Abstract
• Background and Aims Aluminium (Al) stimulates the efflux of citrate from apices of rice bean (Vigna umbellata) roots. This response is delayed at least 3 h when roots are exposed to 50 µm Al, indicating that some inducible processes leading to citrate efflux are involved. The physiological bases responsible for the delayed response were examined here.
• Methods The effects of several antagonists of anion channels and citrate carriers, and of the protein synthesis inhibitor, cycloheximide (CHM) on Al-stimulated citrate efflux and/or citrate content were examined by high-pressure liquid chromatography (HPLC) or an enzymatic method.
• Key Results Both anion channel inhibitors and citrate carrier inhibitors can inhibit Al-stimulated citrate efflux, with anthracene-9-carboxylic acid (A-9-C, an anion channel inhibitor) and phenylisothiocyanate (PI, a citrate carrier inhibitor) the most effective inhibitors. A 6 h pulse of 50 µm Al induced a significant increase of citrate content in root apices and release of citrate. However, the increase in citrate content preceded the efflux. Furthermore, the release of citrate stimulated by the pulse treatment was inhibited by both A-9-C and PI, indicating the importance of the citrate carrier on the mitochondrial membrane and the anion channel on the plasma membrane for the Al-stimulated citrate efflux. CHM (20 µm) also significantly inhibited Al-stimulated citrate efflux, confirming that de novo protein synthesis is required for Al-stimulated citrate efflux.
• Conclusions These results indicate that the activation of genes possibly encoding citrate transporters plays a critical role in Al-stimulated citrate efflux.
Keywords: Aluminium resistance, anion channel, citrate carrier, inhibitor, organic acid anions, protein synthesis, rice bean, toxicity, transporter, Vigna umbellata
INTRODUCTION
Aluminium (Al) toxicity is one of the major constraints for reduced crop productivity in acid soils. Micromolar concentrations of Al can inhibit root elongation and consequently influence water and nutrient uptake, resulting in poor plant growth (Delhaize and Ryan, 1995; Kochian, 1995). On the other hand, some plant species have evolved sophisticated mechanisms to cope with Al stress. Much of the current evidence argues that Al-stimulated efflux of organic acids such as citrate, malate and oxalate from roots is an important Al resistance mechanism, although some reports demonstrated that organic acid effux plays a minor role such as in signalgrass (Wenzl et al., 2001) and spinach (Yang et al., 2005a) or is not the only mechanism for Al resistance such as in maize (Piñeros et al., 2005) and buckwheat (Zheng et al., 2005). Recently, an Al resistance gene from wheat, ALMT1, has been cloned, and identified as a gene encoding an Al-activated malate transporter, and expression of this gene in other genotypes increased malate efflux and enhanced Al resistance (Delhaize et al., 2004; Sasaki et al., 2004).
Two patterns of Al-stimulated efflux of organic acids have been proposed on the basis of the timing of efflux (Ma, 2000). In pattern I, no discernible delay is observed between the addition of Al and the onset of organic acid efflux such as in tobacco (Delhaize et al., 2001), wheat (Ryan et al., 1995) and buckwheat (Zheng et al., 1998). Thus, Al may activate an already expressed organic acid transporter in such species. However, in plant species such as rye (Li et al., 2000), triticale (Ma et al., 2000) and Cassia tora (Yang et al., 2005b), the efflux of organic acids was delayed by several hours, and Al might induce the expression of genes and synthesis of proteins involved in organic acid biosynthesis or transport which are necessary for efflux of organic acids across the root cells. However, there is not much direct experimental evidence to support this supposition. Recently, we demonstrated the different responses of buckwheat, a typical pattern I plant, and C. tora, a typical pattern II plant, to treatment with cycloheximide (CHM), a protein synthesis inhibitor, before, simultaneously and after Al exposure, and provided the first experimental evidence that both de novo synthesis and activation of an anion channel are needed for Al-induced efflux of citrate in C. tora, but that in buckwheat the plasma membrane protein responsible for oxalate efflux pre-existed (Yang et al., 2005b).
In an attempt to gain better understanding of the physiological and biochemical processes responsible for the efflux of organic acids, some reports have correlated this efflux with changes in enzyme activities involved in organic acid biosynthesis. However, the results remain ambiguous and still a matter of debate (Kochian et al., 2004). In the present study, we found that rice bean roots can specifically release citrate to alleviate Al toxicity, and the efflux was delayed by at least 3 h. In order to determine the key step involved in the Al-stimulated citrate efflux, several anion channel inhibitors and citrate carrier inhibitors as well as a protein synthesis inhibitor were used. Our results indicated that de novo protein synthesis (possibly of the citrate carrier and anion channel themselves) rather than citrate biosynthesis is the critical step leading to citrate efflux in roots of rice bean.
MATERIALS AND METHODS
Reagents
Niflumic acid (NIF), phenylisothiocyanate (PI), anthracene-9-carboxylic acid (A-9-C), phenylglyoxal (PG) and CHM were obtained from Wako Chemical (Osaka). Pyridoxal 5′-P (PP) and mersalyl acid (MA) were purchased from Sigma Chemical Company (St Louis. MO, USA). Stock solutions (10 mm) of NIF and PI were prepared in ethanol, CHM, PP and MA were prepared in de-ionized water and A-9-C was dissolved in 1 m NaOH.
Plant material and growth conditions
Seeds of rice bean [Vigna umbellata (Thunb.) Ohwi & Ohashi ‘Jiangnan’] were collected from Quzhou (acid soil region, Zhejiang Province, China). Seeds were soaked in de-ionized water overnight, and germinated at 26 °C in the dark. After germination, the seeds were transferred to a floating tray with a net bottom suspended in a 5·0 L solution of 0·5 mm CaCl2 (pH 4·5). The solution was renewed daily. On d 3, seedlings of a similar size were transplanted into a 1 L plastic pot (12 seedlings per pot) containing aerated nutrient solution. One-fifth strength of Hoagland solution was used, which contained the macronutrients in mm: KNO3 (1·0), Ca(NO3)2 (1·0), MgSO4 (0·4) and (NH4)H2PO4 (0·2), and the micronutrients in µm: NaFeEDTA (20), H3BO3 (3), MnCl2 (0·5), CuSO4 (0·2), ZnSO4 (0·4) and (NH4)6Mo7O24 (1). The solution was adjusted to pH 4·5 by HCl and renewed every other day. The plants were grown in a greenhouse for 2 weeks, and 2 days before the treatments the pots were moved to a controlled-environment room with a 14 h/26 °C day and a 10 h/22 °C night regime, a light intensity of 150 µmol photons m−2 s−1 and a relative humidity of 65 %. All the experiments were repeated at least once, and the results from a set of experiment are presented.
Collection of root exudates
Before various treatments, the roots were cleaned by placing them in 0·5 mm CaCl2 solution at pH 4·5 overnight in the same pots. Seedlings (12 d old) were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3. Root exudates were collected every 3 h for 12 h. The collected exudates were passed through a cation-exchange column (16 mm × 14 cm) filled with 5 g of Amberlite IR-120B resin (H+ form, Muromachi Chemical, Tokyo, Japan), followed by an anion-exchange column (16 mm × 14 cm) filled with 2 g of Dowex 1 × 8 resin (100–200 mesh, formate form). The organic acids retained on the anion-exchange resin were eluted by 15 mL of 1 m HCl, and the eluate was concentrated to dryness by a rotary evaporator (40 °C). The residue was redissolved in 1 mL of Milli-Q water and subjected to determination of organic acids.
Location of secretion site
In order to study the spatial distribution of citrate exudation along the root, either apical 5 or 10 mm root segments of 3-d-old seedlings were excised. The segments were transferred into 8 mL centrifuge tubes containing 5 ml of 0·5 mm CaCl2 solution (pH 4·5). Tubes with root segments were placed on a shaker for 1 h to remove organic acids leaked from cut cells. The washing solution was then removed from each tube and a further 5 mL of 0·5 mm CaCl2 solution (pH 4·5) added to rinse the root segments. Al treatment was initiated by replacing the Ca solution with 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3 for 6 h.
Effect of anion channel and citrate carrier inhibitors
In anion channel inhibitor experiments, seedlings (12 d old) were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 50 µm AlCl3 in the presence or absence of 20 µm NIF, PG or A-9-C. In citrate carrier inhibitor experiments, seedlings (12 d old) were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 50 µm AlCl3 in the presence or absence of 20 µm PI, PP or MA. Root exudates were collected after 9 h treatments. In another experiment, seedlings (12 d old) were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3 for 6 h, and then transferred to the 0·5 mm CaCl2 solution (pH 4·5) (+Ca), 0·5 mm CaCl2 solution (pH 4·5) containing 20 µm A-9-C (+A-9-C), 20 µm PI (+PI) or 20 µm A-9-C plus 20 µm PI (+PI +A-9-C). Root exudates were collected every 3 h, and apical 5 mm root segments were excised for determination of endogenous citrate content.
Effect of protein synthesis inhibitor
Seedlings (12 d old) were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 50 µm AlCl3 in the presence or absence of 20 µm CHM. After 9 h, root exudates were collected.
Determination of citrate
For endogenous citrate analysis, root apices were homogenized on ice, in 1 mL of 0·6 n perchloric acid. The extract was centrifuged at 15 000 g for 5 min, and 0·9 mL of supernatant was collected and neutralized with 80 µL of 5 m K2CO3. The neutralized solution was centrifuged at 15 000 g for 5 min, and the supernatant was used for the measurement of citrate.
Citrate in root tissues (Fig. 5B) and released from root apices (Fig. 2) was determined by an enzymatic method (Delhaize et al., 1993), and citrate in root exudates was analysed by high-performance liquid chromatography (HPLC) according to Zheng et al. (2005).
RESULTS
Effect of Al on citrate efflux
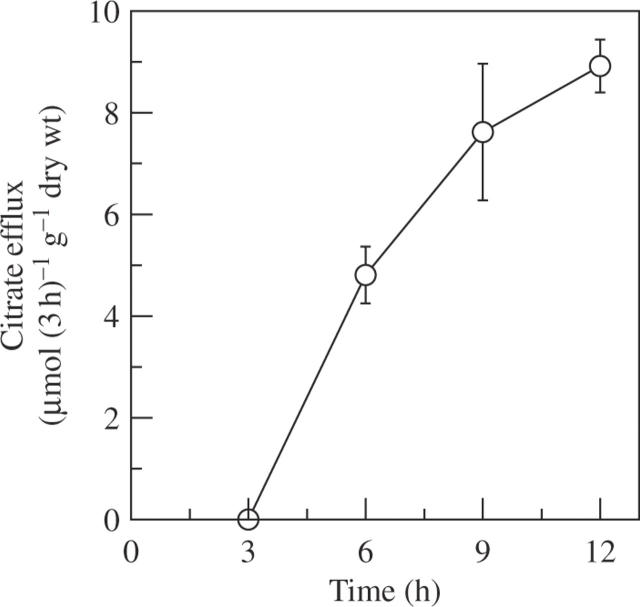
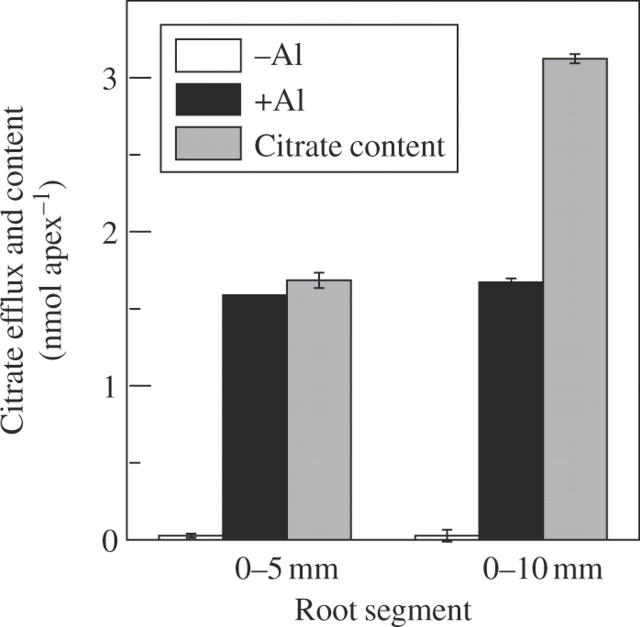
Despite many kinds of organic acids present in root tissues, only a large amount of citrate was released from the roots of rice bean when exposed to 50 µm Al (other organic acids were not detected), but the efflux was delayed by at least 3 h after the initiation of Al treatment (Fig. 1). This delay of citrate efflux by Al stress is a typical pattern II response. Al-stimulated citrate efflux was largely confined to the apical 5 mm of roots, and the citrate content in the apical 10 mm root was approx. 2-fold greater than in the apical 5 mm root (Fig. 2). On a root length basis, approx. 95 % of the citrate was released from the apical 5 mm of roots compared with the apical 10 mm of roots.
Fig. 1.
Time course of Al-stimulated release of citrate in rice bean. Seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3. Root exudates were collected every 3 h after initiation of Al treatment. Organic acids were analysed by HPLC. Error bars represent ± s.d. (n = 3).
Fig. 2.
Citrate exudation in excised root segments of rice bean. Either the terminal 5 or 10 mm of the root was used for citrate exudation experiments. Root segments were washed in 0·5 mm CaCl2 solution (pH 4·5) for 1 h and then incubated in 0·5 mm CaCl2 solution (pH 4·5) with 0 or 50 µm AlCl3 for 6 h. Root segments in 0·5 mm CaCl2 solution without Al (pH 4·5) after incubation were used for tissue extraction and citrate analysis. Citrate was determined enzymatically. Error bars represent ± s.d. (n = 3).
Effect of anion channel and citrate carrier inhibitors on citrate efflux
Anion channel inhibitors (NIF, A-9-C and PG at 20 µm) produced different effects on Al-stimulated citrate efflux. PG had no effect, while NIF and A-9-C inhibited the release by 29 and 43 %, respectively (Fig. 3). Three kinds of citrate carrier inhibitors (PP, PI and MA at 20 µm), which have been reported as inhibitors of citrate carrier on the mitochondrial membrane (Genchi et al., 1999), also showed different effects on citrate efflux. MA and PI inhibited the release by 34 and 45 %, respectively, but PP had no effect (Fig. 4).
Fig. 3.
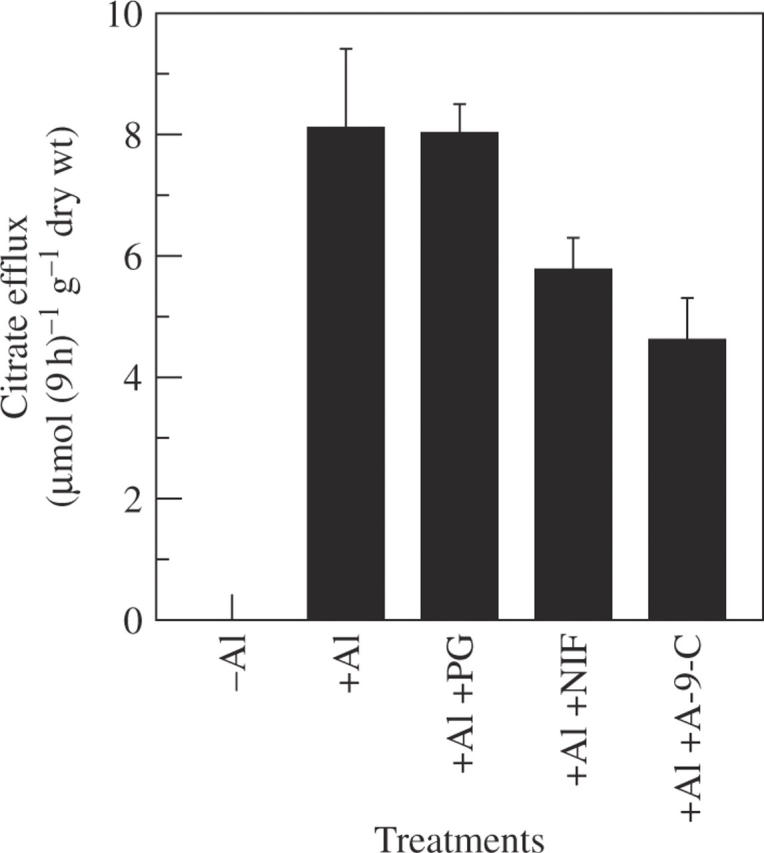
Effect of anion channel inhibitors on Al-stimulated release of citrate in rice bean. Seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3 in the presence or absence of each inhibitor (20 µm). Root exudates were collected after 9 h of treatment. Organic acids were analysed by HPLC. Error bars represent ± s.d. (n = 3).
Fig. 4.
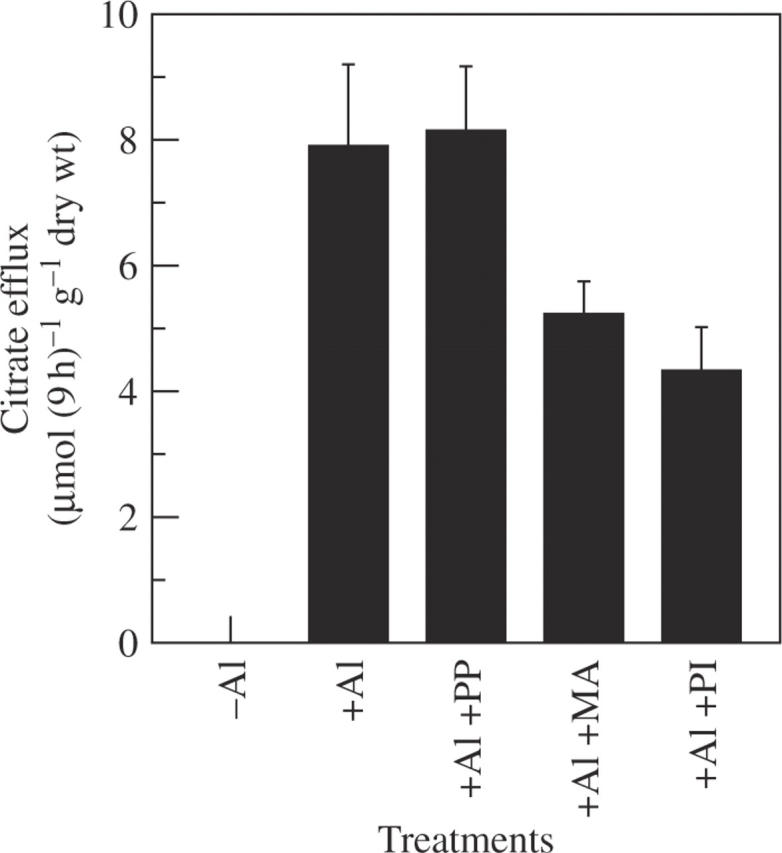
Effect of citrate carrier inhibitors on Al-stimulated release of citrate in rice bean. Seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 50 µm AlCl3 in the presence or absence of each inhibitor (20 µm). Root exudates were collected after 9 h of treatment. Organic acids were analysed by HPLC. Error bars represent ± s.d. (n = 3).
A 6 h pulse with 50 µm Al also induced significant citrate efflux after 3 h, but it was decreased after that (Fig. 5A). However, the efflux of citrate was significantly inhibited by 20 µm PI and A-9-C, and even more when exposed to both (Fig. 5A). The citrate content in root apices increased with exposure time, and was maintained at least for 6 h when transferred to Ca solution (Fig. 5B), but the citrate content in root apices treated with Ca solution did not change over time (data not shown). Furthermore, PI and A-9-C had no effect on the citrate content of root apices (Fig. 5B), indicating that the decreased citrate efflux was not due to the citrate content but to the inhibitory effects of PI and A-9-C on citrate transporters.
Fig. 5.
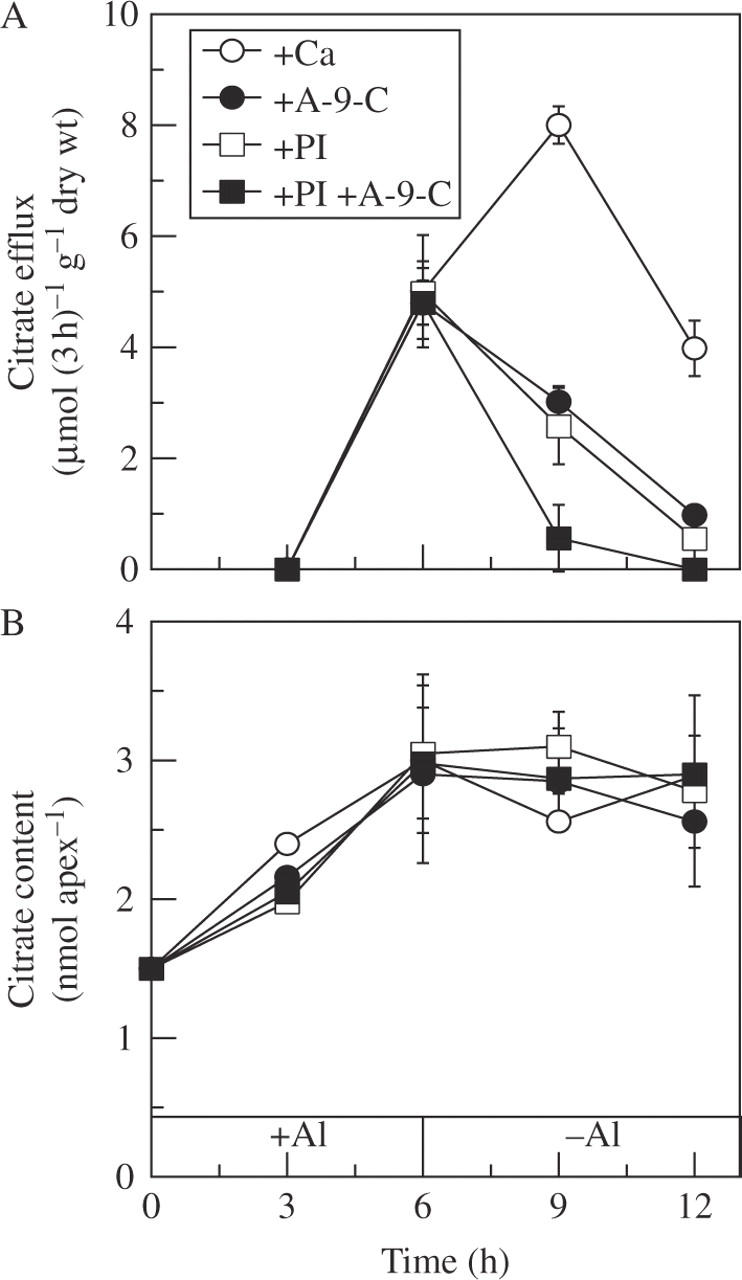
Effect of A-9-C and PI on the citrate efflux (A) and content (B) after 6 h pulse treatment with 50 µm AlCl3. Seedlings were exposed to 0.5 mm CaCl2 solution (pH 4.5) containing 50 µm AlCl3 for 6 h and then transferred to 0.5 mm CaCl2 solution (pH 4.5) containing 20 µm A-9-C, 20 µm PI or both. Root exudates were collected every 3 h for determination of citrate efflux, and apical 5 mm root apices were excised for endogenous citrate content analysis. Error bars represent ± s.d. (n = 3).
Effect of protein synthesis inhibitor on citrate efflux
When compared with Al treatment, the efflux of citrate was inhibited by 87 % after 9 h of treatment with 20 µm CHM (Fig. 6).
Fig. 6.
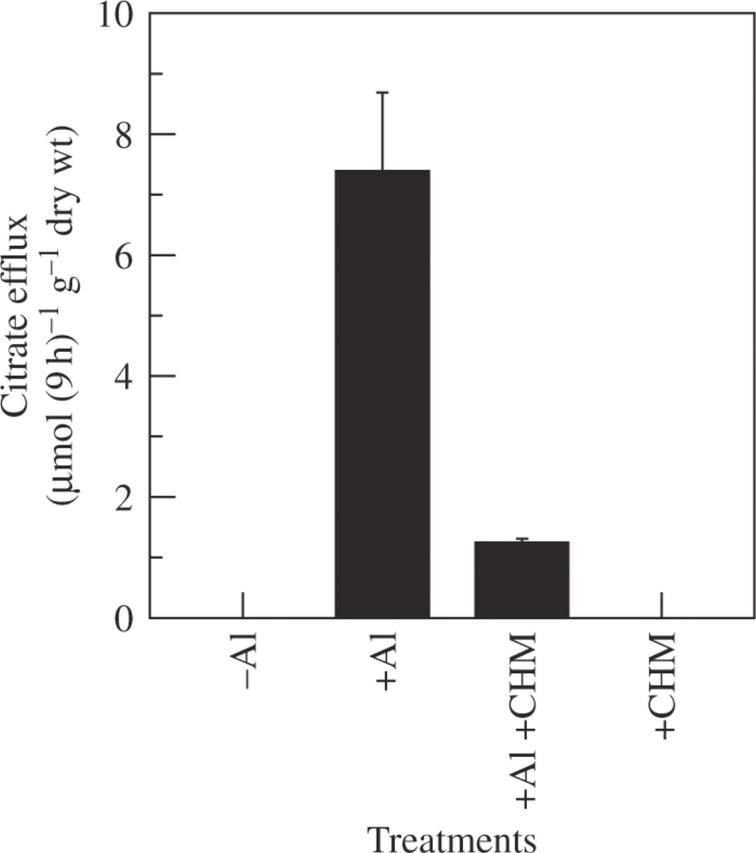
Effect of the protein synthesis inhibitor CHM on Al-stimulated release of citrate in rice bean. Seedlings were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing no Al (–Al), 50 µm AlCl3 (+Al), 50 µm AlCl3 plus 20 µm CHM (+Al +CHM) or 20 µm CHM (+CHM). Root exudates were collected after 9 h of treatment. Organic acids were analysed by HPLC. Error bars represent ± s.d. (n = 3).
DISCUSSION
Al-stimulated efflux of organic acids has been considered an important mechanism leading to Al resistance. We found that citrate was released from the roots of rice bean, but could not be detected during the first 3 h exposure to Al stress (Fig. 1). Furthermore, neither P deficiency nor LaCl3 stimulated the release of citrate (data not shown), indicating that the efflux was selective to Al stress. The efflux of citrate was largely confined to the apical 5 mm root zone (Fig. 2) where Al caused the greatest damage to root cells (Ryan et al., 1993), indicating the importance of citrate efflux in detoxifying Al. Although it is difficult to estimate how much the citrate efflux contributes to Al resistance in rice bean, there are ample examples to suggest that organic acids play an important role in improving Al resistance (for reviews, see Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004). The delayed efflux of citrate in response to Al stress is characteristic of a pattern II response (Ma et al., 2001), indicating that some inducible processes such as gene activation might be involved in the efflux of citrate. The same characteristic has been reported in a number of other plant species including C. tora (Yang et al., 2005b), rye (Li et al., 2000) and triticale (Ma et al., 2000).
Ma et al. (2001) postulated that the delayed efflux of organic acids in response to Al stress might be related to Al-activated gene expression and de novo protein synthesis. These genes may be involved in the metabolism (organic acids biosynthetic and/or turnover enzymes) of organic acids, the anion channel on the plasma membrane or transport of organic acids out of the mitochondria (Ma, 2000). However, the experimental evidence supporting the speculations is lacking, and most current work is focused on relating the organic acid efflux to changes in enzyme activities. For example, Al induced an increase of citrate content in rye and soybean, and the increase was accompanied by an increase in citrate synthase (Li et al., 2000; Yang et al., 2001). In line with this, genetic manipulation of plants to overexpress enzymes involved in organic acid biosynthesis showed increased organic acid contents, efflux and Al resistance (de la Fuente et al., 1997; Tesfaye et al., 2001; Anoop et al., 2003). Others, however, have shown poor correlations between efflux and the activities of biosynthetic enzymes (Ryan et al., 1995; Delhaize et al., 2001; Hayes and Ma, 2003; Zhao et al., 2003; Yang et al., 2005b). In the present study, we found that Al treatment increased citrate content in root apices (Fig. 5B). However, it is not adequate to state that Al-regulated citrate metabolism plays an important role in Al-stimulated citrate efflux, and in the present study we even argue that citrate metabolism plays at most a minor role in the efflux of citrate. The reasons supporting this rely on the following two lines of evidence. One is that the Al-induced increase in the content of citrate in root apices preceded the efflux (Fig. 5B). The other is that a 6 h pulse with 50 µm Al also significantly stimulated citrate efflux after 3 h and this was decreased thereafter when roots were transferred to the Ca solution (Fig. 5A), but the citrate content in root apices remained steady (Fig. 5B). Furthermore, studies using the patch–clamp technique have demonstrated that anion-permeable channels allowing the passive flow of organic anions from the cytosolic side of the membrane to the apoplasm are involved in the efflux of organic anions (Ryan et al., 1997; Kollmeier et al., 2001; Piñeros and Kochian, 2001; Zhang et al., 2001). Although we did not investigate the relationship between enzyme activities and citrate biosynthesis, the present results clearly imply that the transport of citrate across the membrane might constitute the critical step in the efflux of citrate in roots of rice bean.
Citrate is produced in mitochondria through the tricarboxylic acid or Krebs cycle, and citrate carrier, an intrinsic protein of the inner mitochondrial membrane, plays a vital role in exporting citrate out of the mitochondria. Since the pH of the cell cytosol is near neutral, the concentration of the undissociated citric acid is very low. Thus the thermodynamically passive transport of citrate anions across the plasma membrane can be mediated by anion channels (Ryan et al., 2001). In the present study, although different inhibitors showed different effects on the efflux, either anion channel inhibitors or citrate carrier inhibitors inhibited the Al-stimulated citrate efflux from roots of rice bean (Figs 3 and 4), indicating the possible involvement of both the citrate carrier and anion channel in the efflux process. It has also been reported that anion channel inhibitors can effectively inhibit malate efflux in wheat (Ryan et al., 1995) and maize (Jorge and Menossi, 2005). Li et al. (2000) found that two citrate carrier inhibitors effectively inhibited citrate efflux in rye. In the present study, the fact that a 6 h pulse with 50 µm Al was sufficient to induce citrate efflux (Fig. 5A) indicated that the opening of transporters can be maintained at least for 3 h. However, the addition of either PI (a citrate carrier inhibitor) or A-9-C (an anion channel inhibitor) inhibited the efflux of citrate greatly, and efflux was almost completely inhibited when the roots were exposed to both inhibitors (Fig. 5A), further confirming the cooperative roles of both transporter proteins in the regulation of citrate efflux.
In a previous study, we demonstrated that a protein synthesis inhibitor, CHM, inhibited Al-induced citrate efflux in C. tora (reported as a pattern II plant), and concluded that de novo synthesis of the anion channel is involved in the Al-induced secretion of citrate in C. tora (Yang et al., 2005b). In the present study, we also found that the Al-stimulated citrate efflux was significantly inhibited by 20 µm CHM (Fig. 6), indicating that de novo protein synthesis is also required for citrate efflux in rice bean. Given that other proteins are not required for the activation of transporter proteins mediating citrate efflux, we proposed that Al first activated gene expression, then these genes encode both citrate carrier and anion channel protein synthesis. However, the possibility still remains that it is de novo synthesis of other proteins rather than the transporters themselves that is required for the activation of transporter proteins. For example, Osawa and Matsumoto (2001) demonstrated that a 48 kDa K-252a-sensitive protein kinase might be involved in Al-stimulated malate efflux in wheat. Shen et al. (2004) related the Al-stimulated citrate efflux in soybean to abscisic acid biosynthesis. Recently, they further demonstrated that upregulation of plasma membrane H+-ATPase activity was associated with the citrate efflux in soybean roots (Shen et al., 2005).
In conclusion, these results suggest that Al-stimulated citrate efflux from roots of rice bean is mediated by both citrate carrier and anion channel. The possible involvement of other factors associated with the opening of transporter proteins has yet to be investigated.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China and Program for New Century Excellent Talents in University from the Chinese Ministry of Education.
LITERATURE CITED
- Anoop VA, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ. 2003. Modulation of citrate metabolism alters aluminium tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiology 132: 2205–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. 1995. Aluminum toxicity and tolerance in plants. Plant Physology 107: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. 1993. Aluminum tolerance in wheat (Triticum aestivum L.) II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiology 103: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Hebb DM, Ryan PR. 2001. Expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco is not associated with either enhanced citrate accumulation or efflux. Plant Physiology 125: 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. 2004. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proceedings of the National Academy of Science of the USA 101: 15249–15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente JM, Ramirez-Rodríguez V, Cabrera-Ponce JL, Herrera-Estrella L. 1997. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276: 1566–1567. [DOI] [PubMed] [Google Scholar]
- Genchi G, Spagnoletta A, Santis AD, Stefanizzi L, Palmieri F. 1999. Purification and characterization of the reconstitutively active citrate carrier from maize mitochondria. Plant Physiology 120: 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Ma JF. 2003. Al-induced efflux of organic acid anions is poorly associated with internal organic acid metabolism in triticale roots. Journal of Experimental Botany 54: 1753–1759. [DOI] [PubMed] [Google Scholar]
- Jorge RA, Menossi M. 2005. Effect of anion channel antagonists and La3+ on citrate release, Al content and Al resistance in maize roots. Journal of Inorganic Biochemistry 99: 2039–2045. [DOI] [PubMed] [Google Scholar]
- Kochian LV. 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 46: 237–260. [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology 55: 459–493. [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Dietrich P, Bauer SC, Horst WJ, Hedrich R. 2001. Aluminium activates a citrate-permeable anion channel in the aluminium-sensitive zone of the maize root apex. A comparison between an aluminium-sensitive and an aluminium-resistant cultivar. Plant Physiology 126: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Ma JF, Matsumoto H. 2000. Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiology 123: 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF. 2000. Role of organic acids in detoxification of Al in higher plants. Plant and Cell Physiology 44: 383–390. [DOI] [PubMed] [Google Scholar]
- Ma JF, Taketa S, Yang ZM. 2000. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiology 122: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. 2001. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science 6: 273–278. [DOI] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H. 2001. Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiology 126: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Kochian LV. 2001. A patch–clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiology 125: 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Carvalho VM, Kochian LV. 2005. Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiology study. Plant Physiology 137: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. 1993. Aluminium toxicity in roots: investigation of the spatial sensitivity and the role of the root cap in Al tolerance. Journal of Experimental Botany 44: 437–446. [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. 1995. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196: 103–110. [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyerman SD. 1997. Aluminum activates an anion channel in the apical cells of wheat roots. Proceedings of the National Academy of Science of the USA 94: 6547–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. 2001. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology 52: 527–560. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. 2004. A wheat gene encoding an aluminum-activated malate transporter. Plant Journal 37: 645–653. [DOI] [PubMed] [Google Scholar]
- Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan XL, Matsumoto H. 2004. Effect of K-252a and abscisic acid on the efflux of citrate from soybean roots. Journal of Experimental Botany 55: 663–671. [DOI] [PubMed] [Google Scholar]
- Shen H, He LF, Sasaki T, Yamamoto Y, Zheng SJ, Ligaba A, et al. 2005. Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Up-regulation of transcription, translation, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiology 138: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA. 2001. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiology 127: 1836–1844. [PMC free article] [PubMed] [Google Scholar]
- Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM. 2001. The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiology 125: 1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZM, Nian H, Sivaguru M, Tanakamaru S, Matsumoto H. 2001. Characterization of aluminum-induced citrate secretion in aluminum-tolerant soybean (Glycine max) plants. Physiologia Plantarum 113: 64–71. [Google Scholar]
- Yang JL, Zheng SJ, He YF, Matsumoto H. 2005a. Aluminium resistance requires resistance to acid stress: a case study with spinach that exudes oxalate rapidly when exposed to Al stress. Journal of Experimental Botany 56: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Yang JL, Zheng SJ, He YF, You JF, Zhang L, Yu XH. 2005b. Comparative studies on the effect of a protein-synthesis inhibitor on aluminum-induced secretion of organic acids from Fagopyrum esculentum Moench and Cassia tora L. roots. Plant Cell and Environment doi:10.1111/j.1365–3040.2005.01416.x. [DOI] [PubMed]
- Zhang WH, Ryan PR, Tyerman SD. 2001. Malate-permeable channels and cation channels activated by aluminium in the apical cells of wheat roots. Plant Physiology 125: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Ma JF, Sato K, Takeda K. 2003. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 217: 794–800. [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H. 1998. High aluminum resistance in buckwheat: I. Al-induced special secretion of oxalic acid from root tips. Plant Physiology 117: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H. 2005. Immobilization of aluminum with phosphorus in roots is associated with high Al resistance in buckwheat. Plant Physiology 138: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]