Abstract
• Background and Aims The timing of flowering and summer dormancy induction plays a central role in the adaptation of Mediterranean geophytes to changes in the length of the growth season along rainfall gradients. Our aim was to analyse the role of the variation in the responses of flowering and summer dormancy to vernalization, daylength and growth temperature for the adaptation of Poa bulbosa, a perennial geophytic grass, to increasing aridity.
• Methods Flowering and dormancy were studied under controlled daylengths [9 h short day (SD) vs. 16 h long day (LD)] and temperatures (16/10, 22/16 and 28/22 °C day/night) in four ecotypes originating in arid, semi-arid and mesic habitats (110, 276 and 810 mm rain year−1, respectively) and differing in flowering capacity under natural conditions: arid–flowering, semi-arid–flowering, semi-arid–non-flowering and mesic–non-flowering.
• Key Results Flowering and dormancy were affected in opposite ways by daylength and growth temperature. Flowering occurred almost exclusively under SD. In contrast, plants became dormant much earlier under LD than under SD. In both daylengths, high temperature and pre-chilling (6 weeks at 5 °C) enhanced dormancy imposition, but inhibited or postponed flowering, respectively. Induction of flowering and dormancy in the different ecotypes showed differential responsiveness to daylength and temperature. Arid and semi-arid ecotypes had a higher proportion of flowering plants and flowering tillers as well as more panicles per plant than mesic ecotypes. ‘Flowering’ ecotypes entered dormancy earlier than ‘non-flowering’ ecotypes, while the more arid the site of ecotype origin, the earlier the ecotype entered dormancy.
• Conclusions Variation in the flowering capacity of ecotypes differing in drought tolerance was interpreted as the result of balanced opposite effects of daylength and temperature on the flowering and dormancy processes.
Keywords: Poa bulbosa, bulbous blue-grass, daylength, temperature, flowering, summer dormancy, aridity, ecotype
INTRODUCTION
The timing of phenological transitions during the life cycle of geophytes inhabiting regions with seasonal changes in growth conditions is of key importance for their survival and reproduction. These transitions, i.e. activation and sprouting of regeneration buds, flowering and dormancy, are usually caused by seasonal changes in environmental factors, such as daylength and temperature (LeNard and De Hertogh, 1993). We argue that ecotypic adaptation of geophytes along gradients of climatic conditions, such as increasing aridity, involves variation in the timing of flowering and dormancy onset due to changes in the responsiveness of these processes to the relevant regulatory environmental factors. Here we study this hypothesis in ecotypes of Poa bulbosa occurring along a steep rainfall gradient and differing in drought tolerance (Ofir and Kigel, 2003). Poa bulbosa is a summer-dormant, small perennial grass geophyte, widely distributed in the Mediterranean and adjacent phytogeographic regions (Davis, 1985). In regions with a Mediterranean type of climate (i.e. mild, rainy winters and dry, hot summers), its small bulbs re-sprout after the first rains in the autumn. The plants grow and flower during the winter and produce new bulbs at the base of the tillers, just below the soil surface, as the plants become dormant at the end of their active growth phase, in early spring (Ofir and Kerem, 1982; Ofir and Dorenfeld, 1992). In some populations, apomictic seeds and/or small vegetative propagules (i.e. bulbils) develop in the inflorescences (Youngner, 1960; Heyn, 1962; Davis, 1985). Summer dormancy in P. bulbosa is induced by long days and accelerated by high temperature (Ofir and Kerem, 1982), while pre-exposure to short days and low temperature enhances dormancy induction by long days (Ofir and Kigel, 1999). A similar mode of environmental control of summer dormancy occurs in Allium cepa (Brewster, 1990) and A. sativum (Kamenetsky et al., 2004). Flowering in P. bulbosa occurs relatively early during the growth season, in late winter, and is highly variable among and within populations, with flowering and non-flowering plants co-occurring in the same population (Heyn, 1962; Ofir and Kerem, 1982; Ofir and Kigel, 2003). In some populations, a high proportion of the plants flower, while in others flowering is absent or rare, and reproduction is mainly vegetative, by tillering and basal tiller bulbs. Since tillering ceases when plants become dormant, variation in the onset of dormancy directly affects flowering potential, as well as the balance between seed and vegetative reproduction.
Previous work with P. bulbosa showed wide variation in the timing of dormancy imposition and in the flowering capacity of populations along a rainfall gradient (Ofir and Kigel, 2003). Furthermore, ecotypes showed a negative relationship between age at dormancy onset and flowering: dormancy was earlier and flowering capacity was higher with increasing aridity at the site of population origin. Here we propose that this ecotypic variation in dormancy onset was due to changes in the responsiveness to long days and increasing temperature, the main regulatory environmental factors leading to summer dormancy in P. bulbosa. On the other hand, little information is available on the daylength and temperatures required for flowering in P. bulbosa (Youngner, 1960), nor on differential responses of ecotypes varying in flowering capacity to daylength, temperature and vernalization. In most Festucoid perennial grasses from temperate regions, flowering has a dual induction requirement: a primary induction by vernalization and/or short days, and a secondary induction that requires a transition from short to long days and is enhanced by moderately high temperatures (Heide, 1994). However, a wide spectrum of flowering responses to daylength and vernalization has been found among annual and perennial populations of P. annua and in the related P. infirma and P. supina (Johnson and White, 1997a, b; Heide, 2001), raising doubts about the nature of the environmental factors controlling flowering in P. bulbosa. Thus, the main goals of our research were (a) to study the effects of vernalization, daylength and growth temperature on flowering and dormancy induction in ecotypes differing in flowering capacity and occurring along a rainfall gradient; and (b) to analyse ecotypic differences in responsiveness of flowering and dormancy to the environmental factors controlling these processes.
MATERIALS AND METHODS
Plant material
Populations of P. bulbosa L. were sampled along a North–South (latitudes 33°03′ to 31°15′) rainfall gradient (810–110 mm rain year−1) in Israel during April 2000 (Ofir and Kigel, 2003). Collected clumps (‘parent plants’) were planted in loess soil in 4 L pots and kept outdoors under the natural climate conditions in a net-house at the Faculty of Agriculture in Rehovot, Israel. The parent plants became dormant every spring (March to April) and started active growth after the first autumn rains (October to November). Flowering was recorded for each parent plant during 3 years, from 2000 to 2003. Populations differed in the degree of flowering in the net-house and in their response to dormancy induction by long days under controlled conditions: the percentage of flowering plants was higher and onset of dormancy was earlier, the more arid the site of population origin (Ofir and Kigel, 2003). From this collection, four contrasting ecotypes from three sites across the rainfall gradient and differing in their flowering capacity, i.e. consistent yearly flowering or lack of flowering in the net-house, were taken for this study (Table 1): (1) arid (110 mm rain year−1), flowering ecotype (‘A–F’); (2) semi-arid (276 mm year−1), flowering ecotype (‘SA–F’); (3) semi-arid (276 mm year−1), non-flowering ecotype (‘SA–NF’); and (4) mesic (810 mm rain year−1), non-flowering ecotype (‘M–NF’). In the semi-arid site, the flowering and non-flowering ecotypes co-occur. Three large parent plants were chosen from each ecotype and 160–300 dry, dormant bulbs were separated from each parent plant in April 2003. These bulbs were dry stored in paper bags at 40 °C in the dark for 6 weeks, to reduce dormancy and facilitate simultaneous sprouting after planting (Ofir, 1986).
Table 1.
Characterization of four P. bulbosa ecotypes differing in the rainfall at the site of origin, flowering capacity and dormancy: arid and flowering (‘A–F’); semi-arid and flowering (‘SA–F’), semi-arid and non-flowering (‘SA–NF’); and mesic and non-flowering (‘M–NF’)
| Panicles per plant |
|||||||
|---|---|---|---|---|---|---|---|
| Ecotype | Site of origin | Parent plant | Annual rainfall (mm) | Age at dormancy (d) | 2001 | 2002 | 2003 |
| A–F | Road from Arad to Dead Sea | 1 | 110 | 55 | 22 | 11 | 23 |
| 2 | 9 | 6 | 14 | ||||
| 3 | 7 | 3 | 51 | ||||
| SA–F | Har Amasa | 1 | 276 | 88 | 8 | 1 | 9 |
| 2 | 7 | 4 | 4 | ||||
| 3 | 18 | 12 | 28 | ||||
| SA–NF | Har Amasa | 1 | 276 | 114 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||
| 3 | 0 | 0 | 0 | ||||
| M–NF | Montfort | 1 | 810 | 118 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||
| 3 | 0 | 0 | 0 | ||||
SA–F and SA–NF ecotypes co-occur in the same habitat. Flowering was recorded outdoors in a net-house under similar natural conditions. Time to onset of dormancy was determined under inductive conditions (16 h LD and 22/16 °C; Ofir and Kigel, 2003). Age at onset of dormancy is the mean value for the three parent plants of each ecotype. Latitudes of the sites of origin ranged between 31°15′ (road from Arad to Dead Sea) and 33°03′ (Montfort).
Controlled experiment conditions
Differences among ecotypes in flowering and dormancy responses to low temperature pre-treatment (i.e. pre-chilling), daylength and growth temperature were studied under controlled environmental conditions in a phytotron, in glass-covered growth rooms transmitting 80 % of outside solar radiation (Ofir and Kigel, 2003). Day/night growth temperatures were 16/10, 22/16 and 28/22 °C, controlled within ± 0·5 °C. Relative air humidity during the day period was set to 64, 75 and 82 %, respectively, to ensure the same vapour pressure deficit (0·66 kPa) at the different growth temperatures. Day temperature and humidity were maintained from 07:00 to 16:00 h. Changes between day and night temperature and humidity were gradual, spanning 3 h. The daylengths used were short day (SD), 9 h (07:00–16:00 h) of natural light, and long day (LD), 16 h (04:00–20:00) attained by extending the natural daylength with supplementary lighting (3–5 µmol m−2 s−1 PAR at plant level), using 75 W incandescent tungsten lamps (LM960 Osram GmbH, München, Germany). Plants were grown singly in 0·8 L drained plastic pots, in a substrate of volcanic tuff gravel, vermiculite no. 4 and peat (2 : 1 : 1 v/v/v), irrigated alternately with 50 % Hoagland's nutrient solution and tap water, once every 1–2 days to avoid salt accumulation.
Experimental design
A factorial experiment with a completely randomized design was carried out with the following treatment combinations: four ecotypes, pre-chilling at 5 °C vs. non-chilled bulbs, followed by growth under SD or LD photoperiods, at 16/10, 22/16 and 28/22 °C. In the low temperature pre-treatment, bulbs from each parent plant were planted at 5 °C in a moist substrate (vermiculite–tuff gravel 1 : 1 v/v) for 6 weeks (pre-chilling, LT6). The bulbs sprouted during the low temperature treatment and were illuminated for 8 h daily with cool white fluorescent lamps (100 µmol m−2 s−1 PAR at plant level) until the end of the treatment. In the control treatment (without pre-chilling, LT0) the bulbs were dry stored at room temperature (20–25 °C) for 6 weeks. At the end of the pre-chilling and dry storage periods, respectively, bulbs were transplanted singly to 0·8 L pots in a wet substrate in the phytotron, at SD and LD at the three growth temperatures. Eight to 12 daughter plants from each of the three parent plants, making up 24–36 replicates, were included in every treatment combination of ecotype, pre-chilling, photoperiod and growth temperature.
Flowering and dormancy parameters
A plant was considered as flowering when at least one of its tillers had an emerged panicle, and as dormant when most of its leaves were yellowing and drying and most of its tillers had bulbs. Plant age at flowering and dormancy was recorded individually for each plant. Plant age in LT6 treatments was measured from planting day in the phytotron of already sprouted, pre-chilled plants with 2·4 ± 0·1 leaves. Age in LT0 plants was taken from day 7 after sprouting, when plants had about two leaves, as in the pre-chilled plants. Dormant plants were routinely removed and the total number of tillers and proportions of bulbing tillers recorded. The cumulative percentage of dormant LD plants vs. time was plotted for each ecotype, in each treatment combination. The rate of dormancy imposition was determined from the nearly linear portion of the resulting sigmoid curve, between one-sixth and five-sixths of the final percentage of dormant plants. All linear regressions were highly significant (r > 0·96, P < 0·01). In SD treatments, the proportions of flowering and dormant plants were recorded at age 3 and 5 months (end of the experiment). The number of panicles per plant was recorded 5 months after planting.
Statistical analyses
Statistical analyses were carried out with JMPIN (version 4·04 SAS Institute Inc.). Means were compared using the LSMEANS test. Analysis of variance (ANOVA) of data presented as proportions (percentage of bulbing, dormant and flowering tillers) was carried out after arcsin transformations. The results of categorical type (e.g. flowering vs. non-flowering plants, dormant vs. non-dormant plants) were analysed using contingency analysis and Pearson's χ2-test (Sokal and Rohlf, 1995).
RESULTS
Flowering
Flowering (i.e. emergence of panicles) under LD was rare and sporadic. Pre-chilling (LT6) resulted in 3–9 % flowering in A–F, SA–F and SA–NF ecotypes at 16/10 and 22/16 °C. Without pre-chilling (LT0), 15 % of SA–F and SA–NF plants flowered at 16/10 °C (n = 30–33). No flowering was observed in the M–NF ecotype at all the growth temperatures. None of the ecotypes flowered at 28/22 °C.
Under SD, in contrast, a high proportion of the plants flowered in all ecotypes, depending on the growth temperature. The age at flowering was earlier (Fig. 1) and the percentage of flowering was higher at 16/10 °C compared with at 22/16 °C, but nearly no flowering occurred at 28/22 °C (Fig. 2). Even ecotypes that did not flower in the net-house under outdoor conditions (SA–NF and M–NF) reached flowering in SD 16/10 °C. The ‘flowering’ ecotypes A–F and SA–F showed earlier flowering (by approx. 30 d), particularly at 16/10 °C and without pre-chilling (Fig. 1). The mesic ecotype M–NF did not flower at 22/16 °C. These effects of ecotype and growth temperature on flowering age were consistent and highly significant (Table 2). In contrast, the effect of pre-chilling (LT6) on flowering was complex, and changed with plant age (Figs 1 and 2; Tables 2 and 3). Pre-chilling delayed flowering in the ecotypes from the arid and semi-arid habitats (A–F, SA–F and SA–NF), but not in the mesic, M–NF ecotype (Fig. 1). The effects of pre-chilling on the percentage of flowering 3 months after planting were not consistent (Fig. 2A and B; Table 3): flowering was increased by pre-chilling in SA–NF at 16/10 °C (χ2 = 11·04, P = 0·0009) and in SA–F at 22/16 °C (χ2 = 11·62, P = 0·0007), but was reduced in A–F at 22/16 °C (χ2 = 6·88, P = 0·0087). Differences in percentage of flowering due to pre-chilling mostly disappeared 5 months after planting (Fig. 2C and D), except for a reduction in flowering of A–F and SA–NF at 22/16 °C (χ2 = 8·57, P = 0·0034 and χ2 = 12·14, P = 0·0005, respectively). In spite of these inconsistencies, pre-chilling generally postponed flowering and diminished the proportion of flowering plants.
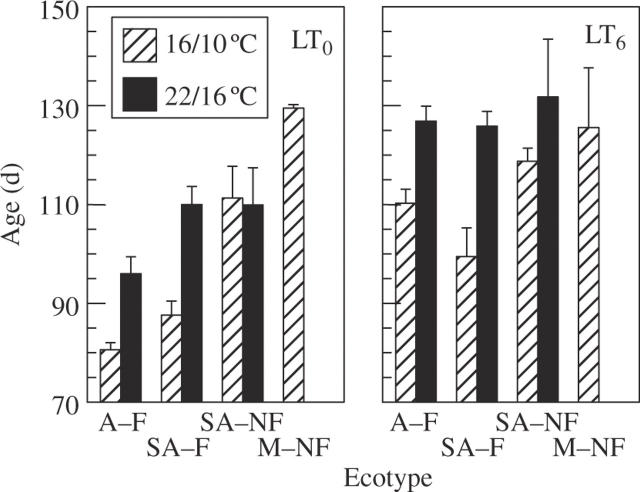
Fig. 1.
Effects of pre-chilling and growth temperature on the age of flowering in P. bulbosa plants growing under SD (9 h). Pre-chilled bulbs (LT6) were planted in wet substrate at 5 °C for 6 weeks; control bulbs (LT0) were stored dry at 20–25 °C for 6 weeks. Sprouted plants from both treatments continued growth at 16/10, 22/16 and 28/22 °C (day/night). Data for 28/22 °C and for M–NF at 22/16 °C are not given due to the lack or scarcity (<13 %) of flowering. Ecotypes differed in rainfall at the site of origin and flowering capacity: plants were from an arid habitat (110 mm rain year−1), of ‘flowering’ type (A–F); semi-arid ‘flowering’ and semi-arid ‘non-flowering’ (276 mm rain year−1: SA–F and SA–NF, respectively) and M–NF, mesic (810 mm rain year−1) ‘non-flowering’. SA–F and SA–NF ecotypes co-occur in the same habitat. Data are means and s.e. n = 22–30 plants. Age is presented as days from 7 d after sprouting (LT0), or from the end of pre-chilling (LT6), both 2·5 leaves per plant.
Fig. 2.
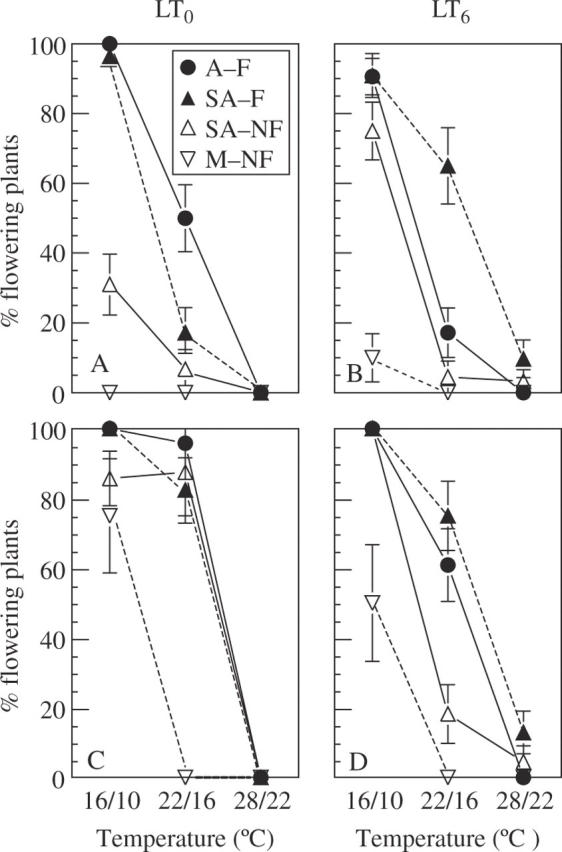
Effects of pre-chilling and growth temperature on the percentage of flowering plants of P. bulbosa growing at SD (9 h), at age 3 months (A and B) and 5 months (C and D), of four ecotypes differing in rainfall at the site of origin and flowering capacity: arid habitat and ‘flowering’ (A–F); semi-arid, ‘flowering’ (SA–F); semi-arid, ‘non-flowering’ (SA–NF); and mesic, ‘non-flowering’ (M–NF). SA–F and SA–NF ecotypes co-occur in the same habitat. Pre-chilled bulbs (LT6) were planted in a wet substrate at 5 °C for 6 weeks; control bulbs (LT0) were stored dry at 20–25 °C for 6 weeks. Sprouted plants from both pre-treatments continued growth at 16/10, 22/16 and 28/22 °C (day/night). Data are means ± s.e. n = 22–30 plants.
Table 2.
Statistical analyses (ANOVA) of the effects of ecotype, pre-chilling (5 vs. 20–25 °C) and growth temperature (16/10, 22/16 and 28/22 °C) on age of flowering, percentage of flowering tillers and number of panicles per plant in SD (9 h), and age of dormancy in LD (16 h)
| SD |
LD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age at flowering |
% flowering tillers |
Panicles plant−1 |
Age at onset of dormancy |
|||||
| Factor | F | P | F | P | F | P | F | P |
| Ecotype (E) | 12·5 | 0·0001 | 82·0 | 0·0001 | 36·7 | 0·0001 | 189·0 | 0·0001 |
| Pre-chilling (Pr) | 23·0 | 0·0001 | 0·2 | 0·7 | 1·5 | 0·2 | 19·7 | 0·0001 |
| Temperature (T) | 22·8 | 0·0001 | 130·2 | 0·0001 | 114·7 | 0·0001 | 391·7 | 0·0001 |
| Interactions | ||||||||
| E × Pr | 4·4 | 0·005 | 0·4 | 0·7 | 3·4 | 0·02 | 3·8 | 0·01 |
| T × Pr | 1·4 | 0·24 | 0·8 | 0·5 | 3·3 | 0·04 | 0·2 | 0·813 |
| E × T | 3·2 | 0·04 | 8·5 | 0·0001 | 14·1 | 0·0001 | 3·5 | 0·002 |
| d.f.error | 244 | 402 | 412 | 748 | ||||
Flowering under LD was nil or very low. Therefore, daylength was not included as a factor in the ANOVA. Ecotypes were arid and flowering, semi-arid and flowering, semi-arid and non-flowering, and mesic and non-flowering. Most plants did not flower under LD and did not become dormant under SD.
Table 3.
Statistical analyses (contingency analysis and Pearson's χ2-test) of the effects of ecotype, pre-chilling (5 vs. 20–25 °C) and growth temperature (16/10, 22/16 and 28/22 °C) on the percentage of flowering and the percentage of dormant P. bulbosa plants in SD (9 h), at age 3 months and 5 months after planting
| 3 months SD |
5 months SD |
|||||||
|---|---|---|---|---|---|---|---|---|
| % flowering plants |
% dormant plants |
% flowering plants |
% dormant plants |
|||||
| Factor | χ2 | P | χ2 | P | χ2 | P | χ2 | P |
| Ecotype | 87·1 | 0·0001 | 61·5 | 0·0001 | 17·8 | 0·0005 | 32·4 | 0·0001 |
| Pre-chilling | 4·9 | 0·027 | 32·6 | 0·0001 | 0·3 | 0·6 | 4·6 | 0·03 |
| Temperature | 217·6 | 0·0001 | 137·4 | 0·0001 | 268·5 | 0·0001 | 136·7 | 0·0001 |
| d.f.error | 644 | 644 | 448 | 448 | ||||
Ecotypes were arid and flowering, semi-arid and flowering, semi-arid and non-flowering, and mesic and non-flowering.
Differences among ecotypes in flowering response to growth temperature were particularly evident after 3 months of growth (Fig. 2A, B). In the ‘flowering’ ecotypes (A–F and SA–F), 90–100 % of the plants flowered at 16/10 °C, compared with the ‘non-flowering’ ecotypes that reached 30 and 75 % flowering in SA–NF and 0 and 10 % in M–NF, in the LT0 and LT6 pre-treatments, respectively. At 22/16 °C, flowering occurred mainly in the ‘flowering’ ecotypes. The percentage of flowering increased in all ecotypes after 5 months of growth at 16/10 and 22/16 °C, but remained practically nil at 28/22 °C (Fig. 2C, D), even in ecotypes that flowered profusely at 16/10 °C. M–NF plants that did not flower at 3 months reached considerable flowering under 16/10 °C at 5 months, though at 22/16 °C they did not flower. Thus, at 16/10 °C, all ecotypes reached >75 % flowering, with and without the pre-chilling treatments (except M–NF LT6 with 50 % flowering). The effect of pre-chilling (LT6) on the percentage of flowering at age 5 months was negative, particularly for ecotypes A–F and SA–NF at 22/16 °C and M–NF at 16/10 °C.
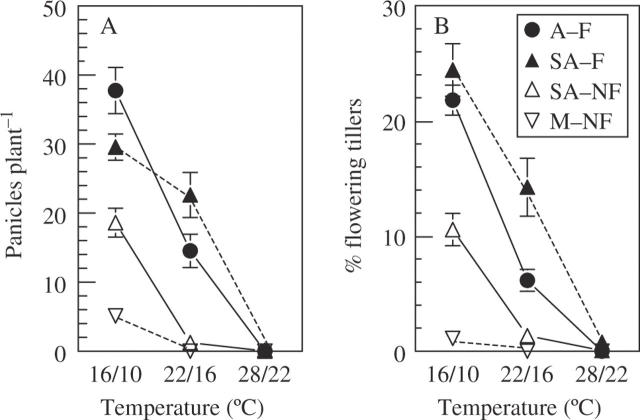
The number of panicles per plant and the percentage of flowering tillers were additional criteria to evaluate ecotypic differences in flowering response to temperature treatments under SD. Both parameters were unaffected by pre-chilling (Table 2). Therefore, data from LT0 and LT6 treatments were pooled (Fig. 3). The effects of ecotype and growth temperature on both parameters were highly significant (Table 2). Panicles per plant (Fig. 3A) and percentage of flowering tillers (Fig. 3B) were highest at 16/10 °C, decreased markedly at 22/16 °C and were almost nil at 28/22 °C. ‘Flowering’ ecotypes A–F and SA–F had a higher percentage of flowering tillers and more panicles per plant than ‘non-flowering’ ecotypes, particularly at 22/16 °C where very few tillers (<2 %) flowered in the ‘non-flowering’ ecotypes. Furthermore, both parameters were significantly higher in SA–F than in the co-occurring SA–NF ecotype (panicles per plant, P = 0·003 and P < 0·0001 for 16/10 and 22/16 °C, respectively; percentage of flowering tillers, P < 0·0001 for both temperatures). The M–NF ecotype had the lowest percentage of flowering tillers and panicles per plant.
Fig. 3.
Effects of growth temperature (16/10, 22/16 and 28/22 °C day/night) on the number of panicles per plant (A) and the percentage of flowering tillers (B) in four ecotypes of P. bulbosa growing at SD (9 h), age 5 months, differing in rainfall at the site of origin and flowering capacity: arid habitat and ‘flowering’ (A–F); semi-arid, ‘flowering’ (SA–F); semi-arid, ‘non-flowering’ (SA–NF); and mesic, ‘non-flowering’ (M–NF). SA–F and SA–NF ecotypes co-occur in the same habitat. Data of pre-chilled and non-pre-chilled (control) treatments were pooled, since differences between the two pre-treatments were not significant. Data are means ± s.e. n = 30 plants.
Dormancy
Under LD, plants became dormant much earlier than under SD, as shown by the percentage of dormant plants at age 3 months (Fig. 4A, B, and C, D). Moreover, while LD plants of all ecotypes and temperature treatments became dormant between age 1 and 4 months (Fig. 5), most SD plants from all ecotypes were still growing actively at the end of the experiment (age 5 months) at 16/10 and 22/16 °C (Fig. 4E, F). LD plants became dormant earlier the higher the growth temperature and after pre-chilling, compared with unchilled plants. These effects were highly significant (Fig. 5, Table 2). The enhancing effect of pre-chilling was significant only in the ‘non-flowering’ ecotypes, that entered dormancy late (SA–NF and M–NF; P = 0·001), while in the ‘flowering’ ecotypes (A–F and SA–F), the pre-chilling effect on dormancy imposition was not significant (P = 0·57 and 0·06, for A–F and SA–F, respectively).
Fig. 4.
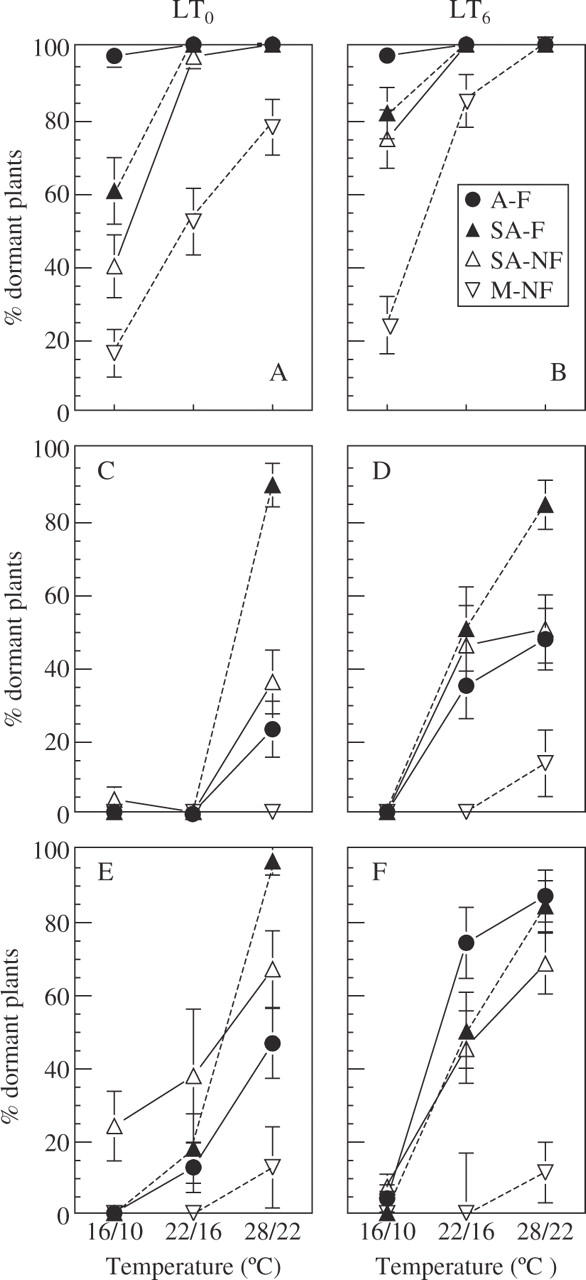
Effects of pre-chilling and growth temperature on the percentage of dormant P. bulbosa plants at age 3 months, under LD (A and B) and SD (C and D), and at age 5 months of SD plants (E and F), in ecotypes differing in rainfall at the site of origin and flowering capacity: arid habitat and ‘flowering’ (A–F); semi-arid and ‘flowering’ (SA–F); semi-arid, ‘non-flowering’ (SA–NF); and mesic, ‘non-flowering’ (M–NF). SA–F and SA–NF ecotypes co-occur in the same habitat. Pre-chilled (LT6) bulbs were planted in wet substrate at 5 °C for 6 weeks; control bulbs (LT0) were stored dry at 20–25 °C for 6 weeks. Sprouted plants from both pre-treatments continued growth at 16/10, 22/16 and 28/22 °C (day/night). Data are means ± s.e. n = 22–30 plants.
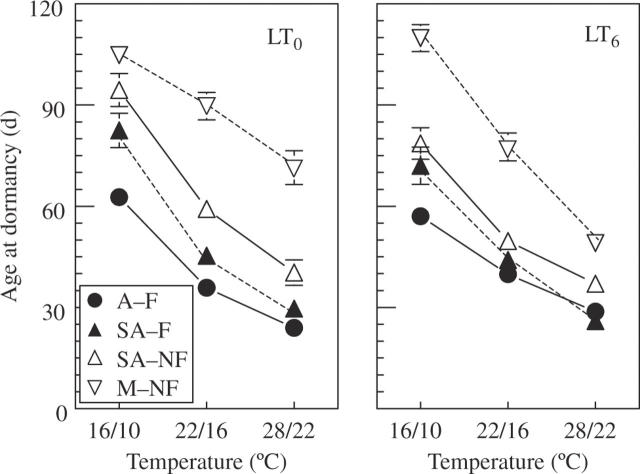
Fig. 5.
Effects of pre-chilling and growth temperature on age at dormancy of P. bulbosa at LD (16 h), in ecotypes differing in rainfall at the site of origin and flowering capacity: arid habitat and ‘flowering’ (A–F); semi-arid, ‘flowering’ (SA–F); semi-arid, ‘non-flowering’ (SA–NF); and mesic, ‘non-flowering’ (M–NF). SA–F and SA–NF ecotypes co-occur in the same habitat. Pre-chilled bulbs (LT6) were planted in wet substrate at 5 °C for 6 weeks; control bulbs (LT0) were stored dry at 20–25 °C for 6 weeks. Sprouted plants from both pre-treatments continued growth at 16/10, 22/16 and 28/22 °C (day/night). Data are means ± s.e. n = 27–33 plants. Age is presented as days from 7 d after sprouting (LT0), or from the end of pre-chilling (LT6), both 2·5 leaves per plant.
Under SD, dormancy imposition recorded at age 3 and 5 months was also greatly enhanced by increasing growth temperature and by pre-chilling (Table 3; Fig. 4C–F). At 16/10 °C, no or few dormant plants (<25 %) were observed in all ecotypes, even after 5 months of growth, while at 28/22 °C the proportion of dormant plants was high. Dormancy promotion by pre-chilling (Table 3) was clearly seen at 22/16 and 28/22 °C. At 22/16 °C, all ecotypes were non-dormant in the LT0 treatment at age 3 months, compared with 30–50 % in the A–F, SA–F and SA–NF ecotypes in the LT6 treatment.
Ecotypes differed in patterns of dormancy response under both LD and SD. Under LD, ecotypes whose parent plants flowered in the net-house (A–F and SA–F) became dormant earlier than those from ‘non-flowering’ ecotypes (SA–NF and M–NF) (Fig. 5; Table 2). The differences between A–F and SA–F vs. M–NF ecotypes were highly significant (P < 0·0001) in all growth temperature and pre-chilling combination treatments. Moreover, plants of the ‘flowering’ SA–F ecotype became dormant earlier than plants of the ‘non-flowering’ SA–NF, even though they co-occur in the same site. The M–NF ecotype became dormant considerably later than the SA–NF in five out of the six growth temperature–pre-chilling combinations (P < 0·001). The enhancing effect of increasing growth temperature on earliness of dormancy onset was similar in the four ecotypes, as shown by the weak, though significant, temperature × ecotype interaction (Table 2).
Similar but less well defined ecotypic trends in patterns of dormancy imposition were found in SD. At 28/22 °C, the highest percentage of dormancy at age 3 months occurred in the SA–F ecotype (80–90 %). In A–F and SA–NF, the percentage of dormant plants was intermediate (20–40 and 40–50 % in the LT0 and LT6, respectively;Fig. 4A, B). The M–NF ecotype remained non-dormant at 16/10 and 22/16 °C and only approx. 15 % dormancy was reached at 28/22 °C, even after 5 months of growth (Fig. 4).
The time course for dormancy imposition was followed under LD. Earlier onset of dormancy was negatively correlated with a higher rate of dormancy imposition in both LT0 (y = 4·70 – 0·027x; r = 0·749) and LT6 (y = 5·18 – 0·037x; r = 0·760) pre-treatments (d.f. = 10, P = 0·01, in both control and pre-chilled plants). The difference between the two regressions was not significant.
DISCUSSION
Flowering and dormancy in P. bulbosa were affected in opposite ways by daylength and growth temperature. Flowering was rare under LD, but readily occurred after 3 months of growth in SD and was enhanced by low temperature (Figs 1 and 2). Dormancy, in contrast, was induced by LD and postponed by low growth temperature (Figs 4 and 5), as previously reported (Ofir and Kerem, 1982; Ofir and Dorenfeld, 1992; Ofir and Kigel, 1999). Plants also became dormant under SD, but at a much later stage and mostly under high temperature (Fig. 4). Thus, high growth temperature inhibited flowering under SD (Fig. 2) and enhanced dormancy under both LD and SD (Figs 4 and 5). Furthermore, exposure of plants to low temperature pre-treatment (6 weeks at 5 °C) accelerated dormancy imposition in LD (Figs 4A, B, and 5; Ofir and Kigel, 1999) and in some ecotypes also in SD (Fig. 4C–F), but lacked consistent effects on flowering (Figs 1 and 2).
Similar trends of earlier flowering in short days, minor or no effect of vernalization and a delay of flowering (i.e. more leaves to flowering) due to increasing growth temperature have been reported for high latitude and alpine populations of perennial types of P. annua (Heide, 2001) and for perennial P. annua ‘reptans’ populations (Johnson and White, 1997a, b). However, in contrast to P. bulbosa that did not reach flowering in long days, perennial types of P. annua flowered in long days at a later plant age compared with short days, particularly under relatively low growth temperatures, or after vernalization treatments (Heide, 2001). In P. bulbosa, long days induced early dormancy and only a few plants ‘escaped’ dormancy and reached flowering at low growth temperatures. The fact that flowering mostly occurred in short days, together with the lack of clear response to vernalization, suggests that in regard to flowering, P. bulbosa is a short day plant.
It may also be argued that P. bulbosa is a facultative long day plant like most Festucoid grasses, but the early transition to the dormant state under LD arrests reproductive development prematurely, before any morphological changes at the shoot apex have occurred. Moreover, even if a shift from short day to long day is required for flowering, as in other temperate grasses (Heide, 1994), in P. bulbosa this shift will enhance the induction of dormancy (Ofir and Kigel, 1999), thus strengthening the inhibition of flowering under these conditions. Suppression of flowering due to dormancy enhancement by the same environmental factors required for flowering induction and inflorescence development has probably also occurred in garlic (A. sativum) and onion (A. cepa), due to selection since historical times for increased storage in the bulbs and for earliness of maturation (Brewster, 1990; Etoh and Simon, 2002). In some bolting types of garlic, the transition of the shoot apex to the reproductive state can occur under both short and long days. However, under short days, the inflorescence fails to elongate, while under long days the fate of the inflorescence depends on the strength of long day induction. Increasing the number of long days leads from normal elongation and flowering to development of dormant vegetative bulbils, instead of flowers (Kamenetsky et al., 2004). Similarly, in the short day C4 perennial grass Bouteloua eriopoda, continued exposure to inductive short days leads to early abortion of the inflorescence (Schwartz and Koller, 1975). This response is probably related to the fact that in this species the rest period occurs during the dry winter. Altogether, it can be argued that natural or artificial selection for increased responsiveness to environmental factors inducing flowering could result in the loss of flowering capacity that is associated with morphological and physiological processes leading to dormancy. Moreover, since similar environmental factors induce summer dormancy in P. bulbosa as well as flowering in many other Festucoid grasses (i.e. long day, pre-exposure to short day or to low temperature), it is conceivable that the control of these phenological transitions can be channelled through inter-related developmental pathways. Thus, the inhibition of flowering in P. bulbosa by high temperature can be attributed to enhanced responsiveness to induction of dormancy by long days or to direct inhibition of flowering (Heide, 1994).
It is noteworthy that flowering does not depend on vernalization but is promoted by short days in perennial types of Poa from contrasting habitats, such as alpine and high latitude populations of P. annua that grow during the summer (Heide, 2001), as well as in Mediterranean populations of P. bulbosa that grow in the winter season. Despite the large climatic differences, both habitats are characterized by a relatively short growth season. In the Mediterranean and adjoining arid phytogeographic regions, P. bulbosa grows on shallow soils with a low moisture-holding capacity, imposing a short growth period in the winter that is followed by a long dry summer. These were probably factors in the evolution of the ability to achieve flowering in short days at low temperatures, together with the early transition to the dormant state.
Flowering of P. bulbosa ecotypes along the steep rainfall gradient occurs during late winter and early spring, when the daylength ranges between 11 and 12 h. This range is also the threshold daylength for dormancy induction (Ofir and Kigel, 1999). Since the rainfall gradient along which the ecotypes were collected spans a narrow latitude range, it may be assumed that the ecotypes in the field are exposed to the same daylength and quite similar temperatures along the gradient. Thus, the ecotypic variation in the capacity and timing of flowering with increasing aridity can be interpreted as the result of balanced opposite effects of daylength and temperature on the flowering and dormancy processes.
In the non-flowering mesic and semi-arid ecotypes, flowering did not occur during 3 years in outdoor conditions in a net-house (Table 1), but they flowered in the phytotron under SD at the lower temperatures (M–NF at 16/10 °C; SA–NF at 16/10 and 22/16 °C, Fig. 2). Thus, lack of flowering in the field doees not necessarily indicate a loss of flowering capacity. These non-flowering ecotypes probably have an increased responsiveness to the inhibitory effect of higher temperature on flowering. It is conceivable that in the field, these ecotypes could reach flowering in years with early onset of the rainy season and relatively cold and prolonged winters. Thus, they may produce seeds or inflorescence bulbils only in particularly favourable years, relying on tiller bulbs for reproduction in the less favourable years. In this case, the balance between local reproduction and persistency by basal bulbs vs. dispersible seeds or bulbils produced by inflorescences is determined by the responsiveness of the ecotype to the relevant environmental conditions and the frequency with which these conditions occur. The facts that arid and semi-arid ecotypes (A–F and SA–F) flowered every year (Table 1), had a larger proportion of flowering plants than the mesic ecotype (M–NF) even at the intermediate temperature (i.e. 22/16 °C) and also underwent earlier flowering (Figs 1 and 2) support the idea that a higher level of dispersal by sexual and/or asexual propagules (i.e. seeds and bulbils) has an advantage for P. bulbosa populations under arid conditions (Ofir and Kigel, 2003). At the same time, higher responsiveness of these populations to long day and high temperature induction of dormancy reduces the risk of death due to early drought.
We conclude that the variation in the flowering capacity of P. bulbosa ecotypes differing in drought tolerance can be seen as the result of balanced opposite effects of daylength and temperature on the flowering and dormancy processes, thus regulating reproductive capacity, the balance between seed and vegetative reproduction and adaptation to prolonged drought during the summer.
LITERATURE CITED
- Brewster JL. 1990. Physiology of crop growth and bulbing. In: Rabinowitch HD, Brewster JL, eds. Onions and allied crops, Vol. 1. Boca Raton: CRC Press, 53–88.
- Davis PH. 1985. Flora of Turkey, Vol. 9. Edinburgh: Edinburgh University Press.
- Etoh T, Simon PW. 2002. Diversity, fertility and seed production of garlic. In: Rabinowitch HD, Currah L. eds. Allium. Crop science: recent advances. Wallingford, UK: CAB International, 101–117.
- Heide OM. 1994. Control of flowering and reproduction in temperate grasses. New Phytologist 128: 347–362. [DOI] [PubMed] [Google Scholar]
- Heide OM. 2001. Flowering responses of contrasting ecotypes of Poa annua and their putative ancestors Poa infirma and Poa supina. Annals of Botany 87: 795–804. [Google Scholar]
- Heyn CC. 1962. Studies of bulbous Poa in Palestine. 1. The agamic complex of Poa bulbosa. Bulletin of the Research Council of Israel 11D: 117–126. [Google Scholar]
- Johnson PG, White DB. 1997a. Vernalization requirements among selected genotypes of annual bluegrass (Poa annua L.). Crop Science 37: 1538–1542. [Google Scholar]
- Johnson PG, White DB. 1997b. Flowering responses of selected annual bluegrass genotypes under different photoperiod and cold treatments. Crop Science 37: 1543–1548. [Google Scholar]
- Kamenetsky R, Shafir IL, Zemah H, Barzilay A, Rabinowitch HD. 2004. Environmental control of garlic growth and florogenesis. Journal of the American Society of Horticultural Science 129: 144–151. [Google Scholar]
- Le Nard M, De Hertogh AA. 1993. Bulb growth and development and flowering. In: De Hertogh AA, Le Nard M, eds. The physiology of flower bulbs. Amsterdam: Elsevier Science, 29–43.
- Ofir M. 1986. Seasonal changes in the response to temperature of summer-dormant Poa bulbosa L. bulbs. Annals of Botany 58: 81–89. [Google Scholar]
- Ofir M, Dorenfeld Y. 1992. Induction of summer dormancy in Poa bulbosa L. under natural environment and subsequent controlled photo-thermal conditions. Israel Journal of Botany 41: 265–277. [Google Scholar]
- Ofir M, Kerem D. 1982. The effects of temperature and photoperiod on the onset of summer-dormancy in Poa bulbosa L. Annals of Botany 50: 259–264. [Google Scholar]
- Ofir M, Kigel J. 1999. Photothermal control of the imposition of summer-dormancy in Poa bulbosa, a perennial grass geophyte. Physiologia Plantarum 105: 633–640. [Google Scholar]
- Ofir M, Kigel J. 2003. Variation in the onset of summer dormancy and flowering capacity along an aridity gradient in Poa bulbosa L., a geophytic perennial grass. Annals of Botany 91: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Koller D. 1975. Photoperiodic control of shoot-apex morphogenesis in Bouteloua eriopoda. Botanical Gazette 136: 41–49. [Google Scholar]
- Sokal RR, Rohlf FJ. 1995. Biometry, 3rd edn. New York: Freeman and Co.
- Youngner VB. 1960. Environmental control of initiation of the inflorescence, reproductive structures and proliferations in Poa bulbosa. American Journal of Botany 47: 753–757. [Google Scholar]