Abstract
• Background and Aims Germination and establishment of seeds are complex traits affected by a wide range of internal and external influences. The effects of parental temperature preconditioning and temperature during germination on germination and establishment of Arabidopsis thaliana were examined.
• Methods Seeds from parental plants grown at 14 and at 22 °C were screened for germination (protrusion of radicle) and establishment (greening of cotyledons) at three different temperatures (10, 18 and 26 °C). Seventy-three accessions from across the entire distribution range of A. thaliana were included.
• Key Results Multifactorial analyses of variances revealed significant differences in the effects of genotypes, preconditioning, temperature treatment, and their interactions on duration of germination and establishment. Reaction norms showed an enormous range of plasticity among the preconditioning and different germination temperatures. Correlations of percentage total germination and establishment after 38 d with the geographical origin of accessions were only significant for 14 °C preconditioning but not for 22 °C preconditioning. Correlations with temperature and precipitation on the origin of the accessions were mainly found at the lower germination temperatures (10 and 18 °C) and were absent at higher germination temperatures (26 °C).
• Conclusions Overall, the data show huge variation of germination and establishment among natural accessions of A. thaliana and might serve as a valuable source for further germination and plasticity studies.
Keywords: Arabidopsis thaliana, germination, establishment, natural variation, preconditioning, temperature, plasticity
INTRODUCTION
Germination is known to be a complex trait that is affected by interactions between genetic determinants and environmental factors. Whether a seed will germinate or remain dormant is regulated by a wide range of promoting and repressing genes. Several studies have shown that the plant hormones ethylene, gibberellic acid and brassinosteroids promote germination of seeds whereas abscisic acid acts as a strong repressor of germination (Finkelstein et al., 2002; Brady and McCourt, 2003; Gubler et al., 2005). In Arabidopsis, many genes have already been identified that regulate the transition from embryogenesis to seedling growth. Among them are genes that repress germination and maintain embryo dormancy, such as ABI1, 3 and 4 (Abscisic acid insensitive1, 3 and 4), FUS3 (FUSCA3), LEC1 and 2 (Leafy cotyledon1 and 2), and DOG1–7 (Delay of germination), and genes that promote germination, such as CTS (Comatose), SLY1 (Sleepy1) and EIN2 (Ethylene insensitive2) (Steber et al., 1998; Russell et al., 2000; Koornneef et al., 2002; Alonso-Blanco et al., 2003).
The growth potential of the embryo and thus germination can also be influenced by external factors. The mother plant may affect its offspring by one or more mechanisms: genetics, non-Mendelian inheritance (e.g. extrachromosomal or cytoplasmic inheritance), or through information which is passed from the mother to the offspring via chemicals produced by the mother (Roach and Wulff, 1987; Baskin and Baskin, 1998). Furthermore, interactions with the environment experienced by the mother plant, so-called preconditioning effects, are known to alter germination and dormancy behaviour of seeds (reviewed in Baskin and Baskin, 1998).
Large variations in dormancy and germination can be found among and within plant species, which are considered to be adaptations to particular environments (Koornneef et al., 2002). To survive in a particular location, plants have developed mechanisms that ensure seed germination at the most convenient season of the year. As a result of natural fluctuations in the climate, these mechanisms are often plastic and can respond to the actual environment of the seed. Colonizing species such as A. thaliana, which is expanding its geographical range (Hoffmann, 2002), are expected to show plasticity of germination to establish successfully in a new location. Natural selection on germination can be a strong force that filters out many genotypes in the earliest stage of colonization (Donohue et al., 2005b).
Natural variation in A. thaliana has thus far been described for pathogen resistance, plant growth and flowering time (reviewed by Alonso-Blanco and Koornneef, 2000; Mitchell-Olds, 2001; Koornneef et al., 2004).
Studies of recombinant inbred lines (RILs) of A. thaliana between the accessions Ler and Cvi (Alonso-Blanco et al., 2003) revealed seven quantitative trait loci (QTLs) for seed dormancy, the failure of a viable seed to germinate even when given favourable environmental conditions. Van der Schaar et al. (1997), using RILs between Col and Ler, tested seeds that were preconditioned in different environments and stored for 2, 4–5 and 5–8 weeks, for germination in light, in the dark and on paclobutrazol, an inhibitor of gibberellin biosynthesis, respectively. Fourteen QTLs were identified in that study, nine of which were detected in all germination tests. However, five were found only under specific germination conditions, acting on germination in the presence of paclobutrazol with a much lower or zero effect when germination was tested in the darkness and/or light or vice versa. Two other QTLs were found between the accessions Ler and Sha that influenced germination speed (Clerx et al., 2004). Donohue et al. (2005a, b, c) created 120 RILs between Cal and Tac that showed an enormous variability and plasticity of germination in a field study in two different locations using two different seed dispersal times.
In this study the response of germination and establishment of A. thaliana to temperature preconditioning and germination temperature was investigated. For a comprehensive survey, 73 geographically widely distributed accessions were included, taken from parental plants grown at either 14 or 22 °C (preconditioning) and germination was tested at three different germination temperatures (10, 18 and 26 °C). The influence of dormancy on germination was reduced by a minimum after-ripening time of 245 d and a cold treatment at 4 °C for 7 d for the imbibed seeds. Time and percentage germination and establishment were measured and correlated with the accession's geographical origin and its environmental variables, and with seed parameters for possible ecological or physiological correlations.
This extensive survey of germination and establishment and their plasticity in A. thaliana provides basic data for the investigation of the adaptation of natural accessions to their environment as well as the identification and acceleration of the roles of known or newly identified genes in this process.
MATERIALS AND METHODS
Plant material
Accessions either were obtained as single seed descent from the Nottingham Arabidopsis Stock Centre (NASC) or were collected in the wild (Table 1). Accession names are given in italics in the text. Seeds used for the germination screen were obtained from plants grown at 14 and 22 °C under long-day light conditions in controlled environments (Hoffmann et al., 2005). Harvested seeds were stored at room temperature (20–22 °C). The mean storage period, i.e. the period from harvesting to germination, was nearly the same for both preconditioned sets and ranged, depending on the accession, from 35 to 52 weeks. Seeds were placed in 5·5-cm Petri dishes on filter paper soaked with purified water. Plated seeds were chilled at 4 °C for 7 d to break dormancy and then transferred to long-day conditions under three different temperature regimes: 10, 18 and 26 °C. In total, 50 seeds per accession for both preconditioning and germination temperature were screened every day at the same time using two replications with 25 seeds per accession and Petri dish. The same growth chamber was used for all experiments, and accessions were randomly distributed. Germination (emergence of the radicle) and establishment (greening of the cotyledons) was scored each day at the same time. Established seedlings were removed from the Petri dishes.
Table 1.
Mean percentage germination and establishment over two repetitions with 25 seeds each of the 73 accessions used
| Germination |
Establishment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Accession | NASC number | Location | 10 °C | 18 °C | 26 °C | 10 °C | 18 °C | 26 °C |
| Ag-0 | N936 | N45·1′, E1·6′ | 21/25 | 18·5/25 | 16/23·5 | 20·5/24·5 | 18·5/25 | 15/23·5 |
| Alc-0 | N1656 | N40·3′, E3·2′ | 8/22 | 9/21·5 | 2/14·5 | 8/22 | 9/19·5 | 0/14·5 |
| Bad | Badetz, Germany | N51·6′, E11·6′ | 20/24 | 15·5/25 | 18/25 | 20/24 | 15/25 | 17/25 |
| Bla-1 | N970 | N41·1′, E2·5′ | 17·5/24·5 | 24/25 | 21·5/23 | 16·5/24·5 | 22·5/25 | 20·5/23·5 |
| Br-0 | N994 | N49·1′, E16·4′ | 16/24 | 18·5/24 | 16/23 | 15·5/24 | 18/24 | 15·5/23 |
| Bs-2 | N998 | N47·6′, E7·5′ | 13/21·5 | 15/21·5 | 15·5/18·5 | 12·5/21·5 | 14·5/21·5 | 15/16 |
| Bur-0 | N1028 | N53·1′, E9·1′ | 7/5 | 12/16 | 22·5/24 | 7/5 | 11·5/16 | 22·5/24 |
| Can-014 | N1064 | N28′, E15·35′ | 23/– | 23·5/– | 21·75/– | 23/– | 23·5/– | 21·5/– |
| Chi-0 | N1072 | N53·6′, E34·6′ | 23·5/20·5 | 23/23 | 24·5/20·5 | 23·5/20·5 | 22·5/23 | 24·5/20 |
| Chi-122 | N1074 | N53·6′, E34·6′ | –/23·25 | –/23 | –/24·75 | –/23·25 | –/22·5 | –/24 |
| Cit-014 | N1080 | N43·2′, E2·3′ | 16·75/– | 17·75/– | 12·75/– | 16·25/– | 16·5/– | 12·75/– |
| Co | N3180 | N40·1′, E-8·3′ | 23·5/25 | 24/24 | 23/24 | 23/25 | 24/24 | 22/23·5 |
| Col+ | Columbia, Poland | N52·4′, E15·2′ | 17·5/23·5 | 17/24 | 15·5/24·5 | 16·5/23·5 | 16·5/24 | 15/24·5 |
| Ct-122 | N1094 | N37·2′, E15′ | –/19·75 | –/23 | –/25 | –/19·25 | –/22·25 | –/25 |
| Cvi-0 | N902 | N15·3′, E-23·2′ | 20/25 | 25/24·5 | 23·5/24 | 19·5/25 | 25/24·5 | 23·5/24 |
| Di-1 | N1108 | N47·1′, E5·1′ | 21·5/24 | 22·5/25 | 22/24·5 | 21/23·5 | 21·5/25 | 21·5/24·5 |
| Dül | Dülmen, Germany | N51·5′, E7·2′ | 5·5/17·5 | 12/23·5 | 25/25 | 4·5/17·5 | 11/23·5 | 15/25 |
| Edi-0 | N1122 | N55·6′, E-3·1′ | 13/25 | 8·5/25 | 10/25 | 12/25 | 8·5/25 | 10/25 |
| Ely | N6031 | N52·2′, E0·2′ | 20/24·5 | 24·5/24·5 | 18/25 | 20/23·5 | 24·5/24·5 | 16/25 |
| Es-0 | N1145 | N60·1′, E24·4′ | 7/23 | 10/25 | 23/24·5 | 6·5/23 | 10/24·5 | 23/24·5 |
| Est-0 | N1148 | N59′, E25′ | 6·5/17 | 8/23·5 | 12/25 | 6·5/17 | 7/23 | 10/24·5 |
| Flo | N6002 | N43·5′, E11·2′ | 12/24·5 | 8/25 | 16/24·5 | 11·5/24·5 | 7·5/25 | 13/24 |
| Gat | Gatersleben, Germany | N51·5′, E11·2′ | 19·5/24 | 19·5/25 | 21/24 | 19/23·5 | 19·5/25 | 21/24 |
| Gie | Gievenbeck, Germany | N51·6, E7·3′ | 12·5/23·5 | 21/25 | 17/25 | 12·5/22·5 | 20·5/25 | 17/25 |
| Gre-0 | N1210 | N43·1′, E-85·2′ | 24·5/24 | 25/24·5 | 22·5/25 | 24/24 | 25/24·5 | 22/25 |
| Han | Handorf, Germany | N51·6′, E7·4′ | 6·5/20·5 | 14/23·5 | 16·5/24·5 | 6·5/20·5 | 12·5/23·5 | 16·5/24·5 |
| HOG | N922 | N38·5′, E68·5′ | 15/19 | 15·5/21·5 | 20/22·5 | 14·5/18·5 | 15/21 | 19·5/21·5 |
| In-0 | N1238 | N47·2′, E11·2′ | 14·5/25 | 15·5/25 | 18/23·5 | 14/24·5 | 15/25 | 16·5/23·5 |
| Ita-014 | N1244 | N34·1′, E-4·1′ | 22·5/– | 22/– | 23·75/– | 22·5/– | 21·5/– | 23·75/– |
| Kaz-1 | N22458 | N49·5′, E73·1′ | 9·5/21 | 16/21 | 15·5/23·5 | 9·5/20 | 16/21 | 15/23·5 |
| Kaz-2 | N22459 | N49·5′, E73·1′ | 7/21 | 13/22 | 15·5/22·5 | 7/20·5 | 13/22 | 13·5/22 |
| Kaz-3 | N22460 | N49·5′, E73·1′ | 15/24 | 15·5/24 | 15/22·5 | 14/23·5 | 14·5/23·5 | 13/22 |
| KEN | N8142 | N41·5′, E-72·4′ | 18·5/25 | 17/24·5 | 19/24·5 | 18/25 | 17/24·5 | 18/24·5 |
| Kent | N6007 | N51·2′, E0·4′ | 17/24·5 | 18/24·5 | 15/23·5 | 16/24·5 | 17/24·5 | 9/23·5 |
| Kga | N22493 | N62·1′, E34·2′ | 16/17 | 18/21 | 20·5/23·5 | 16/17 | 17·5/21 | 20·5/23 |
| Kn-0 | N1286 | N54·5′, E23·5′ | 17·5/23·5 | 15/22 | 18·5/24 | 16·5/23 | 15/21 | 16·5/21 |
| Ko-214 | N1288 | N55·4′, E12·3′ | 20·75/– | 21·25/– | 23·75/– | 20·5/– | 21/– | 22·75/– |
| Köl | N6003 | N50·6′, E6·6′ | 5/20·5 | 4/21 | 7·5/24·5 | 3·5/20·5 | 2·5/20·5 | 3·5/24 |
| Kondara | N916 | N38·5′, E68·5′ | 17·5/25 | 19·5/25 | 19/24·5 | 17/25 | 19·5/25 | 17/24·5 |
| La-0 | N1298 | N52·4′, E15·2′ | 14/24 | 17·5/24·5 | 20·5/24 | 14/24 | 16/24·5 | 20/24 |
| Lim | N8070 | N40·3′, E-75·3′ | 14/24 | 16/22·5 | 20/25 | 14/24 | 15·5/22·5 | 18/24 |
| Lip-0 | N1336 | N53·1′, E20·1′ | 3/18 | 6/22·5 | 16·5/25 | 3/18 | 6/22·5 | 15·5/25 |
| Lis | N1342 | N52·2′, E4·3′ | 12/25 | 12/25 | 11/25 | 11·5/25 | 12/25 | 10·5/25 |
| Ll-2 | N1342 | N41·6′, E2·5′ | 23·5/25 | 23/25 | 24/24 | 23·5/25 | 22·5/25 | 24/24 |
| Mir-0 | N1378 | N45·4′, E13·5′ | 20·5/25 | 14·5/25 | 17·5/24 | 20·5/25 | 14·5/25 | 14/24 |
| Mt-022 | N1381 | N32·4′, E22·5′ | –/8 | –/21·25 | –/24·5 | –/7·75 | –/21 | –/24·5 |
| Oph | Ophain, Belgium | N50·4′, E4·1′ | 21/25 | 19/24 | 22·5/24 | 20·5/24·5 | 17·5/24 | 21·5/24 |
| Oy-022 | N1436 | N60·23′, E6·1′ | –/23·25 | –/22·5 | –/25 | –/23·25 | –/22·5 | –/24 |
| Pa-1 | N1438 | N38·1′, E13·2′ | 18·5/24·5 | 24·5/25 | 16·5/22·5 | 18·5/24·5 | 24·5/25 | 16·5/22 |
| Par | Paris, France | N48·5′, E2·2′ | 11/25 | 7·5/25 | 3/18 | 11/25 | 7·5/25 | 2/17·5 |
| Pog-0 | N1476 | N49·2′, E-123·1′ | 20·5/25 | 12·5/25 | 16/24·5 | 19·5/25 | 11·5/25 | 15·5/24·5 |
| Rsch-0 | N1490 | N56·2′, E34·2′ | 10/21 | 12/23 | 17/24·5 | 9·5/20·5 | 11·5/22·5 | 16/24·5 |
| Rub-1 | N927 | N49′, E38·2′ | 19/23·5 | 19/24·5 | 22·5/25 | 18·5/23·5 | 17·5/24·5 | 22/25 |
| Ryb | N22479 | N61·2′, E35·3′ | 10·5/22 | 13/20 | 13·5/22·5 | 10/22 | 13/20 | 13·5/21·5 |
| Sah-014 | N1500 | N38·5′, E-3·1′ | 17·5/– | 17·75/– | 2·75/– | 17·5/– | 17·75/– | 2·25/– |
| Sea | N6187 | N47·4′, E-122·2′ | 13·5/23·5 | 13·5/25 | 16/23·5 | 13/23·5 | 13/25 | 12·5/22·5 |
| Sed | N6024 | N50·4′, E-3·1′ | 20/24·5 | 20/24·5 | 15/23 | 18/24·5 | 19·5/24 | 15/23 |
| Sha | N929 | N37·3′, E71·3′ | 23/25 | 22/25 | 24·5/24·5 | 23/25 | 22/25 | 23/24·5 |
| Sie | Siena, Italy | N43·2′, E11·2′ | 13·5/25 | 7·5/24·5 | 15/25 | 13·5/25 | 7·5/24·5 | 14·5/25 |
| Sij-1 | Sijjak, Uzbekistan | N41·4′, E70·1′ | 22/24·5 | 25/25 | 24/25 | 22/24·5 | 24·5/24·5 | 23/25 |
| Sij-2 | Sijjak, Uzbekistan | N41·4′, E70·1′ | 23·5/24·5 | 23/25 | 23·5/24·5 | 23/24·5 | 22·5/25 | 23·5/24·5 |
| Sij-322 | Sijjak, Uzbekistan | N41·4′, E70·1′ | –/23 | –/23·5 | –/23·75 | –/23 | –/23 | –/23·5 |
| Sij-4 | Sijjak, Uzbekistan | N41·4′, E70·1′ | 18/24·5 | 22/25 | 20·5/23·5 | 18/24·5 | 21·5/24·5 | 19/23·5 |
| Stoc | N3114 | N59·2′, E18′ | 19·5/25 | 24·5/24·5 | 24/25 | 19·5/25 | 23·5/24·5 | 24/25 |
| Stw-0 | N1538 | N52·6′, E36′ | 17·5/24 | 19·5/25 | 21/24 | 17·5/23·5 | 18·5/25 | 20/22·5 |
| Tol-1 | N8021 | N41·4′, E-83·3′ | 22/25 | 22·5/24·5 | 22·5/21·5 | 21·5/25 | 22·5/24·5 | 22·5/20 |
| Tsar | N22489 | N62′, E34·1′ | 16/21 | 17/24 | 23·5/22 | 16/20·5 | 17/23·5 | 23·5/22 |
| Tsu-0 | N1564 | N34·4′, E136·3′ | 16·5/24 | 19·5/25 | 19/23 | 16·5/24 | 19·5/25 | 18/23 |
| Wa-1 | N1586 | N52·2′, E21′ | 11·5/19 | 17/20·5 | 18/23 | 11/19 | 17/20 | 18/23 |
| Wil-1 | N1594 | N54·4′, E25·2′ | 10/17 | 14/24 | 23/25 | 10/17 | 12·4/24 | 23/24 |
| Ws* | Wassilevskaja, Ukraine | N47·3′, E35·2′ | 13/19 | 15/19·5 | 22/21 | 12/19 | 14·5/19·5 | 20·5/19 |
| Wt22 | Wittenberg, Germany | N51·5′, E12·4′ | –/23·75 | –/22·25 | –/23·25 | –/23·25 | –/21·25 | –/22·25 |
| Yo-014 | N1622 | N37·5′, E-119·3′ | 22·25/– | 23/– | 24/– | 22·25/– | 23/– | 22·75/– |
Accessions without NASC number or indication were collected in the wild. Mean values for germination and establishment are given for 14 °C preconditioned seeds/22 °C preconditioned seeds.
NASC, Nottingham Arabidopsis Stock Centre.
Accessions were kindly supplied by H. Bäumlein, IPK Gatersleben.
22Only seeds from 22 °C progeny were available; 14 only seeds from 14 °C progeny were available.
Seed size measurements
Seeds from mother plants grown at 14 and 22 °C were harvested, and the mean seed length and width of ten seeds per plant (measured under a stereo microscope equipped with an ocular calibrated in 0·2-mm units) was estimated. Seed parameters were measured for the following accessions: Ws, Sie, Col, Oph, Han, Ag-0, Alc-0, Bla-1, Br-0, Bur-0, Chi-0, Co, Di-0, Edi-0, Es-0, Est-0, Ely, Flo, Kent, Kn-0, La-0, Lis, Ll-2, Rsch-0, Rub-1, Sed, Sha, Sij-2, Stw-0, Tsu-0, Kaz-2 and Kaz-3.
Calculations
Percentage germination and establishment were calculated based on the data screened on the 38th day.
Germination percentage and speed of germination of the 61 accessions, for which both preconditioned seed sets were available, were combined into the Timson's index (Timson, 1965): Σn, where n is the cumulative daily germination percentage for each day of the study. The index ranges between 0 (no germination within the 38 d of the experiment) and 3800 (100 % germination within the first 24 h). For better visualization, the quotient of the Timson indices was estimated for 14 and 22 °C preconditioned seeds for each germination temperature (10, 18 and 26 °C). Values ranged from below 1 (14 °C preconditioned seeds germinated less and more slowly than 22 °C preconditioned seeds), around 1 (seed sets germinated with similar percentage and speed) to above 1 (14 °C preconditioned seeds germinated better and faster than 22 °C preconditioned seeds). Additionally, the Timson index was calculated separately for minimum and maximum differences between the two repetitions in each experiment to test for differences between the two preconditioned seed sets. Resulting differences between the two preconditionings were tested with the Kruskal–Wallis test for significance.
Genotypic effects (accession), preconditioning effects (mother plants grown at 14 or 22 °C) and influence of temperature during germination (10, 18, 26 °C) on the duration of germination and establishment of the seedlings were analysed using a multifactorial analysis of variance (ANOVA). The two repeats (Petri dishes) per accession were used to measure germination and establishment and one value was obtioned for each Petri dish. The day when at least 50 % (=13 seeds) of the 25 seeds in both Petri dishes used for one accession were germinated or established was chosen.
Because the Kolmogorov–Smirnov test and the Levene test rejected normal distribution and homology of variances in the data set, respectively, the significance level of F-values were increased to P = 0·01 (SPSS, 1999).
ANOVAs were not calculated for the total percentage of germination because a majority of samples germinated to more than 90 %, so that the estimated variances would all be close to zero.
The non-parametric Spearman-rank correlation was used to calculate correlations between the percentage germination (appearance of the radicle), establishment (greening of cotyledons), seed dimensions (length and width), and geographical latitude and longitude.
RESULTS
For some accessions no progeny could be obtained from either the 14 °C or the 22 °C maternal plants (Table 1). These losses were not correlated with the latitude or longitude or the temperature at the point of origin (data not shown).
Percentage of germination and establishment
Germination
The percentage germination of the two preconditioned sets differed between the three temperature regimes (Table 1). It was in the range 4–100 % in the 10 °C experiment, 12–100 % in the 18 °C experiment and 0–100 % in the 26 °C experiment. The 14 °C preconditioned set of most accessions had a lower and more diverse germination rate between accessions than the 22 °C preconditioned set in all three temperature treatments (Fig. 1). Nevertheless, there were some exceptions. The 14 °C set germinated better than the 22 °C set for the following accessions: Chi-0 and Gre-0 at the 10 °C treatment; Cvi-0 and Gre-0 at the 18 °C treatment; and Chi-0, Ws, Tol-1 and Tsar at the 26 °C treatment. In none of the tested accessions germinated did the 14 °C set better than the 22 °C set in all three temperature treatments.
Fig. 1.
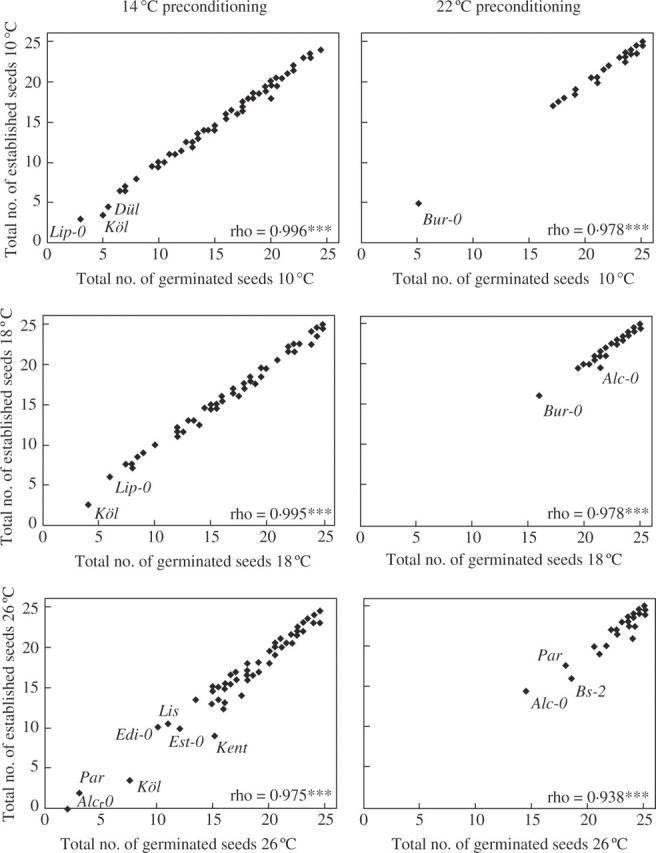
Scatterplots of germination and establishment for the 61 accessions, where 14 °C and 22 °C preconditioned seed sets were available. The mean number of the two repetitions is plotted separately for each combination of preconditioning and germination temperature. Spearman-rank correlation coefficient between germination and establishment is indicated for each plot separately. Accessions with low germination/establishment are indicated.
Accessions Köl and Lip-0 had a low germination over all treatments when preconditioned at 14 °C but this did not occur when Köl and Lip-0 were preconditioned at 22 °C. The 22 °C preconditioned seeds of Bur-0 showed reduced germination and establishment at lower germination temperatures, whereas this reduction occurred in Alc-0 when germinated at higher temperatures (Fig. 1).
Establishment
As expected, establishment was closely associated with germination (Table 1, Fig. 1) and it ranged from 4 to 100 % in the 10 °C experiment, 8 to 100 % in the 18 °C experiment and 0 to 100 % in the 26 °C experiment. The rate of germination and establishment for the 22 °C preconditioned set was higher than 95 % in all three temperature treatments for the 18 following accessions: Oph, Bad, Cvi-0, Edi-0, Gre-0, In-0, KEN, Kondara, La-0, Ll-2, Stoc, Mir-0, Flo, Lis, Pog-0, Sha, Sij-1 and Sij-2. Remarkably there was no accession for which the 14 °C preconditioned set showed germination and establishment higher than 95 % in all three temperature treatments. There were, however, large differences between the treatments and preconditioned sets within the same accession. For example, Alc-0 showed no establishment at all at 26 °C when preconditioned at 14 °C (8 % germination, 0 % establishment), but a reasonable establishment at 26 °C (58 % germination, 58 % establishment) after preconditioning at 22 °C (Fig. 1).
Germination speed and rate
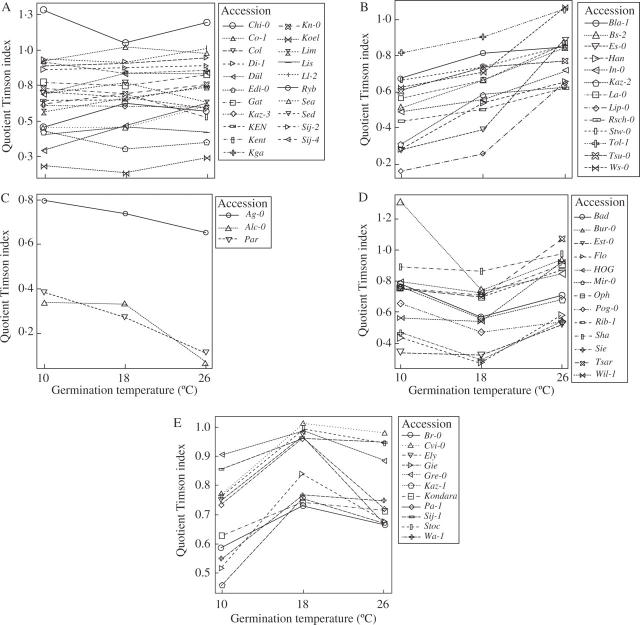
The Timson index, combining germination percentage and speed, was estimated among the 61 accessions for which seeds preconditioned at both temperatures were available. For better visualization of the data, the quotient of the Timson index was calculated separate from the estimations of the Timson index for 14 and 22 °C preconditioned seeds (Fig. 2). Twenty-one accessions showed no significant differences at the 5 % level for either of the three temperature treatments (Fig. 2A). Chi-0 was the only accession that had a quotient above 1·0 for all three temperature treatments, indicating that the 14 °C preconditioned seeds germinated faster and to a higher percentage than the 22 °C preconditioned seeds. All other accessions, which showed no significant differences, had quotients around or below 1·0 for all three temperature treatments, indicating that the 14 °C preconditioned seeds germinated at equal speed and percentage to the 22 °C preconditioned seeds or performed worse. A quotient of less than 0·25 for Köl revealed a much higher and faster germination for the 22 °C preconditioned seeds for all temperature treatments.
Fig. 2.
Timson's index (Timson, 1965) for 61 accessions, where 14 °C and 22 °C preconditioned seed sets were available. The index was estimated separately for the 14 and 22 °C preconditioned sets and the quotient of (14 °C Timson index)/(22 °C Timson index) is shown. Accessions are sorted according to their response patterns. (A) Quotient is not significantly different from 1 (P = 0.05, Kruskal–Wallis test) in either of the three temperature regimes (10, 18, 26 °C). (B–E) Quotient is significantly different from 1 (P = 0.05, Kruskal–Wallis test) in at least one of the three temperature regimes. Accessions are separated according to their gradient in (B–E).
There were significant differences for all other accessions for at least one temperature treatment. Thirteen accessions had a positive gradient (Fig. 2B), indicating a better performance of the 14 °C preconditioning at the higher temperature treatments, whereas only three accessions had a negative gradient (Fig. 2C), two of which had very low quotients of around 0·3. Thirteen accessions gave a concave (Fig. 2D) and 11 accessions a convex shape (Fig. 2E), indicating an increasing or decreasing effect of 14 °C preconditioning on germination percentage and speed for intermediate temperatures (18 °C), respectively.
Duration of germination and establishment
As might be expected, there were differences in the duration of germination between the three temperature experiments. The majority of seeds in the 10 °C experiment germinated on the 4th and 5th day after chilling and established 2–3 d later. In the 18 °C experiment the majority of seeds had already germinated on the 2nd and 3rd day and established only 1 d later. The rate of germination and establishment increased further in the 26 °C experiment, in which the majority of seeds germinated on the 1st and 2nd day after chilling and established 1 d later (data not shown).
To determine whether differences in genotype (accession), treatment (10, 18 and 26 °C) and preconditioning (14 and 22 °C) significantly influenced the duration of germination and establishment, multifactorial ANOVAs were carried out. The number of days when at least 50 % of the seeds (=13 of the 25 seeds per repetition for each accession) were germinated or established in both repetitions was used as a trait. This was reached for all precondition and temperature regimes for the following 31 accessions: Ag-0, Bad, Bla-1, Br-0, Chi-0, Co, Cvi-0, Di-1, Ely, Gat, Gre-0, HOG, In-0, KEN, Kent, Ll-2, Mir-0, Oph, Pa-1, Rub-1, Sed, Sha, Sij-1, Sij-2, Sij-4, Stoc, Stw-0, Tol-1, Tsar, Tsu-0, Ws. Accessions were used as fixed and as random factors in the ANOVAs.
Duration of germination
Accession, preconditioning (14, 22 °C), temperature treatment (10, 18, 26 °C), accession × preconditioning, and temperature × accession showed a significant influence on the duration of germination (Table 2). The interaction temperature × preconditioning had no significant effect in the test set. This indicates that the difference for both preconditioned sets (14 and 22 °C) was similar for all three temperature treatments. Therefore, temperature preconditioning did not influence the duration of germination for the accessions included. The temperature during germination had the same effect on the selected accessions for both preconditioned sets.
Table 2.
Multifactorial ANOVA for the duration of germination and establishment
| Duration of germination |
Duration of establishment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of squares | d·f· | Mean square | F | Significance | Sum of squares | d·f· | Mean square | F | Significance |
| Accession | 550·79 | 30 | 18·36 | 6·74 | 0·000 | 255·06 | 28 | 9·11 | 3·35 | 0·000 |
| Preconditioning | 237·12 | 1 | 237·12 | 87·08 | 0·000 | 260·35 | 1 | 260·35 | 95·87 | 0·000 |
| Temperature | 1026·50 | 2 | 513·25 | 188·48 | 0·000 | 1869·17 | 2 | 934·59 | 344·17 | 0·000 |
| Accession × preconditioning | 319·63 | 30 | 10·65 | 3·91 | 0·000 | 185·40 | 28 | 6·62 | 2·44 | 0·000 |
| Temperature × accession | 385·50 | 60 | 6·43 | 2·36 | 0·000 | 196·99 | 56 | 3·52 | 1·30 | 0·105 |
| Temperature × preconditioning | 11·82 | 2 | 5·91 | 2·17 | 0·117 | 4·02 | 2 | 2·01 | 0·74 | 0·478 |
| Temperature × accession × preconditioning | 357·18 | 60 | 5·95 | 2·19 | 0·000 | 195·48 | 56 | 3·49 | 1·29 | 0·112 |
| Error | 506·50 | 186 | 2·72 | 472·50 | 174 | 2·72 | ||||
| Total | 3395·04 | 371 | 3438·97 | 347 | ||||||
Number of days was used where 13 or more seeds were germinated or cotyledons were unfolded. Accessions were only used when both progenies in all repetitions and temperature treatments showed germination or establishment of 13 seeds.
The significance levels in Table 2 are not changed if accessions are treated as random factor in the ANOVA (assuming that a representative sample of each accession was analysed).
Duration of establishment
Accessions Ws and Kent established less than 50 % (13 seeds per repetition) and therefore they were not included in the analysis. The remaining 29 accessions were used to estimate the time to 50 % establishment. Considering all three factors for estimating the ANOVA, again accession, preconditioning (14, 22 °C), temperature treatment (10, 18, 26 °C) and accession × preconditioning had a significant influence on the duration of establishment of the seedlings (Table 2). In contrast to germination, the interaction temperature × accession had no significant influence on the duration of establishment. As for duration of germination, no significant influences of the other interactions were found.
Again, the significance levels in Table 2 are not changed if accessions are treated as random factor in the ANOVA.
Plasticity of germination
Influence of preconditioning at 14 and 22 °C
Plasticity of the two preconditioned sets (14, 22 °C) revealed a similar pattern for germination in the three different temperature treatments for most accessions (Fig. 3A). Only accession Bur-0 showed a different behaviour in the 22 °C preconditioned seed set for the lower germination temperatures (Fig. 3B).
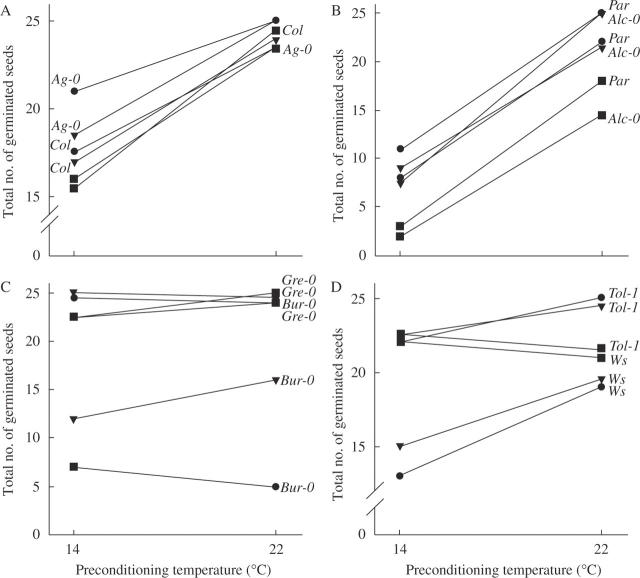
Fig. 3.
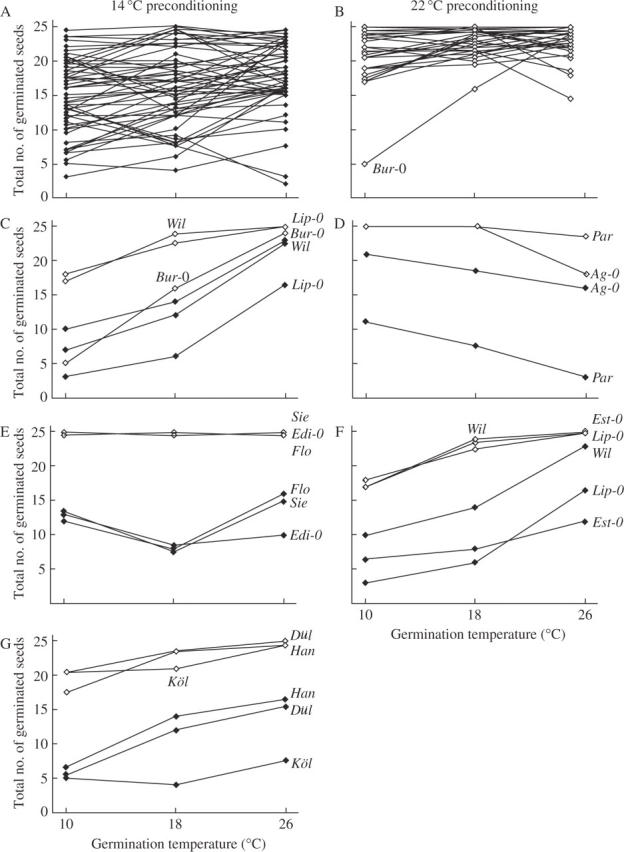
Reaction norms for preconditioning at 14 and 22 °C. Only accessions for which both preconditioned seed sets were available were used. Filled diamonds indicate 14 °C preconditioned seed set, open diamonds indicate 22 °C preconditioned seed set. (A) An overview of all accessions with 14 °C preconditioning; (B) an overview of all accessions with 22 °C preconditioning; (C–G) chosen accessions for comparisons among each other.
Some accessions showed a constant positive gradient for both preconditioning temperatures (e.g. Bur-0, Lip-0 and Wil in Fig. 3C), indicating that plasticity of germination increases with increasing temperatures up to 26 °C. Accessions with a constant negative gradient were Ag-0 and Par (Fig. 3D), indicating a higher plasticity of germination at lower temperatures up to 10 °C. Very plastic accessions were Co, Col, Di-1, Kaz-3, KEN, Ll-2, Lis, Sha and Sij-2 (data not shown), which showed little change in the gradients of the curves for both germination and preconditioning.
Other accessions were highly different between the two preconditioned sets. The most striking differences between the pattern of plasticity between the 14 and 22 °C set were: (1) the 14 °C set gradient was concave whereas the 22 °C set gradient was nearly zero (e.g. Edi-0, Flo and Sie in Fig. 3E); (2) the 14 °C progeny was concave, whereas the 22 °C was convex (e.g. Est-0, Lip-0 and Wil in Fig. 3F); and (3) the gradient of plasticity was similar, but the level of plasticity was different between the 14 and 22 °C set (e.g. Dül, Han and Köl in Fig. 3G).
Influence of preconditioning temperature during germination at 10, 18 and 26 °C
The plasticity of the accessions in the three different temperature treatments was similar (e.g. Ag-0 and Col in Fig. 4A). For most accessions the gradient was very steep between the two preconditioned sets (e.g. Alc-0 and Par in Fig. 4B). Exceptions to this, having a low gradient for all three temperatures, were Di-1, Gat, Kga, Ll-2, Oph, Sha and Sij-2 (data not shown). Different gradients of plasticity between the three temperatures were observed for Bla-1, Bur-0, Co, Cvi-0, Ely, Es-0, Lip, Stoc, Tol-1, Wil, Ws and Tsar. Among these, the following accessions showed a change in the gradient of plasticity for one of the three tested temperatures:
Bur-0 had a negative gradient at 10 °C and a positive gradient at 18 and 26 °C (Fig. 4C), indicating a higher plasticity of the 14 °C set at low temperatures (10 °C). The opposite was true for Tol-1 and Ws, which showed a positive gradient up to 18 °C and then showed a negative gradient at 26 °C (Fig. 4D), indicating a higher plasticity of the 14 °C set at high temperatures (26 °C).
Gre-0 had a negative gradient at lower temperatures (up to 18 °C) and a positive one at 26 °C (Fig. 4C), indicating a higher plasticity of the 22 °C set at high temperatures (26 °C).
Bla-1, Co, Cvi-0, Ely, Sij-1 and Stoc had a positive gradient of plasticity at 10 and 26 °C but a negative one at 18 °C, indicating a higher plasticity of the 22 °C set at moderate temperatures (18 °C; data not shown).
Fig. 4.
Reactions norms for germination temperatures at 10, 18 and 26 °C. Only accessions for which both preconditioned seed sets were available were used. Diamonds indicate germination at 10 °C, triangles germination at 18 °C and squares germination at 26 °C. (A–D) Chosen accessions for comparison among each other.
Correlations of germination and establishment with geographical coordinates, temperature and precipitation of origin and seed length and width
The correlation with geographical coordinates, temperature and precipitation at the origin of each accession was reduced to the 63 accessions from Eurasia. Among these, Wa-0 and Stoc were also excluded because they are tetraploid (Schmuths et al., 2004). Spearman-rank correlations between the percentage germination and establishment with the geographical latitude and longitude revealed significant correlations (Table 3). The percentage germination and establishment in the 14 °C preconditioned set was negatively correlated with the longitude of origin of the accessions. This means the germination and establishment of 14 °C preconditioned seeds decreased the further east that the accession originated. Over all temperature treatments this effect was significant at the P = 0·001 level (Table 3). The effect was more obvious in the lower temperature treatments (10 and 18 °C), whereas it was not detectable in the 26 °C temperature treatment.
Table 3.
Spearman rank correlation of germination and establishment percentages at different preconditionings with the longitude of origin of the accessions and seed length and width
| Longitude of origin correlated with: |
Seed width correlated with: |
Seed length correlated with: |
||||
|---|---|---|---|---|---|---|
| Percentage germination after 14 °C preconditioning | Percentage establishment after 14 °C preconditioning | Percentage germination after 22 °C preconditioning | Percentage establishment after 22 °C preconditioning | Percentage germination after 22 °C preconditioning | Percentage establishment after 22 °C preconditioning | |
| 10 °C treatment | −0.405*** | −0·411*** | 0·488** | 0·491** | 0·104 | 0·193 |
| 18 °C treatment | −0·365** | −0·364** | 0·489** | 0·414 | 0·354* | 0·348 |
| 26 °C treatment | −0·06 | 0·028 | −0·219 | −0·089 | 0·297 | 0·331 |
| All treatments | −0·240*** | −0·240*** | 0·275** | 0·304*** | 0·237* | 0·282** |
Significant at *5 % level, **1 % level, ***0.1 % level.
Germination and establishment of the 14 °C preconditioned seed set were positively correlated with the temperatures occurring from April to October at the place of origin, with P-levels between 0·05 and 0·01 (Spearman-rank correlation). Precipitation at the site of origin between January–April and October–December was positively correlated with P levels between 0·05 and 0·01. Thus, the higher the temperature and precipitation during these months, the higher the observed germination and establishment. The significant correlations found for the 14 °C preconditioned seed were mostly due to the 10 °C treatment, were reduced for the 18 °C treatment and were absent when seeds were germinated at 26 °C.
The 22 °C preconditioned seeds showed positive Spearman-rank correlations between germination and establishment and the temperatures from January to May and August to December as well as precipitation during March, April and October–December (P between 0·01 and 0·05). Again, these effects were mainly due to the 10 °C treatment, and there were no correlations at the two higher germination temperatures.
Seed length and width were measured for 32 accessions (see Materials and Methods) and resulted in a positive Spearman's rho when correlated with germination and establishment of the 22 °C preconditioned seed set with seed width (Table 3). The wider the seeds, the higher their percentage germination and establishment. Again, this correlation could only be observed for the colder treatments, but was absent for the 26 °C treatment (Table 3). Across all treatments the effect was significant at P = 0·01. A positive correlation was found between seed length and germination and establishment of the 22 °C preconditioned seed set. This effect was significant over all treatments and for germination at 18 °C (Table 3).
DISCUSSION
Screening 73 temperature-preconditioned accessions from across the entire distribution range of A. thaliana for germination and establishment at three different temperatures revealed huge natural variability for these traits. According to Sultan (2004), growing differently preconditioned seed sets in contrasting environmental conditions rather than in a single ‘control’ environment enabled us to study an adequate range of plasticity of the accessions.
After-ripening time and conditions are known to effect germination. Cvi-0, one of the most dormant natural accessions identified so far, needed between 74 and 185 d of after-ripening depending on storage conditions (Alonso-Blanco et al., 2003). To minimize the effect of dormancy, we used a minimum after-ripening time of 245 d at room temperature and stratified imbibed seeds at 4 °C for 7 d. Stratification is well known to break dormancy. Four days of cold treatment broke dormancy completely for accession Cvi-0 (Ali-Rachedi et al., 2004), and dormant Ler seeds showed germination of about 55 % after 2 d and nearly 75 % after 4 d (Russell et al., 2000). Clerx et al. (2004) detected four QTLs on different chromosomes of A. thaliana affecting the dormancy of seeds in RILs between Ler and Sha. Interestingly, the Sha alleles of all four loci increased seed dormancy. In the experiments here, Sha showed a continuous high percentage of germination and establishment of more than 85 % for all tested treatments. Therefore, the dormancy-breaking treatments used in this study should have been sufficient to break dormancy and thus enabled study of germination rather than dormancy of A. thaliana.
Despite the nearly complete breakage of dormancy, a broad range of different germination rates influenced by genotype, preconditioning temperature and germination temperature was found. This might imply that some accessions are so dormant that even the treatments applied do not break dormancy or that these accessions were still dormant and did not respond to cold. Non-germinated seeds might also have fallen into secondary dormancy, a state of dormancy that is induced when environmental conditions are unfavourable for germination of after-ripened seeds (Baskin and Baskin, 1998). Cycling of secondary dormancy on a seasonal basis is known to be a common phenomenon in weed seed banks (Hilhorst and Toorop, 1997).
The study presented here shows that once a seed has germinated, in all probability it will establish. This was true for most accessions, but there were some remarkable exceptions for both preconditioned seed sets when germinated at 26 °C (Fig. 1). It appears that high temperature influences establishment negatively for some accessions (e.g. Kent and Par). This effect seemed to become more pronounced when seeds were preconditioned at 14 °C.
The data generally showed that germination and establishment were significantly influenced by the temperature at which the seeds germinated and by the temperature preconditioning of the mother plant. It is well known that temperature and photoperiod have preconditioning effects on germination response in many plant species. Species from a wide range of plant families, life-cycle types and plant communities exhibit differences in germination characteristics of seed collected in locations with different temperature regimes (Baskin and Baskin, 1998).
Maternal photoperiod can also influence the percentage of seeds that germinated and the speed of germination in A. thaliana (Munir et al., 2001). Short-day preconditioning of RILs between the accessions Cal-0 and Tac increased germination percentage and speed in stratified seeds but inhibited germination in unstratified seeds (Munir et al., 2001).
Here, seeds of A. thaliana preconditioned at 22 °C showed a better germination performance than seeds preconditioned at 14 °C. This confirms previous studies that found that seeds produced by plants grown at high temperatures have higher germination percentages and/or rates than those grown at low temperatures (e.g. Beta vulgaris, Lexander, 1980; Avena fatua, Peters, 1982; Plantago lanceolata, Alexander and Wulff, 1985). The better germination performance of the 22 °C preconditioned seeds agrees with the temperature experienced by most plants in the wild during ripening. Seed maturation of A. thaliana occurs in the wild in May/June, when temperatures are about 20 °C during the day in most parts of the species range (Hoffmann, 2002). This might imply that seeds in the wild are preconditioned in a temperature similar to the 22 °C treatment tested here and may show a better germination performance over a wide range of temperatures in the wild.
In contrast to seeds in natural conditions, the seeds used here were exposed to cold during imbibition. Temperature during imbibition is known to influence germination behaviour. Dormant Cvi-0 seeds did not germinate or germinated poorly when imbibed and grown at 20–27 °C, but germinated efficiently when imbibed and grown at 13 °C (Ali-Rachedi et al., 2004).
For colonizing species such as A. thaliana, which is expanding its geographical range (Hoffmann, 2002), an appropriate dormancy and germination response to varied environmental conditions is the first requirement for successful establishment in a new location. Natural selection on germination can be a strong force that filters out many genotypes in the earliest stage of colonization (Donohue et al., 2005b). Donohue et al. (2005a, b, c) tested 120 RILs between the accessions Tac (Takoma, Washington State) and Cal (Calver, England) in two different natural sites (Rhode Island and Kentucky). Germination timing was inappropriately changed in response to geographical location in their sample, demonstrating an inability to adjust germination to different locations. Furthermore, genotypes with high germination success in one site had low germination success in the other and vice versa.
Conditions experienced after dispersal can determine germination timing (Alexander and Wulff, 1985; Munir et al., 2001). Therefore, germination is highly plastic in response to environmental conditions experienced both during seed maturation and after dispersal (Donohue et al., 2005a). The high plasticity of germination found here among 73 natural accessions may suggest reduced pressure of natural selection on this trait. This might help A. thaliana to survive in the different conditions found in its natural range when it is widely distributed. An identical set of accessions was tested by Hoffmann et al. (2005) for expression of morphological and phenological characters at two different temperatures (14 and 22 °C) and revealed few signs of fixed local adaptation.
Depending on the species, germination response varies with latitude, elevation, soil moisture, soil nutrients and temperature (Baskin and Baskin, 1998). A correlation was found here between germination and establishment with the longitude of the origin of the accessions only in the 14 °C preconditioned set. This indicates that plants originating from further east respond with a higher decrease in germination following 14 °C preconditioning than plants originating further westward. However, this correlation is very weak and three accessions from Uzbekistan (Sij-1, -2 and -4) showed a high percentage of germination in all treatments; this result should therefore not be generalized.
This survey of an adequate sample of A. thaliana provides hints at which naturally occurring accessions might be suitable for more detailed analysis, and depending on to the question of interest, the results might help in choosing appropriate accessions. Further studies will be needed to provide detailed insight into the mechanism of germination and establishment.
Acknowledgments
We would like to thank C. Koch and B. Sperling for excellent technical assistance, and M. Holdsworth, M. Koornneef and an anonymous reviewer for helpful comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft to K.B.
LITERATURE CITED
- Alexander HM, Wulff RD. 1985. Experimental ecology genetics in Plantago. X. The effects of maternal temperature on seed and seedling characters in P. lanceolata. Journal of Ecology 73: 271–282. [Google Scholar]
- Ali-Rachedi S, Bouinot D, Wagner M-H, Bonnet M, Sotta B, Grappin P, Jullien M. 2004. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 219: 479–488. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Koornneef M. 2000. Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends in Plant Science 5: 22–29. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M. 2003. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164: 711–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. 1998. Seeds: ecology, biogeography, and evolution of dormancy and germination. New York: Academic Press.
- Brady SM, McCourt P. 2003. Hormone cross-talk in seed dormancy. Journal of Plant Growth and Regulation 22: 25–31. [Google Scholar]
- Clerx EJM, El-Lithy ME, Vierling E, Ruys GJ, Blankestijn-De Vries H, Groot SPC, Vreugdenhil D, Koornneef M. 2004. Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiology 135: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Dorn LA, Griffith C, Kim E, Aguilera A, Polisetty CR, Schmitt J. 2005a. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 59: 740–757. [PubMed] [Google Scholar]
- Donohue K, Dorn LA, Griffith C, Kim E, Aguilera A, Polisetty CR, Schmitt J. 2005b. The evolutionary ecology of seed germination of Arabidopsis thaliana: variable natural selection on germination timing. Evolution 59: 758–770. [PubMed] [Google Scholar]
- Donohue K, Dorn LA, Griffith C, Kim E, Aguilera A, Polisetty CR, Schmitt J. 2005c. Niche construction through germination cueing: life-history responses to timing of germination in Arabidopsis thaliana. Evolution 59: 771–785. [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. 2002. Abscisic acid signalling in seeds and seedlings. The Plant Cell 14: S14–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV. 2005. Dormancy release, ABA and pre-harvest sprouting. Current Opinion in Plant Biology 8: 183–187. [DOI] [PubMed] [Google Scholar]
- Hilhorst HWM, Toorop PE. 1997. Review on dormancy, germinability, and germination in crop and weed seeds. Advances in Agronomy 61: 111–165. [Google Scholar]
- Hoffmann MH. 2002. Biogeography of Arabidopsis thaliana (L.) Heynh. Brassicaceae). Journal of Biogeography 29: 125–134. [Google Scholar]
- Hoffmann MH, Tomiuk J, Schmuths H, Koch C, Bachmann K. 2005. Phenological and morphological responses to different temperature treatments differ among a world-wide sample of accessions of Arabidopsis thaliana. Acta Oecologica 28: 181–187. [Google Scholar]
- Koornneef M, Bentsik L, Hilhorst H. 2002. Seed dormancy and germination. Current Opinion in Plant Biology 5: 33–36. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D. 2004. Naturally occurring genetic variation in Arabidopsis thaliana. Annual Review in Plant Biology 55: 141–72. [DOI] [PubMed] [Google Scholar]
- Lexander K. 1980. Seed composition in connection with germination and bolting of Beta vulgaris L. (sugar beet). In: Hebblethwaite PD, ed. Seed production. London: Butterworths, 271–291.
- Mitchell-Olds T. 2001. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecology 16: 693–700. [Google Scholar]
- Munir J, Dorn LA, Donohue K, Schmitt J. 2001. The effect of maternal photoperiod on seasonal dormancy in Arabidopsis thaliana (Brassicaceae). American Journal of Botany 88: 1240–1249. [PubMed] [Google Scholar]
- Peters NCB. 1982. The dormancy of wild oat seed (Avena fatua L.) from plants grown under various temperature and soil moisture conditions. Weed Research 22: 205–212. [Google Scholar]
- Pigliucci M. 2002. Ecology and evolutionary biology of Arabidopsis. In: Somerville C, Meyerowitz E, eds. The Arabidopsis book. Rockville, MD: American Society of Plant Biologists, 1–20. [DOI] [PMC free article] [PubMed]
- Roach DA, Wulff RD. 1987. Maternal effects in plants. Annual Review of Ecology and Systematics 18: 209–235. [Google Scholar]
- Russell L, Larner V, Kurup S, Bougourd S, Holdsworth MJ. 2000. The Arabidopsis COMATOSE locus regulates germination potential. Development 127: 3759–3767. [DOI] [PubMed] [Google Scholar]
- Schmuths H, Meister A, Horres R, Bachmann K. 2004. Genome size variation among accessions of Arabidopsis thaliana. Annals of Botany 93: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. 1999. SPSS for Windows. Chicago, IL: SPSS Inc.
- Steber CM, Cooney S, McCourt P. 1998. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149: 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S. 2004. Promising directions in plant phenotypic plasticity. Perspectives in Plant Ecology, Evolution and Systematics 6: 227–233. [Google Scholar]
- Timson J. 1965. New method of recording germination data. Nature 207: 216–217. [Google Scholar]
- Van der Schaar W, Alonso-Blanco C, Leon-Kloosterziel KM, Jansen RC, van Ooijen JW, Koornneef M. 1997. QTL analysis of seed dormancy in Arabidopsis using recombinant inbred lines and MQM mapping. Heredity 79: 190–200. [DOI] [PubMed] [Google Scholar]