Digitaria eriantha and Zea mays. Annals of Botany 63: 433–439. [Google Scholar]
- Ueno O. 1995. Occurrence of distinctive cells in leaves of C4 species in Arthraxon and Microstegium (Andropogoneae-Poaceae) and the structural and immunocytochemical characterization of these cells. International Journal of Plant Sciences 156: 270–289. [Google Scholar]
- Ueno O, Sentoku N. 2006. Comparison of leaf structure and photosynthetic characteristics of C3 and C4 Alloteropsis semialata subspecies. Plant, Cell and Environment 28: (in press), doi: 10.1111/j.1365–3040.2005.01418.x [DOI] [PubMed]
- Uhl D, Mosbrugger V. 1999. Leaf venation density as a climate and/or environmental proxy: a critical review and new data. Palaeogeography, Palaeoclimatology, Palaeoecology 149: 17–30. [Google Scholar]
- Wakayama M, Ueno O, Ohnishi J. 2003. Photosynthetic enzyme accumulation during leaf development of Arundinella hirta, a C4 grass having Kranz cells not associated with veins. Plant and Cell Physiology 44: 1330–1340. [DOI] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N. 2000. Sugar transporters in higher plants: diversity of roles and complex regulation. Trends in Plant Science 5: 283–290. [DOI] [PubMed] [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increase in CO2 from pre-industrial levels. Nature 327: 617–618. [Google Scholar]
- Yamazaki K. 1960. Studies on the connecting strand of the vascular system in rice leaves. Proceedings of the Crop Science Society of Japan 29: 400–403. [Google Scholar]
- Yamazaki K. 1963. Studies on leaf formation in rice plants. II. The development of leaves in relation to their position on a stem. Proceedings of the Crop Science Society of Japan 32: 81–88. [Google Scholar]
- Yoshimura Y, Kubota F, Ueno O. 2004. Structural and biochemical bases of photorespiration in C4 plants: quantification of organelles and glycine decarboxylase. Planta 220: 307–317. [DOI] [PubMed] [Google Scholar]