Abstract
• Background and Aims Silica deposition is one of the important characteristics of the family Poaceae. The distribution, deposition process and physiology of silica in this family have been extensively investigated. Bamboos among members of Poaceae have leaves with a fairly long life span, and the leaves continuously accumulate silica in their tissues throughout their life, not only during the course of leaf opening, but also after opening. It has been revealed that the silica deposition process in relation to ageing of the bamboo leaf after opening differed depending on the cell types comprising the tissues. However, silica deposition has never been examined during the development and maturation periods of bamboo leaves. Hence, to clarify the silica deposition process in a developmental stage of the bamboo leaf, distribution of silica was observed in the abaxial epidermis before the opening of the leaf blades of Pleioblastus chino.
• Methods Abaxial epidermal tissues of leaves were examined using a scanning electron microscope equipped with an energy dispersive X-ray microanalyser.
• Key Results Among seven cell types comprising the abaxial epidermis, three types of cells, guard cells, prickle hairs and silica cells, deposited silica conspicuously, and another four types, cork cells, long cells, micro hairs and subsidiary cells, deposited only a little silica. Among the former group of cell types, silica cells and guard cells deposited silica over their entire surfaces, while prickle hairs deposited silica only in the point-tips. Silica deposition was detected firstly in prickle hairs, and then in silica cells and guard cells. Only silica cells were assumed to deposit silica conspicuously before leaf opening but not conspicuously after opening.
• Conclusions Cell types in leaf epidermis of bamboo are classified into three groups according to the silica deposition pattern. Silica deposition in silica cells may be positive as a part of the physiological activities of leaves.
Keywords: Pleioblastus chino, Poaceae, silica deposition, leaf opening, abaxial epidermis, guard cell, prickle hair, silica cell, scanning electron microscopy, X-ray microanalysis
INTRODUCTION
Silicon is the most abundant element in the earth's crust. Plants absorb silicon in the form of silicic acid from the soil. It is well known that plants of Equisetaceae, Cyperaceae and Poaceae are rich in silicon, which is deposited as amorphous hydrated silica (SiO2.nH2O) in the plant body, although most plants do not accumulate silica noticeably.
Silica deposition is one of the important characteristics of plants in the family Poaceae. Many studies have examined the distribution, deposition and physiology of silica in this family. Various functions for silica accumulation have been proposed (Jones and Handreck, 1967; Raven, 1983; Sangster and Hodson, 1986; Marschner, 1995; Prychid et al., 2003; Richmond and Sussman, 2003; Ma, 2004; Epstein and Bloom, 2005). These include mechanical stability of tissues, protection against fungi, insects and herbivores, resistance to drought, facilitation of light interception, and alleviation of problems caused by nutrient deficiency and excess.
Grasses and bamboos have large deposits of silica in leaf blades and inflorescence bracts. It is reported that in Phyllostachys pubescens and P. bambusoides the silica content in mature leaves increases rapidly during the first early growing season and levels off during the first autumn, and then, in the former species, increases again in the early spring of the following year (Kaneko, 1995), whereas it does not increase further in the latter (Ueda and Ueda, 1961). However, the increase in silica content in the second growing season is still unclear, because the life span of leaves in both species is too short, being only about 1 year. The leaves of Sasa veitchii have a life span of approx. 2 years, and they continuously accumulate silica not only during the developmental process but also after maturation up to the end of life (Motomura et al., 2002). Season-dependent changes in the silica content are clear and the accumulation pattern (rapid in spring and summer and slow in winter) is repeated during the 2-year life span. These facts suggest that water flow in the transpiration stream brings silica dissolved in water from the roots to the leaves.
Most grasses and bamboos accumulate silica in the leaf tissues, but the distribution is not uniform among the various cell types comprising the tissues. In mature leaves of Sasa veitchii, nearly all silica cells conspicuously deposit silica (approx. 100 % in cell ratio) in early as well as in later stages in the life spans of about 2-years following full expansion (Motomura et al., 2004). Motomura et al. (2004) showed that the three cell types in the epidermis (bulliform cells, micro hairs and prickle hairs) deposit silica densely and continuously after leaf expansion, while others (cork cells, guard cells, long cells and subsidiary cells) consistently deposit silica at low levels. Cell types depositing silica after the leaf expansion can therefore be categorized into two groups. Also after leaf maturation fusoid cells in mesophyll tissue deposit silica densely, while chlorenchyma cells consistently deposit little silica. This clearly demonstrates that silica deposition in mature leaves of bamboo is dependent largely on the physiological role of each cell type. However, silica deposition has never been examined during the development and maturation periods of bamboo leaves.
In grass leaves, only silica cells deposit silica during leaf expansion with no further deposition after the full expansion, whilst other types of epidermal cells deposit silica only after leaf expansion (Prat, 1932; Blackman, 1968; Sangster, 1970a, b, 1977). These results suggest that silica deposition processes are different depending on whether the leaf is expanding or mature. In accordance with the results for grass leaves, silica deposition in leaf epidermal tissues of young leaves (about 1–2 months after leaf expansion) of Pleioblastus chino was reported to occur in silica cells and other epidermal cell types both on adaxial and abaxial epidermis throughout the whole leaf (Motomura et al., 2000). These results suggest that the silica deposition in silica cells may occur at an early stage of leaf development not only in grass but also in bamboo. Thus, it is necessary to study silica deposition in leaf tissues of bamboo during leaf expansion. Therefore, by comparing silica deposition in the various cell types of the epidermis of bamboo leaf at different developmental stages, opening, expansion and full differentiation, the silica deposition process will be completely clarified. In the present study, silica distribution was examined in the abaxial epidermis before the opening of leaf blades of P. chino using a scanning electron microscope equipped with an energy-dispersive X-ray analyser (SEM-EDXA).
In bamboos, new leaves develop successively from the primordia that occur on alternate sides underneath a shoot apex, growth being enclosed completely within outer but still differentiating leaves. When a leaf expands and opens it exposes part of the abaxial epidermis of the inner, younger rolled-up leaf to the environment. At this stage, the leaf blade and sheath are indistinguishable. A ligule and oral setae gradually differentiate at the basal portion of a leaf blade, and the leaf blade becomes distinguishable from the leaf sheath to the unaided eye. An intercalary meristem differentiates at the basal portion of the leaf blades, and produces new cells acropetally. Thus, there is a spatial sequence of cellular differentiation from base to tip in each leaf blade during the developing process, and such a spatial sequence within a leaf blade is related to the temporal sequence of cellular development. In this study, therefore, leaf epidermal tissues were examined from base to tip in each developing leaf blade.
MATERIALS AND METHODS
Fresh leaves were collected from Pleioblastus chino Makino growing on the campus of the Forestry and Forest Products Research Institute, Tsukuba, Japan.
For analysis of silica deposition along with the development of leaf epidermal tissue, three leaf blades that exposed the leaf tips were collected before leaf opening from the tops of three newly grown culms in August 1996 and September 1997 (Fig. 1). They had lengths of 6 cm, 7 cm and 10 cm, respectively. Each leaf blade was dissected into equal sections of 1 cm in length. These sections were rapidly frozen in liquid nitrogen, and then freeze-dried for 6 h in a freeze-drying device for chemical analysis (Virtis Co. Inc., New York, USA). They were coated with platinum-palladium in an ion-sputter-coater (JFC-1100, JEOL Co. Ltd, Tokyo, Japan), and investigated in the SEM (JSM-840, JEOL Co. Ltd) equipped with an EDXA system (JED-2110, JEOL Co. Ltd).
Fig. 1.

A shoot with a developing leaf at the top. The rolled leaf that is exposed outside the older leaf sheath is indicated by an arrow.
To study leaf structure in the mature condition, fully expanded leaves were also sampled near the top of each of three shoots in October 1996. From each sample, several pieces of approx. 5 mm2 were cut from the region between the leaf margin and midrib of the middle sections. Some samples were fixed overnight at 4 °C with 3 % glutaraldehyde in 0·1 m phosphate buffer (pH 7·2), dehydrated in an ethanol series, then transferred to propylene oxide and embedded in epoxy resin (Spurr, 1969). Thin sections (2–5 µm thick) were cut from the embedded samples using a rotary microtome equipped with a glass knife, and then stained with safranin. Other samples were cleared in a 1 : 1 solution of glacial acetic acid and 30 % hydrogen peroxide for 48 h at 60 °C, dehydrated in an ethanol series, and then stained with safranin. These cleared pieces and thin sections were observed under a light microscope.
RESULTS
General anatomy of Pleioblastus chino leaf
The matured leaf comprised a single-layered adaxial epidermis (ad), two layers of chlorenchyma cells (ch), a single cell layer of fusoid cells (fu), two layers of chlorenchyma cells (ch) and a single-layered abaxial epidermis (ab) (Fig. 2A and B). Vascular bundles (v) were interspersed among the mesophyll cells (chlorenchyma and fusoid cells), and each was surrounded by a bundle sheath consisting of thin-walled parenchyma cells (bs-pa) (Fig. 2A). The vascular bundles were girded by sclerenchyma (sc) adjacent to both the adaxial and abaxial epidermis (Fig. 2A). Cells composing epidermal tissues were categorized into eight cell types; i.e. bulliform cells (bu), cork cells (co), guard cells (gu) and subsidiary cells (su) comprising stomatal complex (st), long cells (lo), micro hairs (mi), prickle hairs (pr) and silica cells (si) (Fig. 2C and D). It has already been shown that the cellular composition ratio is different between the adaxial and abaxial epidermis (see table 1 and fig. 7 in Motomura et al., 2000). Seven of eight cell types are distributed in the abaxial epidermis in contrast to few types in the adaxial epidermis, so observations were focused only on the abaxial epidermis.
Fig. 2.
Microphotographs of matured leaf blades of Pleioblastus chino. (A) Transverse section; (B) longitudinal section; (C) adaxial side; (D) abaxial side. AB, Adaxial side; AD, abaxial side; M, mesophyll; V, vascular bundle; bs-pa, bundle sheath parenchyma cell; bu, bulliform cell; ch, chlorenchyma cell; co, cork cell; fu, fusoid cell; lo, long cell; mi, micro hair; pr, prickle hair; sc, sclerenchyma cell; si, silica cell. Scale bars = 50 µm.
Cells comprising the abaxial epidermis were categorized into seven cell types minus the bulliform cell type (Fig. 2D). Abaxial epidermal cells were arranged in parallel rows along the longitudinal axis of the leaf. The cellular composition and arrangement was different depending on the rows (Fig. 2D). The rows of the middle portion between the vascular bundles had cork cells (co), prickle hairs (pr), long cells (lo) and silica cells (si). Prickle hairs had short pointed structures with swollen bases. Micro hairs usually consisted of two cells, i.e. cap cell and basal cell, and most cap cells were easily broken during clearing because of their very thin walls even in mature leaf blades. Cork cells were solitary in some cases and in most cases paired with micro hairs, prickle hairs or silica cells. In such pairs, the cork cells were always basal (Fig. 2D). Silica cells were distinguishable morphologically from other cell types with the characteristic that they were filled with refractive materials. These cells of four types (cork cells, micro hairs, prickle hairs and silica cells) and the cell pairs (cork cell coupled with one of three types, micro hairs, prickle hairs or silica cells) alternated with the long cells (Fig. 2D). Long cells were characterized by having several papillae (pa) on the cell walls (Fig. 2D). Although similar cell arrangements were also observed in the areas covering vascular bundles, the shape and size of the cells above the vascular bundles were different from those between vascular bundles (Fig. 2D); e.g. silica cells above the vascular bundles had a rectangular-shape with rounded corners, while those over the mesophyll were crescent-shape and smaller; long cells above the vascular bundles were shorter. In other rows of the abaxial epidermis, long cells and stomata complexes (st) occurred (Fig. 2D). Stomatal complexes comprised two guard cells and two subsidiary cells and also alternated with long cells (Fig. 2D).
Cell differentiation in abaxial surface
In the basal portion of the differentiating leaf blades (Fig. 3A), abaxial surfaces generally shrank, and the epidermal cells were wrinkled externally in the freeze-dried samples. It is assumed that their walls were apparently thinner than those of upper portions and deformed during the inadequate freeze-drying process. Swollen cells are assumed to be micro hairs (mi) and prickle hairs (pr). Stomatal apertures and papillae were not apparently differentiated at this stage (Fig. 3A).
Fig. 3.
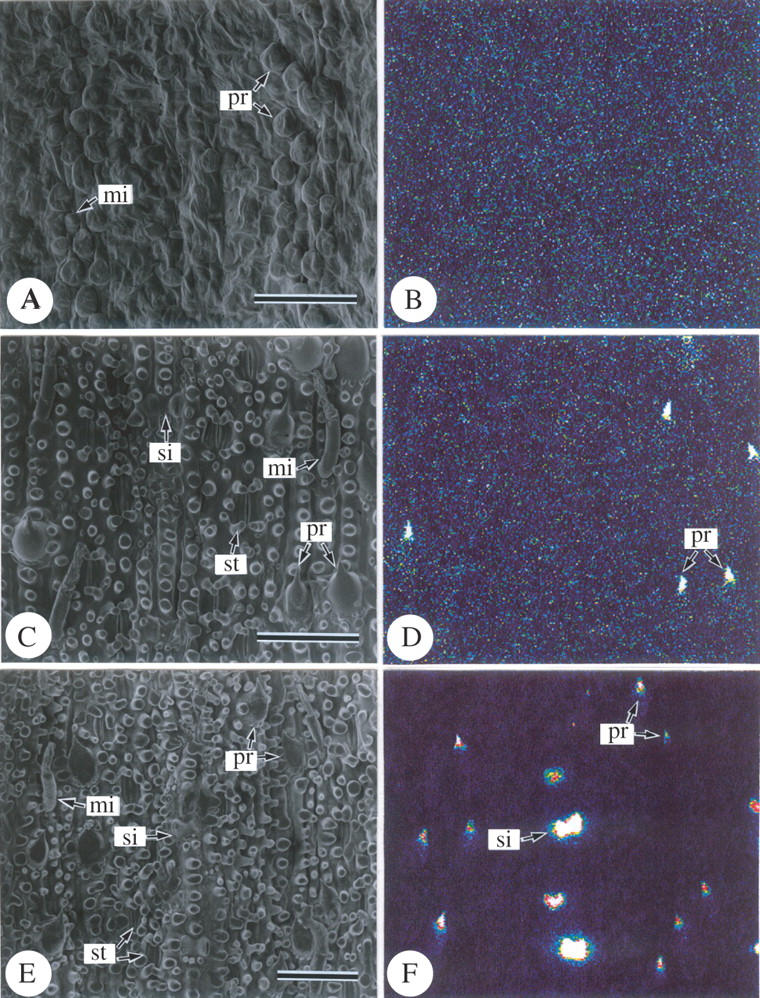
Scanning electron microphotographs of a different area from an abaxial surface of a freeze-dried developing leaf of Pleioblastus chino (10 cm in length). (A, C and E) Secondary electron image; (B, D and F) silicon distribution images by SEM-EDX. Relative silicon density is imaged by colour from very low (dark blue) to very high (white) through sky blue, yellow and red. (A and B) Abaxial epidermis 2 cm above the base of the leaf blade; (C and D) abaxial epidermis 4 cm above the base of the leaf blade; (E and F) abaxial epidermis 5 cm above the base of the leaf blade. mi, Micro hair; pr, prickle hair; si, silica cell. Scale bars = 50 µm.
In the middle portion (Fig. 3C), micro hairs comprising two cell types, and prickle hairs with point-tips had well differentiated shapes, as a result of the acropetal cell differentiation progress. At this stage, stomatal apertures were clearly observed, and long cells had several papillae (pa) on the outer periclinal cell walls (Fig. 3C).
In the upper portion (Fig. 3E), the developmental morphology of the abaxial epidermal cells was similar to that in the middle portion (Fig. 3C). These results indicate that the epidermal cells in the sample leaves are in the differentiating stage at the base portion and are already fully differentiated in the middle.
Silica deposition in differentiating abaxial epidermis
SEM-EDXA studies revealed the density and distribution of silica on the abaxial epidermis in detail (Figs 3 and 4). In the cell-differentiating stage in the basal portion of leaf blades (Fig. 3A and B), the silica deposition was not detected; only background noise was observed (Fig. 3B). In the middle of leaf blades (compare C and D in Fig. 3), where all epidermal cells were fully differentiated, silica deposition was conspicuously detected only at the point-tips of prickle hairs (pr), and was not apparent in other cell types. In the upper portion (Fig. 3E and F), dense silica deposition was found in prickle hairs (pr) and in silica cells (si) that deposited silica over their entire surfaces. Long cells had papillae (pa) on the periclinal cell walls, but no sign of dense silica deposition was observed, although they were already well developed (Fig. 3E and F). Silica deposition was not apparent in other epidermal cell types (Fig. 3E and F). In the top portion (Fig. 4), silica deposition was conspicuously detected over almost the entire surfaces of guard cells (gu) (Fig. 4C and D), while it was not apparent in subsidiary cells. Silica deposition was sparse in cork cells, long cells and micro hairs (Fig. 4). Thus, the conspicuous progress in silica deposition from the middle portion to the upper portion indicates that silica deposition gradually occurs after full-differentiation of epidermal cells.
Fig. 4.
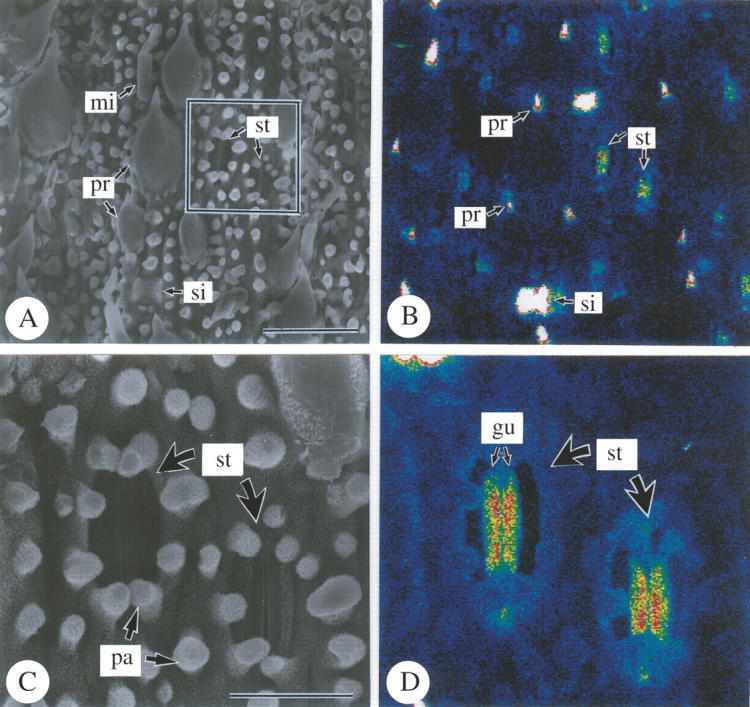
Scanning electron microphotographs of abaxial surfaces of freeze-dried developing leaves of Pleioblastus chino (10 cm in length) 7 cm above the base of leaf blade. (A and C) Secondary electron images; (B and D) silicon distribution images by SEM-EDX; (A and B) abaxial epidermis; (C and D) stomatal complex. gu, Guard cell; mi, micro hair; pa, papillae; pr, prickle hair; si, silica cell; st, stomatal complex. Scale bars: A and B = 50 µm; C and D = 10 µm.
DISCUSSION
It has been reported that in bamboo leaves silica is continuously accumulated in the tissues throughout their life, not only during the course of leaf opening, but also after the opening (Ueda and Ueda, 1961; Kaneko, 1995; Motomura et al., 2002). In the present study, the silica deposition process in each cell type, as observed with SEM-EDXA, revealed that conspicuous silica deposition in the abaxial epidermis of the bamboo leaf before opening occurred first in the prickle hairs, and then in the silica cells and guard cells as the leaves expanded before the opening. This study is the first report for bamboo on silica deposition at the cell-type level in expanding leaves before they open.
The first prominent silica deposition in a leaf of Pleioblastus chino was observed in the point-tips of prickle hairs at the stage just after cell differentiation. In the abaxial epidermis of sugarcane, the first silica deposition was reported in point-tips of prickle hairs as well as in the basal cells of micro hairs at the stage when these types of cell were fully differentiated in very young leaf blades but were still completely enclosed within the next outer leaf (Sakai and Sanford, 1984). Prickle hairs were suggested to have two types of silica deposits with different refractive indices under the light microscope (Baker, 1960; Jones et al., 1963; Parry and Smithson, 1964; Hodson, 1986): one has a transparent deposition in cell walls of prickle hairs, and another has a translucent deposition in the central portion of the hairs or in the lumen surmounted by the aforesaid deposition. Baker (1960) observed that the transparent deposition occurred initially in prickle hairs, although early silica deposition is not easily detected under a light microscope. As it has already been demonstrated by SEM-EDXA, prickle hairs deposit silica densely, not only in the point-tips but also in the body of fully differentiated leaves, about 1–2 months after leaf opening in P. chino (Motomura et al., 2000), the prominent silica deposition restricted only to the point-tips of prickle hairs before leaf opening, shown by SEM-EDXA in the present study, clearly indicated that silica deposition starts at the stage just after cell differentiation and before leaf opening.
Silica cells also deposited silica conspicuously before leaf opening in Pleioblastus chino. In fully differentiated leaves of about 1–2 months after leaf opening, nearly all silica cells have already deposited dense silica (approx. 100 % in cell ratio) in P. chino and Sasa veitchii (Motomura et al., 2000, 2004). Motomura et al. (2004) demonstrated, in S. veitchii, that silica cells in older leaves, 2 years after leaf opening, deposited large amounts of silica. Silica cells usually differentiate in the epidermis of plants in the family Poaceae, and in the grass leaf silica cells deposit silica during leaf expansion with no further deposition after the full expansion (Prat, 1932; Blackman, 1968; Sangster, 1970a, b, 1977). The conspicuous silica deposition in silica cells at leaf opening and expanding stages of P. chino clearly indicates that silica cells deposit large amounts of silica in the early stage of leaf development and none after the opening, whether grass or bamboo.
Among the two cell types forming the stomatal complex, conspicuous but not dense silica deposition progressed in only the guard cells before leaf opening in Pleioblastus chino, while in fully differentiated leaves about 1–2 months after leaf opening of P. chino silica deposition in guard cells was not conspicuous (Motomura et al., 2000), agreeing with the observation that in grass leaves guard cells usually deposit little silica in fully differentiated leaves after the leaf opening. Thus, the observations on guard cells before the leaf opening in this study do not agree with those on fully differentiated leaves after the opening. Motomura et al. (2000) have demonstrated in P. chino that conspicuous but not dense silica deposition occurs over the entire surface of abaxial epidermis in young leaves about 1–2 months after the opening of the leaf blades. This disagreement, which has arisen regarding the guard cells in the leaves before opening and after, is attributed to the fact that conspicuous silica deposition occurs rapidly in the guard cells before other epidermal cell types deposit silica. In contrast, silica deposition in subsidiary cells was not conspicuous in the opening and expanding process of a leaf. Silica deposition in subsidiary cells was usually low in fully differentiated leaves of about 1–2 months in P. chino and S. veitchii (Motomura et al., 2000, 2004). Motomura et al. (2004) demonstrated in S. veitchii that subsidiary cells in leaves aged 2 years deposited silica at a low level. Thus, it can be concluded that subsidiary cells consistently deposit a little silica throughout their long life.
As discussed above, conspicuous silica deposition occurred in three cell types in the abaxial epidermis of leaf blades before opening. In particular, silica deposition of silica cells is noteworthy, as this cell type conspicuously deposits silica before leaf opening but not conspicuously after leaves have opened similar to silica deposition in other types of epidermal cell. In a previous study (Motomura et al., 2004), it was revealed that other cell types in the leaf epidermis of bamboo are categorized into two groups by the silica deposition pattern: (1) silica was deposited densely and continuously after the leaf opening (bulliform cells, micro hairs and prickle hairs); (2) silica was consistently deposited at low levels (cork cells, guard cells, long cells and subsidiary cells). Taking the silica deposition pattern of silica cells into consideration, cell types in the leaf epidermis of bamboo are classified into three groups. Silica deposition in silica cells is deduced to be positive as a part of the physiological activities of leaves in the family Poaceae, although a cytological study on the silica deposition process is still unknown.
Acknowledgments
We thank the Forestry and Forest Products Research Institute for providing facilities. This study was carried out under the collaboration between FFPRI and Prof. Suzuki of Tohoku University. We also thank Dr Chrissie Prychid for critically reviewing the manuscript prior to publication.
LITERATURE CITED
- Baker G. 1960. Hook-shaped opal phytoliths in the epidermal cells of oats. Australian Journal of Botany 8: 69–74. [Google Scholar]
- Blackman E. 1968. The pattern and sequence of opaline silica deposition in rye (Secale cereale L.). Annals of Botany 32: 207–218. [Google Scholar]
- Epstein E, Bloom AJ. 2005. Mineral nutrition of plants, 2nd edn. Sunderland, MA: Sinauer Associates.
- Hodson MJ. 1986. Silicon deposition in the roots, culm and leaf of Phalaris canariensis L. Annals of Botany 58: 167–177. [Google Scholar]
- Jones LHP, Handreck KA. 1967. Silica in soils, plants, and animals. Advances in Agronomy 19: 107–149. [Google Scholar]
- Jones LHP, Milne AA, Wadham SM. 1963. Studies of silica in the oat plant. II. Distribution of the silica in the plant. Plant and Soil 18: 358–371. [Google Scholar]
- Kaneko S. 1995. Seasonal change of nutrient concentrations in Phyllostachys bambusoides and Phyllostachys pubescens. Bamboo Journal 13: 27–33 [in Japanese]. [Google Scholar]
- Ma JF. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition 50: 11–18. [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd edn. San Diego, CA: Academic Press.
- Motomura H, Fujii T, Suzuki M. 2000. Distribution of silicified cells in the leaf blades of Pleioblastus chino (Franchet et Savatier) Makino (Bambusoideae). Annals of Botany 85: 751–757. [Google Scholar]
- Motomura H, Mita N, Suzuki M. 2002. Silica accumulation in long-lived leaves of Sasa veitchii (Carrière) Rehder (Poaceae, Bambusoideae). Annals of Botany 90: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura H, Fujii T, Suzuki M. 2004. Silica deposition in relation to ageing of leaf tissues in Sasa veitchii (Carrière) Rehder (Poaceae: Bambusoideae). Annals of Botany 93: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DW, Smithson F. 1964. Types of opaline silica depositions in the leaves of British grasses. Annals of Botany 28: 169–185. [Google Scholar]
- Prat H. 1932. L'Epiderme des graminées. Annales Sciences Naturelles Botanique 10 série 14: 117–329. [Google Scholar]
- Prychid CJ, Rudall PJ, Gregory M. 2003. Systematics and biology of silica bodies in monocotyledons. The Botanical Review 69: 377–440. [Google Scholar]
- Raven JA. 1983. The transport and function of silicon in plants. Biological Review 58: 179–207. [Google Scholar]
- Richmond KE, Sussman M. 2003. Got silicon? The non-essential beneficial plant nutrient. Current Opinion in Plant Biology 6: 268–272. [DOI] [PubMed] [Google Scholar]
- Sakai WS, Sanford WG. 1984. A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscopy and energy dispersive X-ray analysis. American Journal of Botany 71: 1315–1322. [Google Scholar]
- Sangster AG. 1970a. Intracellular silica deposition in immature leaves in three species of the Gramineae. Annals of Botany 34: 245–257. [Google Scholar]
- Sangster AG. 1970b. Intracellular silica deposition in mature and senescent leaves of Sieglingia decumbens (L.) Bernh. Annals of Botany 34: 557–570. [Google Scholar]
- Sangster AG. 1977. Characteristics of silica deposition in Digitaria sanguinalis (L.) Scop. (crabgrass). Annals of Botany 41: 341–350. [Google Scholar]
- Sangster AG, Hodson MJ. 1986. Silica in higher plants. In: Evered D, O'Connor M, eds. Silicon biochemistry. Chichester: Wiley, 90–111. [DOI] [PubMed]
- Spurr AR. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- Ueda K, Ueda S. 1961. Effect of silicic acid on bamboo-growth. Bulletin of the Kyoto University Forests 33: 79–99 [in Japanese]. [Google Scholar]



