Abstract
Rationale
Food restriction (FR) enhances learned and unlearned behavioral responses to drugs of abuse and increases D-1 dopamine (DA) receptor-mediated activation of ERK 1/2 MAP kinase in nucleus accumbens (NAc). While a role has been established for ERK signaling in drug-mediated associative learning it is not clear whether ERK regulates unconditioned behavioral effects of abused drugs.
Objectives
The purpose of this study was to determine whether blockade of ERK signaling, using the brain-penetrant MEK inhibitor, SL-327, decreases behavioral or NAc cellular responses to acute drug treatment and their augmentation by FR.
Materials and methods
Separate experiments assessed effects of SL-327 (50 mg/kg, i.p.) on (i) the reward-potentiating effect of d-amphetamine in an intracranial self-stimulation protocol, (ii) the locomotor-activating effect of the D-1 agonist, SKF-82958, and (iii) Fos-immunostaining induced in NAc by SKF-82958.
Results
FR rats displayed enhanced responses to drug treatment on all measures. SL-327 had no effect on sensitivity to rewarding brain stimulation or the reward-potentiating effect of d-amphetamine. The MEK inhibitor, U0126, microinjected into NAc was also without effect. The locomotor-activating effect of SKF-82958 was unaffected by SL-327. In contrast, SL-327 decreased NAc Fos-immunostaining and abolished the difference between feeding groups.
Conclusions
These results support the conclusion that ERK signaling does not mediate unlearned behavioral responses to drug treatment. However, the upregulation of ERK and downstream transcriptional responses to acute drug treatment may underlie the reported enhancement of reward-related learning in FR subjects.
Keywords: food restriction, reward, self-stimulation, SKF-82958, d-amphetamine, c-fos, ERK 1/2, SL-327, U0126
INTRODUCTION
It is well established that chronic food restriction (FR) enhances the acquisition and maintenance of drug self-administration behavior in animal subjects (Carroll and Meisch, 1984). Using learning-free measures, it has also been found that the reward-potentiating and locomotor-activating effects of d-amphetamine (as well as a variety of other abused drugs) and the D-1 dopamine (DA) receptor agonist, SKF-82958, are greater in FR than ad libitum fed (AL) rats (Cabeza de Vaca and Carr, 1998; Carr et al., 2000; Carr et al., 2003). Drug treatments that produced stronger behavioral responses also produced greater activation of ERK 1/2 MAP kinase, CREB, c-fos, and preprodynorphin gene expression in nucleus accumbens (NAc) of FR relative to AL subjects (Carr and Kutchukhidze, 2000; Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005a, 2005b). The possibility that increased ERK 1/2 MAPK signaling is necessary for the enhanced behavioral and transcriptional responses to psychostimulant and D-1 DA agonist challenge is consonant with a number of findings. All drugs of abuse examined to date activate ERK throughout the striatum in a D-1 DA receptor-dependent manner (Valjent et al., 2004). Moreover, the ERK cascade activates RSK2, an S6 kinase that activates transcription factors, including CREB, that bind to the promoter region of the c-fos gene (Thomas and Huganir, 2004). MEK inhibitors -drugs that block the kinase upstream of ERK- abolish the conditioned place preference (CPP) otherwise reinforced by cocaine, amphetamine, THC and MDMA (Valjent et al., 2000; Valjent et al., 2001; Salzmann et al., 2003; Gerdjikov et al., 2004).
In the CPP studies, MEK inhibitors were administered in conjunction with a drug of abuse prior to each conditioning session. Consequently, it is not clear whether the blockade of ERK signaling attenuated the unconditioned rewarding effect of drugs or exclusively interfered with consolidation of the learned association between drug and context (Gerdjikov et al., 2004; Miller and Marshall, 2005; Valjent et al., 2006). There have been no behavioral studies designed to assess whether inhibition of ERK signaling attenuates the unconditioned rewarding effect of any abused drug in AL, let alone in FR subjects. This is not an unreasonable question to pose in as much as not all functional consequences of ERK phosphorylation would be expected to require transcription, translation and a long latency to behavioral effects. Upon activation, ERK is not only translocated to the nucleus but is also present in cytoplasm and dendrites, suggesting that ERK controls the phosphorylation state of local targets within or close to synapses, providing a rapid mechanism for modulating synaptic plasticity (Girault et al., 2006; Wang et al., 2007). A specific example is the rapid inactivation of voltage-gated K+ channels (Yuan et al., 2002). Behaviorally, ERK signaling in the NAc core and central amygdala, induced by exposure to cocaine-paired environmental cues, mediates expression of a CPP (Miller and Marshall, 2005) and reinstatement of cocaine-seeking during extinction (Lu et al., 2005). Most germane to the present study, there have been two reports that pretreatment with a MEK inhibitor attenuates the unconditioned motor-activating effects of psychostimulants (Valjent et al., 2000; Shi and McGinty, 2006). Consequently, the first aim of this study was to determine whether the reward-potentiating effect of d-amphetamine, passively administered to FR and AL rats responding in a curve-shift protocol of intracranial self-stimulation testing, is diminished by pretreatment with the brain-penetrant MEK inhibitor, SL-327.
While SL-327 previously decreased the activation of striatal ERK 1/2 induced by intracerebroventricular injection of SKF-82958, and eliminated the enhanced activation otherwise observed in FR subjects (Haberny and Carr, 2005a), the acute behavioral and c-fos responses to SKF-82958 treatment were not measured. Consequently, a second aim of this study was to administer SKF-82958 as in prior studies and determine whether SL-327 decreases the resultant hyperlocomotion, NAc c-fos expression, and/or the augmentation of these responses by FR.
MATERIALS AND METHODS
Subjects and Surgical Procedures
Subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) initially weighing 375–425 g. Food (pelleted Purina rat chow) and water were available ad libitum except when FR conditions applied. Animals were individually housed in clear plastic cages with bedding under a 12 h light:dark photoperiod with lights on at 0700 h. Subjects to be tested for reward-potentiating effects of d-amphetamine were deeply anesthetized with ketamine (100 mg/kg; i.p.) and xylazine (10 mg/kg; i.p.) and stereotaxically implanted with a 0.25 mm diameter monopolar stimulating electrode (Plastics One) in the lateral hypothalamic medial forebrain bundle (coordinates: 3.0 mm posterior to bregma, 1.6 mm lateral to the sagittal suture, and 8.5 mm ventral to skull surface). An anterior ipsilateral stainless steel skull screw served as ground. Subjects to be tested for locomotor-activating effects of SKF-82958 were implanted with a 26-gauge guide cannula (inner diameter: 0.24 mm; Plastics One, Roanoke, VA, USA) in the right lateral ventricle using the following coordinates with bregma and lamda suture landmarks in the same horizontal plane: 1.0 mm posterior to bregma, 1.5 mm lateral to the mid-sagittal suture, and 3.5 mm ventral to skull surface. Subjects to be tested for effects of MEK inhibitor microinjection in NAc were implanted with 26-gauge guide cannulae bilaterally, 2 mm dorsal to the intended injection site at the core/shell border using coordinates: 1.6 mm posterior to bregma, 2.5 mm lateral to the mid-sagittal suture (tips angled 8° toward the midline), and 5.7 mm ventral to skull surface. The electrode and/or cannula(e) were permanently affixed to the skull by flowing dental acrylic around them and four surrounding mounting screws. Patency of guide cannulae was maintained with an occlusion stylet. Five to seven days after surgery, accuracy of lateral ventricular cannula placements was verified by a short latency, sustained drinking response to microinjection of angiotensin II (50 ng in 5 μl). Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Food restriction and habituation training
Beginning two days after angiotensin testing of subjects in the locomotor study, half the rats were switched from ad libitum feeding to a restricted feeding regimen in which a single 10 g meal was delivered at approximately 1700 h each day. FR rats continued to have ad libitum access to water. Once body weight decreased by 20% (approximately 14 days) daily food allotments were titrated to maintain this target body weight for the remainder of the study. Experimental testing was initiated when body weights in this group had been stabilized for one week. During the ~3 week period preceding experimental testing, all rats were habituated, on at least 6 occasions, to transport (along an interior corridor from animal facility to laboratory), handling, microinjection (mock) procedures, and the activity chamber (30 min) to be employed throughout the study. Additional sets of eight AL and eight FR rats, with lateral ventricular cannulas, were habituated to all but the activity chamber and would ultimately be used to assess Fos-immunostaining. Half the subjects in the self-stimulation experiments were food-restricted, though the regimen was only initiated after subjects had achieved certain milestones in self-stimulation training (see below).
Self-stimulation (LHSS) apparatus
Brain stimulation training and testing were conducted in eight standard test chambers (26 × 26 × 21 cm) placed within sound attenuating cubicles. Each chamber had a retractable lever mounted on one wall and a house light mounted on the opposite wall. Four constant current stimulators (PHM-152B/2; Med-Associates, Georgia, VT), with dual outputs, were used to deliver trains of 0.1 ms cathodal pulses, which were conducted to implanted electrodes by way of commutators and flexible cables. Electrical stimulation, contingencies, and data recording were controlled through Dell XPS R400 computers and interface (Med-Associates). All stimulation parameters were monitored on Tektronix (TAS 455) oscilloscopes.
Self-stimulation (LHSS) procedures
After one week of postsurgical recovery, rats were trained to lever press for 0.5 s trains of electrical stimulation at a frequency of 100 pulses per second (pps). The initial stimulation intensity of 120 μA was systematically varied to locate, for each rat, the lowest intensity that maintained vigorous lever pressing. On subsequent days, rats were trained in a discrete-trials procedure. Each training session consisted of twenty-four 60-s trials. Extension of the lever and a 2-s train of ‘priming’ stimulation initiated each trial. Each trial was terminated by retraction of the lever and followed by a 10-s intertrial interval. Each lever press produced a 1-s train of stimulation, except for those presses emitted during the stimulation train, which did not increase reinforcement density. The number of reinforcements was recorded for each trial.
Discrete trials training was followed by rate-frequency training, which continued for approximately two weeks. Rate-frequency curves were generated by presenting 12 trials in which the frequency of brain stimulation decreased over successive trials (approximately 0.05 log units each trial) from an initial frequency of 100 pps to a terminal frequency of 28 pps. At least two such series were presented in each training session. During the second week of training, subjects were divided into two groups matched for body weight and M-50 (the brain stimulation frequency that supported 50% of the maximum reinforcement rate) and one of the groups was placed on the food restriction regimen described above. Training and mock test sessions (see below) continued, at least twice per week, for all rats, during the ensuing ~3 week period during which the FR group achieved and then stabilized at the target body weight (~80% of pre-restriction value).
Self-stimulation testing
Each test session began with a pre-injection test consisting of three rate-frequency series (42 min). The first series in a session was considered a ‘warm-up’ and data were excluded. Injection of SL-327 (50 mg/kg, i.p.; a generous gift from Dr. James Trzaskos and the Bristol Myers Squibb Company) or vehicle (DMSO) was followed, 30 min later, by injection of d-amphetamine (0.5 mg/kg, i.p.) or 0.9% saline vehicle, which was then followed, in 10 min, by a post-injection behavioral test consisting of two rate-frequency series (28 min). For each rate-frequency series, the number of reinforcements delivered as a function of stimulation frequency was recorded. For each rat, the two series from each test were averaged to yield a single rate-frequency function per test.
Groups of 7 AL and 7 FR rats were tested on 4 occasions separated by a minimum of 3 days. Each subject received the following treatment combinations: vehicle/vehicle; vehicle/d-amphetamine; SL-327/vehicle; SL-327/d-amphetamine in a semi-random order matched between feeding groups.
In a follow-up experiment two new groups of 6 AL and 6 FR rats were tested on 4 occasions. In all sessions all subjects were injected with d-amphetamine (0.5 mg/kg, i.p.) which was then followed, in 10 min, by a post-injection test. In the second and third sessions, d-amphetamine injection was preceded, 5 min earlier, by intra-NAc microinjection of U0126 (Sigma-Aldrich) at doses of 0.1 μg and 1.0 μg/0.5 μl bilaterally with half the subjects in each feeding group receiving the lower dose first, and half receiving the higher. In the first and fourth test sessions, d-amphetamine was preceded by intra-NAc microinjection of vehicle (50% DMSO) and results of the two tests were averaged.
Microinjection procedure
For microinjections, solutions were loaded into two 30 cm lengths of PE-50 tubing attached at one end to 25-μl Hamilton syringes filled with distilled water and at the other end to 31-gauge injector cannulae, which extended 2.0 mm beyond the implanted guides. The syringes were mounted on the twin holders of a Harvard 2272 microliter syringe pump which delivered the 0.5 μl injection volumes over a period of 100 sec. One minute following completion of injections, injector cannulae were removed from guides, stylets were replaced, and animals were returned to home cages for 5 min prior to systemic injection of d-amphetamine.
Self-stimulation data analysis
For each test, the rate-frequency function was used to derive three parameters. The maximum reinforcement rate, described by a line that parallels the x-axis, was defined as the mean of all consecutive values within 10% of the highest rate for the curve. All remaining values comprised the descending portion of the curve, with the lowest point being at the highest frequency to produce fewer than 2.5 reinforcements per minute. Regression analysis of the descending portion of the curve was used to calculate the M-50 and theta-0 reward thresholds which are defined as the log pulse frequency sustaining half the maximum reinforcement rate and x-axis intercept of the regression line, respectively. The M-50 is a conventional threshold measure in this protocol, though the theta-0 indicates the lowest frequency at which stimulation becomes rewarding. Changes in the reinforcing efficacy of stimulation produced by drugs of abuse are typically reflected as parallel leftward shifts in the rate-frequency curve with similar effects on the M-50 and theta-0 measures (Wise, 1996). For each parameter, antilog transformations were then applied and natural frequencies used to calculate the percentage change occurring in post-injection tests relative to a pre-injection test. Changes in reward threshold are considered to be reflective of changes in drug reward magnitude, while changes in the maximum rate are reflective of changes in performance capacity (Edmonds and Gallistel, 1974; Miliaressis et al., 1986). In the SL-327 experiment, results for each parameter were analyzed by 3-way ANOVA with repeated measures on two factors. In the U0126 experiment, results for each parameter were analyzed by 2-way ANOVA with repeated measures on one factor.
Motor activity apparatus and data recording
Horizontal activity was measured using the VersaMax System (Accuscan, Columbus, OH) which monitored animal activity via a grid of 16 × 16 infrared light beams that traverse the animal cage (42 × 42 × 30 cm) front to back and left to right. Information about beam status, scanned at a rate of 100 times per second, was stored to disk. Activity was expressed as the total number of beam interruptions in a 30 min session.
Experimental testing
Treatment groups were similar to the self-stimulation experiment except that i.c.v. injection of SKF-82958 was substituted for systemic d-amphetamine. Four test sessions were conducted, 3–4 days apart, in which rats were first injected with SL-327 or vehicle and then placed in the motor activity test chamber for a 30-min period of pre-i.c.v. injection data acquisition. This was followed by i.c.v. injection of vehicle (saline) or SKF-82958 (20 μg/2.5 μl), 10 min after which, animals were returned to the activity chamber for 30-min data acquisition.
Microinjection procedure
As above except for use of a single 31-gauge injector cannula, extending 1.0 mm beyond the implanted guide, through which the 2.5 μl injection volume was delivered over a period of 1 min.
Locomotor data analysis
Results from the 30-min period that preceded i.c.v. injections were analyzed separately from the post-i.c.v. injection period, with results obtained in the post-i.c.v. injection session being expressed as difference scores, and analyzed by 2-way ANOVA with repeated measures on one factor.
C-Fos immunohistochemistry
Subjects were eight AL and eight FR rats. Treatments consisted of a combination of intraperitoneal injection and i.c.v. injection prior to transcardial perfusion. Half the subjects in each feeding condition received vehicle/SKF-82958 (20 μg/2.5μl) and half received SL-327-SKF/82958. Based on the previous observation that Fos-immunostaining in NAc of rats injected i.c.v. with saline vehicle is weak and does not differ between feeding groups (Carr et al., 2003), the present test excluded the i.c.v. vehicle treatment condition.
Perfusion
All injections were made between 1000 and 1500 h and members of the two feeding groups were matched with respect to timing of injections. Thirty min following injection of SL-327 or vehicle, animals were injected i.c.v. with SKF-82958. Ninety min following the second injection they were transcardially perfused with isotonic phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS using a Cole-Parmer Masterflex console drive (model 7518-10). Brains were then removed and maintained in 20% sucrose at 4° C for 48 hours.
Immunostaining
Forty μm coronal sections of each brain were cut on a Reichert-Jung 2800 Frigocut cryostat and collected in a cryoprotective solution. At least six consecutive sections were sampled from a coronal level corresponding to +1.2 − +1.6 mm in the atlas of Paxinos and Watson (1998). Sampling was guided by light microscopic examination to ensure anatomical matching of sections across rats. Immunohistochemical staining was carried out using the avidin-biotin method. Sections were washed in 1% sodium borohydride followed by PBS and incubated for 2 hrs in 4% normal goat serum plus 1% BSA in PBS containing 0.2% Triton X-100 (Sigma) to block nonspecific binding. This was followed by incubation, overnight, with rabbit polyclonal c-fos antiserum (Oncogene Science; 1:5000 dilution). Following several PBS washes, sections were incubated with a secondary antiserum (Vector) for 60 min and subsequently reacted with avidin-biotin complex (ABC) (Vector). The peroxidase reaction was visualized with a chromogen solution containing 100mM nickel sulfate, 125 mM sodium acetate, 10 mM imidazole, 0.03% diaminobenzidine (DAB), and 0.01% hydrogen peroxide at pH 6.5. Sections were then mounted on chrome-alum coated slides, dehydrated, and coverslipped. To control for variation in the immunohistochemical reaction for Fos, tissue from the different treatment groups was reacted together to minimize potential histochemical variability.
Quantification of c-fos immunopositive cells and data analysis
Counting of c-fos positive cells in the NAc core and shell was accomplished by obtaining images using a Nikon Eclipse 50i microscope equipped with a Nikon S5500 digital camera, and counting grains using NIH Image J software. Medial shell and core were demarcated based on the schematics of the Paxinos and Watson atlas (1998). For nuclei counting, the Image J (NIH) program was set to accept black areas between 10 and 100 pixels at a magnification of 100× using a common background. Bilateral grain counts from three to five sections were averaged to arrive at a value for a particular rat, with the large areas counted expected to reduce selection bias and variability. Manual grain counts were taken in several cases to verify accuracy of automatic counts. NAc core and shell were analyzed separately using 2-way ANOVA.
RESULTS
Self-stimulation
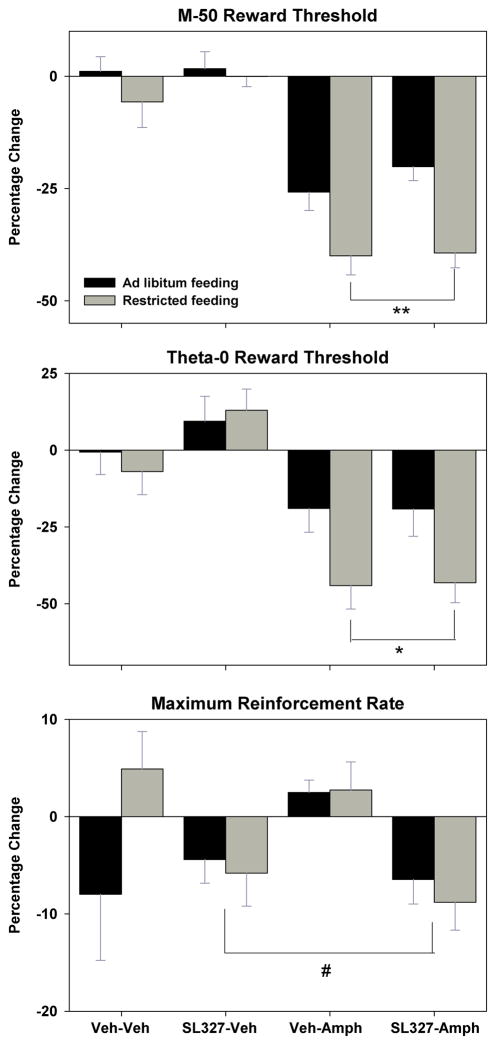
When subjects received intraperitoneal injection of 0.9% saline 10 min prior to the post-injection tests of self-stimulation, the M-50 measure of reward threshold did not change, regardless of whether the saline injection was preceded, 30 min earlier, by injection of DMSO or SL-327 (Figure 1, top panel, left). When subjects were injected with d-amphetamine 10 min prior to post-injection tests, the M-50 measure of reward threshold decreased (main effect of d-amphetamine, F1,11=78.4, p<.001) with FR subjects displaying a significantly greater effect than AL subjects (main effect of feeding condition, F1,11=23.7, p<.001; Figure 1, top panel, right). SL-327 pretreatment had no effect on the threshold-lowering effect of d-amphetamine in either feeding group (Figure 1, top panel, right). The identical pattern of results was obtained for the theta-0 measure of reward threshold (Figure 1, middle panel; main effect of d-amphetamine, F1,11=35.1, p<.001; main effect of feeding condition, F1,11=7.8, p<.02). A different pattern of results was obtained for the maximum reinforcement rate measure, with the one significant effect being a decrease produced by SL-327 treatment (Figure 1, bottom panel; main effect of SL-327, F1,11=7.8, p<.02).
Figure 1.
Mean (± s.e.m.) percentage changes in the M-50 (top) and theta-0 (middle) measures of reward threshold and maximum reinforcement rate (bottom), derived from self-stimulation rate-frequency curves, 10 min following intraperitoneal injection of d-amphetamine (0.5 mg/kg) or vehicle in ad libitum fed (black fill) and food-restricted (gray fill) rats. These injections were preceded, 30 min earlier, by intraperitoneal injection of SL-327 (50 mg/kg) or DMSO vehicle. **p<.001 compared to AL subjects; *p<.02 compared to AL subjects; #p<.02 compared to vehicle pretreatment.
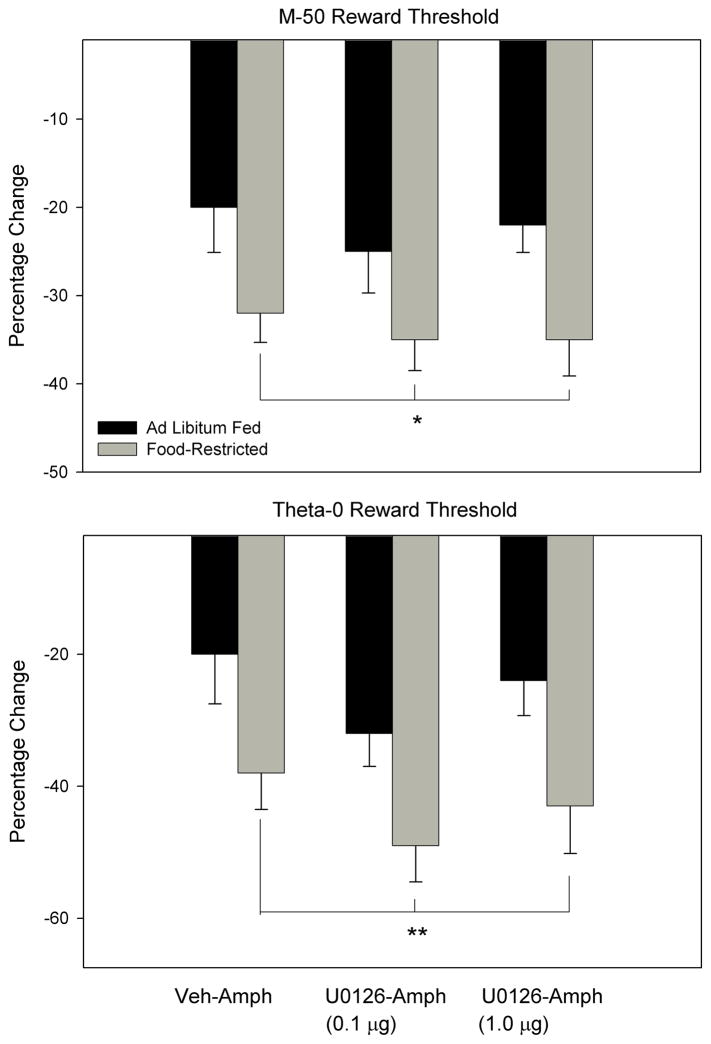
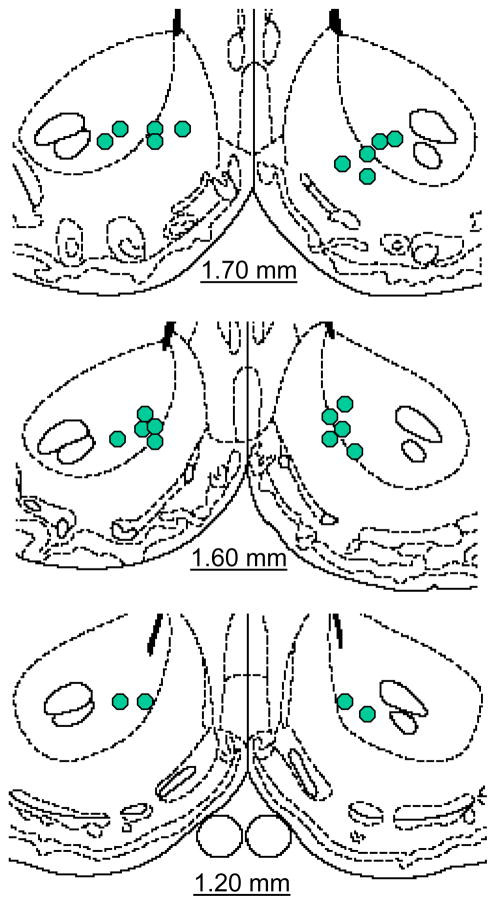
In the follow-up experiment, d-amphetamine produced a greater lowering of the M-50 reward threshold in FR relative to AL subjects (Figure 2, top panel; main effect of feeding condition, F1,10=7.58, p<.025) with no effect of intra-NAc U0126 microinjection. The same result was obtained for the theta-0 measure of reward threshold (Figure 2, bottom panel; main effect of feeding condition, F1,10=10.99, p<.01). Neither feeding condition nor U0126 treatment affected the maximum reinforcement rate (data not shown). Microinjection sites were generally located in the medial shell bordering the core, or in medial core (Figure 3).
Figure 2.
Mean (± s.e.m.) percentage changes in the M-50 (top) and theta-0 (bottom) measures of reward threshold derived from self-stimulation rate-frequency curves, 10 min following intraperitoneal injection of d-amphetamine (0.5 mg/kg) or vehicle in ad libitum fed (black fill) and food-restricted (gray fill) rats. These injections were preceded, 5 min earlier, by intra-nucleus accumbens microinjection of U0126 (0.1 and 1.0 μg/0.5 μl, bilaterally) or vehicle (50% DMSO). *p<.025 compared to AL subjects; **p<.01 compared to AL subjects.
Figure 3.
Schematic diagrams adapted from Paxinos and Watson (1998), indicating sites of U0126 microinjection in nucleus accumbens.
Locomotor activity
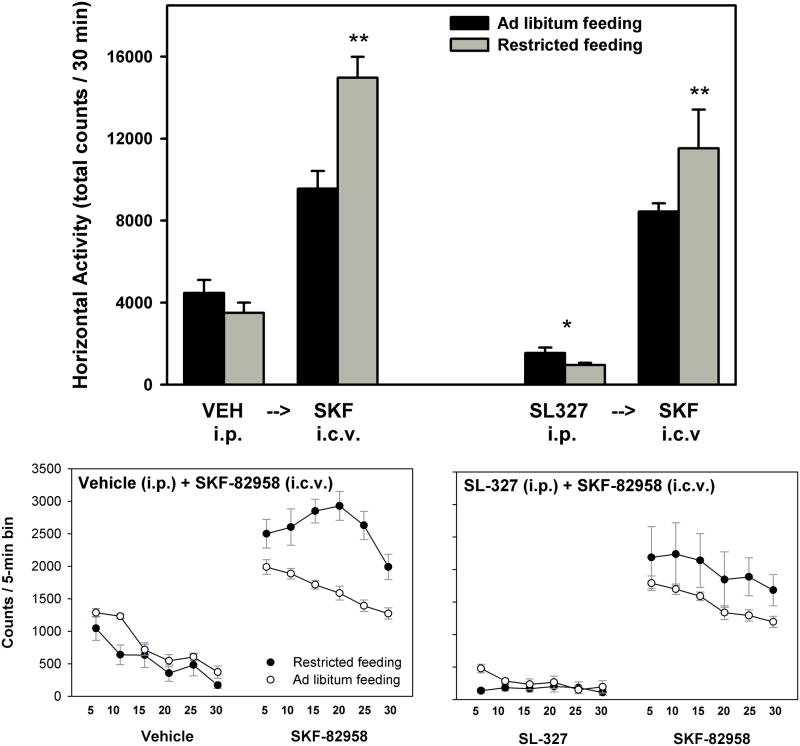
During the initial 30 min period of activity monitoring that followed intraperitoneal injections, no differences were observed between AL and FR subjects. However, SL-327 decreased locomotor activity across feeding conditions (main effect of SL-327, F1,13=70.6, p<.001; Figure 4). Activity scores for the subsequent 30 min period of monitoring, which followed i.c.v. microinjections, were analyzed as difference scores (i.e. post-i.c.v. activity – pre-i.c.v. activity; data not shown). Accordingly, FR subjects displayed significantly greater locomotor activation than AL subjects (main effect of feeding condition, F1,13=19.6, p=.001), with no effect of SL-327 treatment. SL-327 produced uniformly low levels of spontaneous forward locomotion across 5-min time bins of the 30-min monitoring period in both feeding groups (Figure 4, bottom). Nevertheless, the activating effect of SKF-82958 and the difference between FR and AL groups was evident whether preceded by vehicle or SL-327.
Figure 4.
Top: Mean (± s.e.m.) horizontal activity counts in the 30 min period following intraperitoneal injection of DMSO vehicle or SL-327 (50 mg/kg) –indicated by bars to the left of each arrow- and following a subsequent i.c.v. injection of SKF-82958 (20 μg) –indicated by bars to the right of each arrow- in ad libitum fed (black fill) and food-restricted (gray fill) rats. **p<.001 compared to AL subjects; *p<.001 compared to vehicle treatment. Bottom: Horizontal activity counts per 5-min time bin following intraperitoneal injection of vehicle (left side of left graph), SL-327 (left side of right graph), and following subsequent i.c.v. injection of SKF-82958 (right side of both graphs) in ad libitum fed (open symbols) and food-restricted (filled symbols) rats.
The effects described for horizontal activity were also observed as trends in the vertical activity measure, although the difference between feeding groups in response to SKF-82958 was not significant (data not shown).
Fos-immunostaining
In both the NAc shell and core, SKF-82958 produced greater Fos-immunostaining in FR than AL subjects (F(shell)1,12=5.9, p<.05; F(core) 1,12=5.6, p<.05). SL-327 pretreatment suppressed Fos-immunostaining in both regions across feeding conditions (F(shell)1,12=12.4, p<.01; F(core) 1,12=7.3, p<.02; Figure 5). At the coronal levels examined, it was also observed that SL-327 eliminated the relatively weak immunostaining otherwise seen in caudate-putamen and weakened the otherwise strong immunostaining seen in piriform cortex of both feeding groups (not shown).
Figure 5.
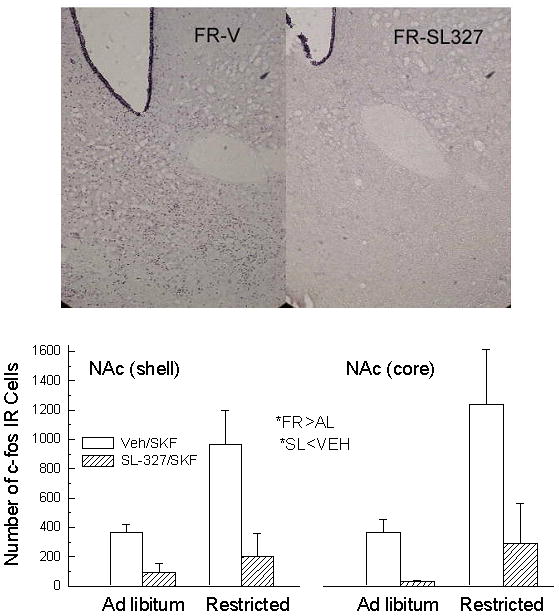
Bottom: Mean (± s.e.m.) bilateral count of Fos-immunoreactive cells in NAc shell (left) and NAc core (right) of ad libitum fed and food-restricted rats following i.c.v. injection of SKF-82958 (20 μg) preceded, 30 min earlier, by intraperitoneal injection of DMSO vehicle (clear bar) or SL-327 (50 mg/kg; hatched bar). Top: Representative photomicrographs depicting Fos-immunostaining in NAc of food-restricted rats injected i.c.v. with SKF-82958 preceded by intraperitoneal injection of vehicle (left) versus SL-327 (right).
DISCUSSION
Striatal ERK phosphorylation in response to D-1 DA agonist and psychostimulant drug treatment is rapid and transient, peaking at about 15–20 minutes post-injection (Valjent et al., 2000; Gerfen et al., 2002). Consequently, the self-stimulation and locomotor testing conducted in this study, which commenced 10 minutes after drug injection and continued for ~ 30 minutes should have coincided with the period of ERK phosphorylation. Yet, administration of a dose of SL-327 similar to those previously used to block D-1 DA agonist and psychostimulant-induced ERK phosphorylation (e.g., Valjent et al., 2000; Gerfen et al., 2002; Haberny and Carr, 2005a; Shi and McGinty, 2006), had no effect on sensitivity to rewarding electrical brain stimulation or the reward-potentiating effect of d-amphetamine, which was markedly greater in FR than AL rats. However, this result does not conclusively indicate that ERK phosphorylation in NAc does not affect the reward-potentiating effect of d-amphetamine; it is possible that an unexpected interaction between MEK inhibition and FR occurred outside of the NAc and precluded detection of an effect in the self-stimulation task. To address this possibility, a follow-up experiment was conducted in which the MEK inhibitor U0126 was microinjected directly into NAc, using a 1.0 μg dose which previously blocked expression of a cocaine conditioned place preference (Miller and Marshall, 2005) and a 0.1 μg dose which has blocked cue-induced cocaine-seeking when injected into central amygdala (Lu et al., 2005). However, neither dose decreased the reward-potentiating effect of d-amphetamine in AL or FR rats. One caveat is that cannulae were generally placed at the medial shell/core border. Should behaviorally significant ERK phosphorylation occur in a region bordering the olfactory tubercle or in lateral core, the present test may not have been adequate to ascertain it. Nevertheless, the result obtained adds support to the conclusion that ERK signaling in NAc does not mediate or modulate the reward efficacy of acute electrical brain stimulation or d-amphetamine. This contrasts with the processes through which associations are formed between environmental contexts and subjective rewarding effects of drugs, and the sensitization that develops with chronic drug treatment - both of which are blocked when each psychostimulant treatment is preceded by administration of SL-327 or other MEK inhibitor (for reviews see: Girault et al., 2006; Lu et al., 2006). The one modest, but significant effect of SL-327 in the self-stimulation experiment was a decrease in maximum reinforcement rate. This is indicative of a decrease in performance capacity and is consonant with the decrease in basal locomotor activity produced by this treatment (see below).
The apparent lack of ERK involvement in the unconditioned behavioral response to DA-enhancing drugs of abuse is further supported by results of the locomotor activity experiment. The dose and route of administration chosen for SKF-82958 were based on the previous observation that this treatment not only resulted in greater locomotor activation in FR than AL subjects, but also greater NAc ERK phosphorylation, CREB phosphorylation, and c-fos and preprodynorphin gene expression (Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005a, 2005b). Most importantly, when preceded by intraperitoneal injection of SL-327 the ERK phosphorylation was markedly decreased and the difference between feeding groups was eliminated (Haberny and Carr, 2005a). Yet, in the present study SL-327 pretreatment did not diminish the locomotor activating effect of SKF-82958, nor alter the difference between feeding groups. However, in the 30 minute period following SL-327 injection prior to SKF-82958 injection, there was a clear decrease in spontaneous locomotor activity across feeding conditions. This observation is consistent with the inferred decrease in performance capacity produced by SL-327 in the self-stimulation experiment. It is unlikely that the depressant effect of SL-327 on basal locomotor activity is reflective of malaise or an aversive state because ICSS thresholds were unaffected by this treatment and aversive/dysphoric states generally increase reward thresholds (for recent review see Carlezon and Chartoff, 2007). Further, systemic injections of SL-327 similar to those used in the present study failed to reinforce a conditioned place aversion (Valjent et al., 2000).
Several studies have examined effects of SL-327 on the unconditioned locomotor-activating effect of psychostimulants in AL mice and rats. While Valjent and coworkers originally reported that SL-327 decreased cocaine-induced motor activation (2000), their subsequent work indicated that doses of SL-327 that were sufficient to block cocaine-induced ERK phosphorylation in striatum did not affect the unconditioned locomotor response to cocaine (Valjent et al., 2006). The same observation was made by Ferguson and coworkers (2006). Similarly, NAc microinjections of the MEK inhibitor, PD98059, blocked acquisition of a d-amphetamine-reinforced CPP but had no effect on d-amphetamine-induced locomotion (Gerdjikov et al., 2004). In contrast, Shi and McGinty (2006) reported that both systemic injection of SL-327 and microinjection of U0126 in caudate-putamen decreased the motor-activating effect of d-amphetamine without affecting basal locomotor activity. The absence of effect of SL-327 on basal activity differs from the present observation and may reflect procedural differences between the two studies. In the present study rats were habituated to the activity chambers on at least 6 occasions prior to the test session while subjects in the Shi and McGinty study were habituated on one occasion. Consequently, novelty-induced exploratory behavior would have been stronger in their study and perhaps less susceptible to a depressant effect of SL-327. Differences in environmental novelty may also have contributed to their uncommon finding that SL-327 decreased drug-induced hyperactivity; amphetamine administration in a novel environment produces an enhanced locomotor response (Crombag et al., 2001) and uniquely recruits a corticostriatal glutamate pathway that leads to ERK-dependent c-fos expression in D-2 DA receptor/enkephalin-expressing neurons of dorsal striatum (Ferguson and Robinson, 2004). Indeed, Shi and McGinty (2006) observed strong, SL-327-sensitive c-fos expression in this neuronal population. In contrast, the SKF-82958 treatment employed in the present locomotor activity study did not induce preproenkephalin gene expression in dorsal striatum of AL or FR rats (Haberny and Carr, 2005b). Consequently, the conclusions drawn from the present effects of SL-327 on SKF-82958-induced motor activity may not generalize to all psychostimulants or contexts; pending additional studies, these results can be taken to indicate that ERK phosphorylation induced by D-1 DA receptor stimulation in a familiar environment contributes little or not at all to the elicited locomotor activity regardless of feeding condition.
The observation that SL-327 markedly decreased NAc Fos-immunostaining induced by SKF-82958, across feeding conditions, is important for two reasons. First, it confirms that the SL-327 treatments used in this study were efficacious in blocking a cellular response to D-1 DA receptor stimulation. Second, it confirms that ERK phosphorylation is necessary for downstream induction of the immediate-early gene (IEG) c-fos, and that the normally upregulated ERK response to D-1 DA receptor stimulation in FR subjects is involved in the correspondingly upregulated expression of c-fos, just as it was shown to be involved in the increased phosphorylation of CREB (Haberny and Carr, 2005a). The inhibitory effect of SL-327 on Fos-immunostaining was dramatic and perhaps surprising given the well established involvement of cAMP/protein kinase A (PKA) in D-1 DA receptor-mediated CREB phosphorylation and c-fos expression (e.g., Das et al., 1997). However, D-1 DA receptor stimulation also leads to increased PKA-dependent phosphorylation of the NMDA receptor NR1 subunit, which increases Ca2+ conductance (Snyder et al., 1998). DA- and PKA-mediated induction of c-fos requires Ca2+ entry via NMDA receptors (Konradi et al., 1996; Dudman et al., 2003), and ERK has been shown to be the primary mediator of Ca2+ -dependent c-fos induction via both CREB and Elk-1 (Vanhoutte et al., 1999). Thus, ERK-dependent activation of CREB and ELK-1 transcription factors may account for a substantial portion of the net c-fos expression in NAc that follows D-1 receptor stimulation.
Overall, the present results support the hypothesis that although drugs of abuse and D-1 DA receptor agonists strongly activate NAc ERK 1/2 upon first exposure, the functional consequences may not be evident until network strengthening effects are probed in a CPP or other associative learning paradigm. Further, the parallel between increased unconditioned behavioral effects of SKF-82958 and increased NAc ERK phosphorylation suggests that drug-mediated associative learning should also be enhanced in FR subjects. In fact, there is a substantial literature indicating that chronic FR enhances learning, memory, and sensorimotor function (Idobro et al., 1987; Ingram et al., 1987; Stewart et al.,1989), with enhanced hippocampal long term potentiation (LTP) as one underlying mechanism (Hori et al., 1992; Goldin et al., 2003). LTP is mediated by increased Ca2+ conductance through the NMDA cation channel which, in turn, activates ERK and CaMK II signaling pathways, both of which are necessary for consequent increases in synaptic strength (Hayashi et al., 2000; Sweatt, 2001; Goldin et al., 2003; Thomas and Huganir, 2004). Importantly, there is increasing evidence that similar mechanisms are present in striatal neurons (Kerr et al., 2001; Wolf et al., 2004; Hyman et al., 2006), and the increased SKF-82958-induced ERK phosphorylation in NAc of FR rats has been shown to be NMDA receptor dependent (Haberny and Carr, 2005a). Thus, the upregulated NAc cell signaling and gene expression observed in FR subjects upon stimulation of the D-1 DA receptor are likely to have important functional consequences for drug-directed behavior, potentially affecting vulnerability to addiction, craving and relapse (Berke and Hyman, 2000; Thomas and Huganir, 2004; Carlezon et al., 2005; Girault et al., 2006). The enhanced acquisition of drug self-administration behavior (Carroll and Meisch, 1984) and drug-reinforced CPP (Bell et al., 1997; Cabib et al., 2000) in FR relative to AL rats are consistent with this expectation, although the contributions of increased drug rewarding effects and enhanced associative learning are conflated in these protocols.
As for the increased behavioral sensitivity of FR subjects to acute drug exposure, this will more likely be explained by changes in medium spiny neuron (MSN) excitability and the way in which convergent DA- and glutamate-coded inputs are integrated by these cells on a subsecond time-scale. D-1 DA receptor stimulation controls MSN firing rates by depressing weak excitatory synaptic signals and augmenting responses to strong glutamatergic inputs, promoting the transition from the hyperpolarized ‘down’ state to the more depolarized ‘up’ state (e.g., Surmeier et al., 2007). Most germane to the behavioral measures taken in the present study, D-1 DA receptor stimulation in NAc shell controls MSN firing rates that are time-locked with and necessary for expression of self-stimulation behavior (Cheer et al., 2007); moreover, microinjection of SKF-82958 in NAc shell exerts reward-potentiating and motor-activating effects that are greater in FR than AL rats (Carr et al., submitted).
The enhanced behavioral responsiveness to acute drug challenge and the upregulation of D-1 DA receptor-mediated cell signaling and transcriptional responses are likely to reflect adaptations to FR that serve energy homeostasis by increasing reward-directed behavior and appetitive associative learning. However, as an extension of the hypothesis that drugs of abuse usurp neuronal circuits that mediate adaptive goal-directed behavior (Kelley and Berridge, 2002; Cardinal and Everitt, 2004; Volkow and Wise, 2005), these neuroadaptations may increase vulnerability to maladaptive forms of goal-directed behavior associated with food restriction, such as restriction-binge cycling and substance abuse (Krahn et al., 1992; Wilson, 1993; Wiederman and Pryor, 1996; Polivy, 2006). Clarification of neuroadaptations that mediate adaptive and maladaptive responses to chronic FR will require a differentiation of mechanisms that modulate unconditioned responses to salient stimuli, reward-related learning, and determination of whether FR simply upregulates mechanisms that prevail under AL feeding conditions or includes mechanisms that are uniquely expressed under the condition of chronic FR.
Acknowledgments
This research was supported by DA03956 from NIDA/NIH.
References
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine- induced conditioned place preference and locomotor activity in rats. Psychopharmacol. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EA. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends in Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, Chau LS. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPA GluR1 antagonist in nucleus accumbens shell are increased by food restriction; possible relevance to enhancement of adaptive and maladaptive reward-directed behavior (submitted) [Google Scholar]
- Carr KD, Kim G-Y, Cabeza de Vaca S. Chronic food restriction augments the central rewarding effect of cocaine and the δ-1 opioid agonist, DPDPE, but not the δ-2 agonist, deltorphin-II. Psychopharmacol. 2000;152:200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N. Chronic food restriction increases fos-like immunoreactivity induced in rat forebrain by intraventricular amphetamine. Brain Res. 2000;861:88–96. doi: 10.1016/s0006-8993(00)02018-7. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol. 1984;4:47–88. [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron. 2007;54:237–244. doi: 10.1016/j.neuron.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Bingeing rats; a model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacol. 2001;24:680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Das S, Grunert M, Williams L, Vincent SR. NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-fos in striatal neurons in primary culture. Synapse. 1997;25:227–233. doi: 10.1002/(SICI)1098-2396(199703)25:3<227::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds DE, Gallistel CR. Parametric analysis of brain stimulation reward in the rat: III Effect of performance variables on the reward summation function. J Comp Physiol Psychol. 1974;87:876–884. doi: 10.1037/h0037217. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacol. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2 MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Girault J-A, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug- induced plasticity? Curr Opin Pharmacol. 2006;6:1–9. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005a;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Comparison of basal and D-1 dopamine receptor agonist- stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Molec Brain Res. 2005b;141:121–127. doi: 10.1016/j.molbrainres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hori N, Hirotsu I, Davis PJ, Carpenter DO. Long-term potentiation is lost in aged rats but preserved by calorie restriction. Neuroreport. 1992;3:1085–1088. doi: 10.1097/00001756-199212000-00013. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Idobro F, Nandy K, Mostofsky DL, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pogment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriat. 1987;6:355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- Konradi C, Leveque J-C, Hyman We. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Subst Abuse. 1992;4:341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdale ERK signaling pathway is critical to incubation of cocaine craving. Nature Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends in Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. An evolutionary perspective on dieting. Appetite. 2006;47:30–35. doi: 10.1016/j.appet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neurosci. 2006;138:1289–1298. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nature Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9- tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault J-A, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier J-V, Guibert B, Pages C, Besson M-J, Hipskind RA, Caboche J. Glutamate induces phosphorylation of elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance abuse and impulsive behaviors among adolescents with eating disorders. Addictive Behav. 1996;21:269–272. doi: 10.1016/0306-4603(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Wilson GT. Binge eating and addictive disorders. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. Guilford Press; New York: 1993. pp. 97–120. [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacol. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]