Abstract
Embryos and developing organs have the remarkable ability of self-regenerating after experimental manipulations. In the Xenopus blastula half-embryos can regenerate the missing part, producing identical twins. Studies on the molecular nature of Spemann’s organizer have revealed that self-regulation results from the battle between two signaling centers under reciprocal transcriptional control. Long-range communication between the dorsal and ventral sides is mediated by the action of growth factor antagonists – such as the BMP antagonist Chordin – that regulate the flow of BMPs within the embryonic morphogenetic field. BMPs secreted by the dorsal Spemann organizer tissue are released by metalloproteinases of the Tolloid family, which cleave Chordin at a distance of where they were produced. The dorsal center secretes Chordin, Noggin, BMP2 and ADMP. The ventral center of the embryo secretes BMP4, BMP7, Sizzled, Crossveinless-2 and Tolloid-related. Crossveinless-2 binds Chordin/BMP complexes, facilitating their flow towards the ventral side, where BMPs are released by Tolloid allowing peak BMP signaling. Self-regulation occurs because transcription of ventral genes is induced by BMP while transcription of dorsal genes is repressed by BMP signals. This assures that for each action of Spemann’s organizer there is a reaction in the ventral side of the embryo. Because both dorsal and ventral centers express proteins of similar biochemical activities, they can compensate for each other. A novel biochemical pathway of extracellular growth factor signaling regulation has emerged from these studies in Xenopus. This remarkable dorsal-ventral positional information network has been conserved in evolution and is ancestral to all bilateral animals.
Keywords: Morphogenetic fields, Embryonic induction, Dorsal-Ventral patterning, BMP, Chordin, Crossveinless-2, Tolloid, Sizzled, Hox genes, Urbilateria
1. Introduction
Within the organism cells do not lead individual lives as they do in a tissue culture Petri dish. They proliferate, differentiate and die as part of groups of hundreds or thousands of cells called morphogenetic fields. Embryology has shown that cells within a field can communicate with each other over long distances, self-regulating pattern to generate the most perfect form possible after experimental perturbations. The molecular mechanisms of cell-cell communication within morphogenetic fields are key to understanding the development and homeostasis of animal tissues and organs, and are the topic of this review. As we will see, the flow of growth factors and their antagonists within the embryonic field is a fundamental property of self-regulating patterning systems.
1.1 Self-regulation
Self-regulation has captured the interest of biologists since the very beginning of experimental embryology. In 1891 Hans Driesch separated the first two cells and in 1936 Sven Horstädius succeeded in separating the first four cells of a sea urchin embryo (Horstädius, 1973). As shown in Figure 1, each cell was able to form a complete sea urchin larva. This tendency of the embryo to form the whole constitutes one of the deepest mysteries in developmental biology.
Fig. 1.
Separation of the first four blastomeres of a sea urchin embryo can give rise to four well-formed pluteus larvae. This powerful regulation was first reported by H. Driesch in 1891, marking the beginning of experimental embryology. It now appears that the self-regulation of embryonic fragments had been reported even earlier, in 1869, by Ernst Haeckel in cnidarian embryos (Sanchez-Alvarado, 2008). The experiment shown here is from Hörstadius and Wolsky, 1936, W. Roux. Arch. Entw. Mech. Org. 135, 69–113, reproduced with permission.
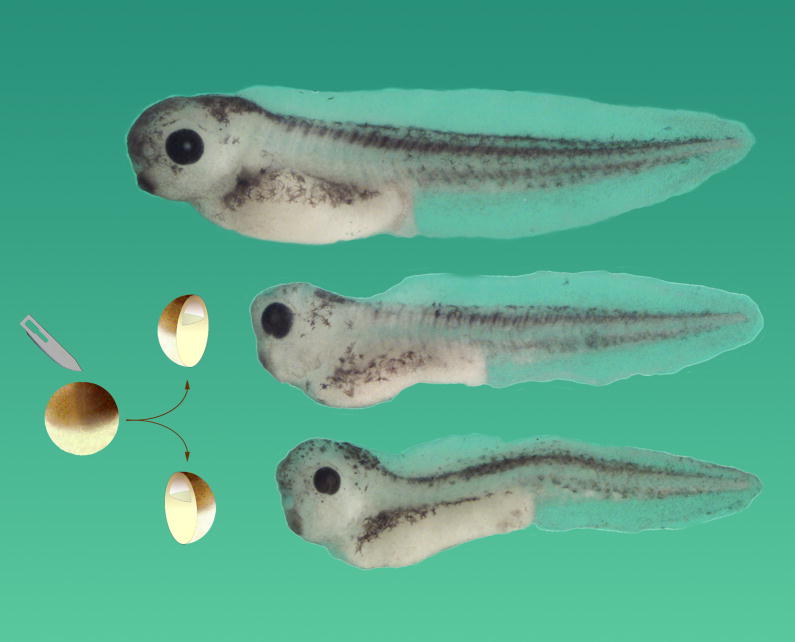
Hans Spemann investigated self-regulation in amphibian embryos gently constricted by fine loops from the hair of his newborn daughter, and was able to generate twins (reviewed in Spemann, 1938). Much later, I realized it is sufficient to bisect a Xenopus embryo at the blastula stage with a scalpel in order to generate identical twins (De Robertis, 2006) (Fig. 2). This simple procedure proved a very useful tool in the investigations discussed below. Twinning after experimental perturbation also takes place in insect embryos (Sander, 1976), and thus self-regulation is a universal phenomenon in animal development.
Fig. 2.
In Xenopus, the blastula constitutes a self-differentiating morphogenetic field, in which cells are able to communicate over long distances. When the blastula is bisected with a scalpel blade, identical twins can be obtained, provided that both fragments retain Spemann’s organizer tissue. Thus a half-embryo can regenerate the missing half. In humans, identical twins are found in three out of 1000 live births, and usually arise from the spontaneous separation of the inner cell mass of the blastocyst into two. A normal tadpole is shown on top, and two identical twins derived from the same blastula below, all at the same magnification. Reproduced from De Robertis, 2006, with permission of Nature Reviews.
1.2 Morphogenetic fields
Natural selection would not have generated self-regulation just in case an inquisitive developmental biologist came by to cut embryos up. Deeper causes must be in play, offering an evolutionary advantage to self-regulating embryos. The tendency to re-form the whole is also observed in later development. During early development (up to gastrulation), we speak about “primary morphogenetic field” regulation, but at later stages experimental embryology has demonstrated that most organs also start their development as “secondary self-regulating morphogenetic fields” (reviewed in Huxley and De Beer, 1934; De Robertis et al., 1991) (Fig. 3).
Fig. 3.
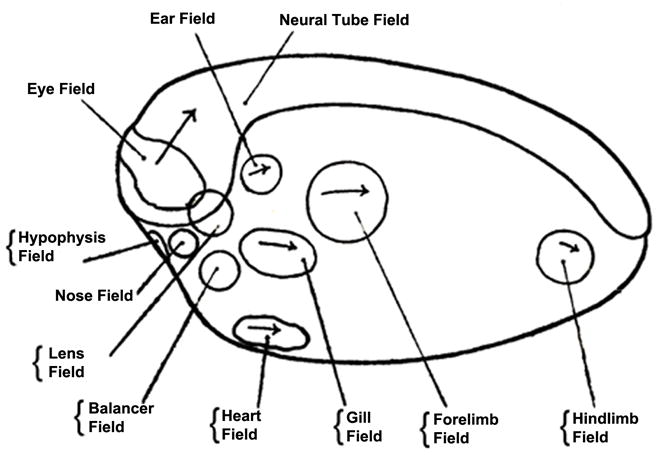
Organ-fields identified by experimental embryologists in the amphibian neurula. The concept of self-regulating morphogenetic fields arose from a transplantation experiment by R. Harrison (1918) using the forelimb field. Reproduced from Huxley and de Beer, 1934, with permission of Cambridge University Press.
The concept of morphogenetic fields was proposed by the famous American embryologist Ross G. Harrison. Working at Yale on embryos of the American salamander Amblystoma punctatum (now renamed Ambystoma maculatum), Harrison showed that a circular region of lateral plate mesoderm would induce the development of forelimbs when transplanted into host embryos. When he cut this region in half, each half could induce a limb. Not a half-limb, but rather an entire limb (Harrison, 1918). Since this experiment a key question in developmental biology has been: How does this regeneration of pattern towards the whole come about?
2. The organizer
2.1 Hans Spemann, Hilde Mangold and the organizer
The way forward in the analysis of self-regulation of pattern came from a transplantation experiment carried out by a graduate student at Freiburg University named Hilde Mangold. Under the direction of Hans Spemann, she grafted the dorsal blastopore lip, the region where gastrulation starts, from a weakly pigmented salamander gastrula to the ventral side of a more pigmented species. This allowed her to distinguish the cells contributed by the graft from those of the host embryo. The lineage-tracing technique used, named heteroplastic transplantation, had been invented by Ross Harrison, who used it to demonstrate that lateral line organ cells of the amphibian tadpole trunk and tail migrate from anterior (auditory) regions of the embryo (Harrison, 1903). Harrison was a close friend of Spemann, hence the use of this lineage tracing method to follow the fate of dorsal lip grafts. During earlier salamander breeding seasons, Spemann had found that the dorsal lip of the blastopore was the only region of the embryo that did not adopt the fate of the surrounding cells when transplanted, but instead kept its own fate giving rise to dorsal tissues (Spemann, 1938).
Hilde Mangold found, and described in exquisite camera lucida drawings of histological sections, that the transplanted dorsal tissue gave rise mostly to notochord, while the neighboring cells from the host were induced to form a Siamese twin containing dorsal tissues such as somites and central nervous system (CNS) (Spemann and Mangold, 1924). This experiment provided the basis for our current view that embryonic development occurs through a succession of cell-cell interactions. Tragically, Hilde Mangold (née Pröscholdt) died shortly afterwards in a kitchen stove accident while warming milk for her recently born baby. She did not live to see her paper published.
Spemann named the inducing activity of the dorsal lip the “organizer”, for it induced a well-formed Siamese twin. Figure 4 shows a Spemann graft in which the transplanted tissue caused the primary embryonic field to become divided almost perfectly in two. This experiment became extremely well known because Spemann was awarded the Nobel Prize for Medicine or Physiology in 1935 for the discovery of embryonic induction by the organizer. However, the demise of Spemann’s organizer was to follow soon afterwards, once the search for the chemical nature of the organizer inducing activity began.
Fig. 4.

The Spemann-Mangold experiment reproduced in Xenopus laevis. A graft of albino dorsal lip was transplanted into the ventral side of the gastrula (bottom right). Signals emanating from this small graft were able to divide the embryonic morphogenetic field of the host into two almost equal parts, which formed a Siamese twin. Note that the D-V and A-P axes are perfectly integrated; this can be seen, for example, in the perfect alignment of somites (segments) of the duplicated axes. Reprinted, with permission, from the Annual Review of Cell and Developmental Biology, Volume 20 (c) 2004 by Annual Reviews www.annualreviews.org.
2.2 The demise of Spemann’s organizer
Spemann thought of the organizer in terms of physics, which was the dominant science of his time. From electromagnetism he adopted terms such as “induction”, “induction potential” and “fields”, and from engineering “double assurance”. Soon some of the best names in embryology, such as Joseph Needham, Conrad Waddington, Jean Brachet and Johannes Holtfreter became interested in isolating the chemical substance that was responsible for the Spemann organizer effect. A great optimism was sweeping through embryology at the time. This was summarized by Huxley and De Beer (1934) at the end of the first chapter of their book as follows: “It may be confidently expected that in time the physiological basis of the organizer’s action will be discovered and accurately described in physic-chemical terms.” However, it was to take another 60-plus years before the chemical nature of Spemann’s organizer could be deciphered.
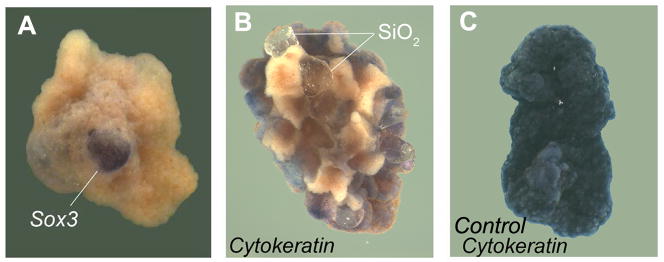
In a burst of investigations in the early 1930s, putative inducing substances were tested by sandwiching between two layers of salamander gastrula ectoderm, which would normally differentiate into epidermis. Dead organizers (killed by heat, alcohol or other methods) and many purified substances such as nucleoproteins (ribosomes), sterols, and even entirely abnormal heterologous inducers such as methylene blue or grains of sand were able to induce CNS (Fig. 5).
Fig. 5.
Sand (SiO2) particles serve as heterologous neural inducers in ectodermal explants of the American salamander Ambystoma maculatum. (A) A single grain of sand sandwiched between two ectodermal explants induces neural tissue marked by Sox3 mRNA. (B) Multiple sand particles cause patches of Cytokeratin-negative cells, which correspond to neural tissue. (C) Ectodermal explants cultured without sand particles, showing that the normal fate of these cells is to form Cytokeratin-epidermal positive cells. Reproduced from Hurtado and De Robertis, 2007, with permission.
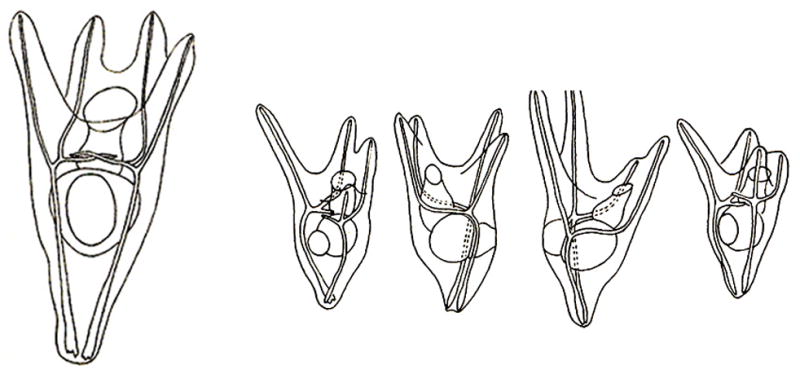
The final nail in the coffin of Spemann’s experimental legacy came when Lester Barth found, and Holtfreter confirmed, that Ambystoma maculatum ectoderm could be coaxed to form CNS in the complete absence of inducer, simply by culturing the ectodermal explants attached to glass (Barth, 1941; Holtfreter, 1944). We repeated these experiments six decades later, and found that neural induction by heterologous inducers is caused by a sustained activation of the activity of the MAPK (Mitogen-Activated Protein Kinase) pathway (Hurtado and De Robertis, 2007). CNS differentiation could be blocked, and epidermal differentiation restored, by addition of a chemical inhibitor of this pathway (Fig. 6). Activation of MAPK causes an inhibitory phosphorylation in the Smad1 transcription factor, and inhibition of Smad1 activity is required for neural differentiation to occur (Pera et al., 2003; Kuroda et al., 2005).
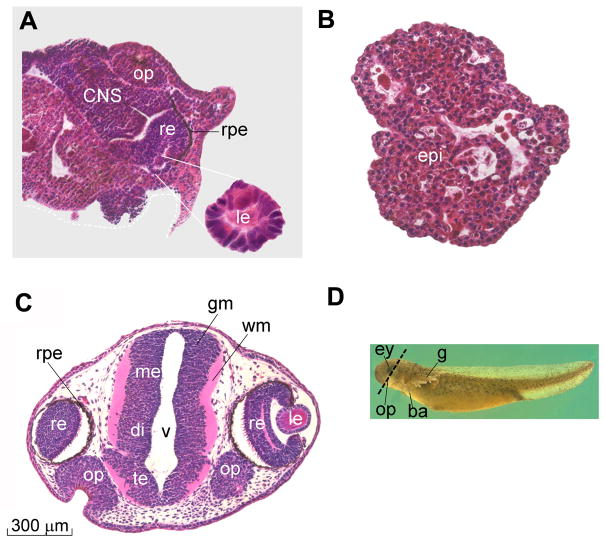
Fig. 6.
CNS differentiations induced by culturing Ambystoma maculatum ectoderm attached to a glass surface (in Holtfreter’s saline solution) can be blocked by addition of UO126, a chemical inhibitor of the MAPK/Erk pathway. (A) Ectoderm cultured attached to glass can develop extensive neural differentiations. After the initial induction of CNS tissue, differentiations of secondary fields also take place, giving rise to olfactory placodes, retina, retinal pigmented epithelium, and lens (of which an enlargement is shown). (B) In the presence of UO126 CNS differentiations are blocked. The explants develop as atypical epidermis (which is called atypical because it contains small cavities containing keratinized cells). (C) Section of a sibling embryo at the same stage of development (9 days) to illustrate the normal histological appearance of CNS tissues. (D) Outside view of Ambystoma maculatum 9-day larva indicating the plane of section. Abbreviations: ba, balancer; CNS, central nervous system; ey, eye; g, gills; gm, gray matter; le, lens; me, mesencephalon; op, olfactory placodes; re, retina; rpe, retinal pigmented epithelium; te, telencephalon; v, ventricle; vm, white matter. Reproduced from Hurtado and De Robertis, 2007, with permission.
It is interesting to note that after CNS differentiation is triggered, ectodermal explants can go on to execute secondary embryonic morphogenetic field organ-differentiation programs, giving rise to well-differentiated forebrain, eye, crystalline lens and olfactory placodes (Fig. 6A). All these secondary CNS differentiations can be blocked if the initial MAPK activation is inhibited with UO126, a chemical that blocks MEK/MAPKK, the enzyme that phosphorylates and activates MAPK/Erk (Fig. 6B).
The finding of heterologous neural inducers brought down the edifice that Spemann had built. Concomitantly, the awesome power of the Drosophila genetics pioneered by Thomas H. Morgan became the dominant force in experimental biology. By the time I was a developmental biologist in training during the 1970s, our professors would teach us that Hans Spemann had set back developmental biology by fifty years. Experimental embryology seemed dead.
2.3 Hamburger to the rescue
In 1988 a remarkable little book by Viktor Hamburger appeared (Hamburger, 1988). He wrote a wonderful memoir about his graduate student days in the Spemann laboratory as a contemporary of Hilde Mangold. Hamburger’s book revived interest in the organizer phenomenon and inspired work in our laboratory and others. Hamburger was well known for discovering that a mammalian cell line caused overgrowth of dorsal root ganglia in chick embryos. He guided Rita Levi-Montalcini in her initial experiments that eventually led to the isolation of Nerve Growth Factor (NGF), the first growth factor. Many regretted that Hamburger was not able to share in the growth factor Nobel prize (Levi-Montalcini, 1986). His book on Hans Spemann and the Organizer, published at age 88, proves that it is never too late for a person to influence the development of scientific ideas.
3. Molecular dissection of Spemann’s organizer
3.1 Cloning Spemann organizer genes
The timing of Hamburger’s book was perfect, because by the late eighties molecular biology, the great equalizer of modern biology, had become practical. We used a Xenopus dorsal lip cDNA library to isolate genes specifically expressed in the organizer (Cho et al., 1991). Other laboratories used different methods to isolate a large number of organizer genes from the gastrula of the South African frog Xenopus laevis (Taira et al., 1992; Dirksen and Jamrich, 1992; Smith and Harland, 1992).
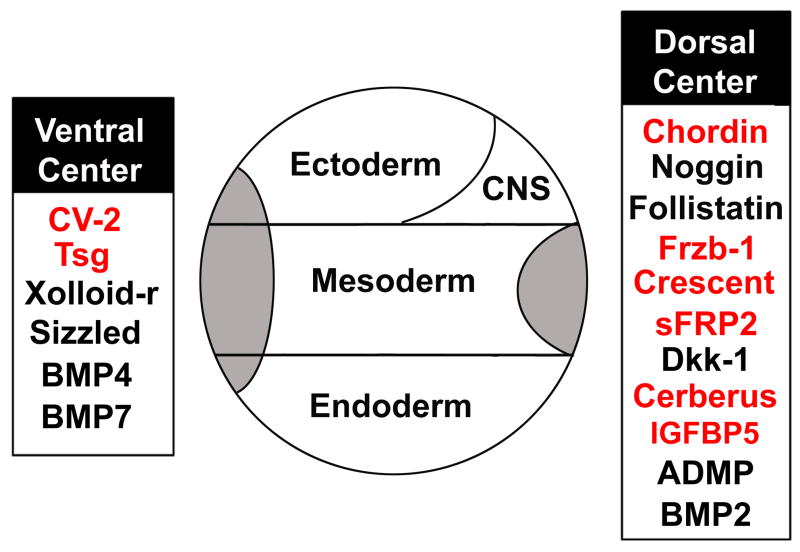
Over the years, a variety of molecular techniques were employed in our laboratory to isolate genes enriched in Spemann organizer tissue (Fig. 7). First, the organizer cDNA library was screened with synthetic DNA oligonucleotides hybridizing to the most conserved region of the homeobox, a sequence conserved among many developmental-controlling genes. This gave us goosecoid (Cho et al. 1991) and Xnot-2 (Gont et al., 1993). We next screened the dorsal lip library with labeled cDNA from Lithium chloride (LiCl) treated embryos. LiCl added at the 16 to 32-cell stage mimics the early embryonic Wnt signal, causing “dorsalized” embryos in which the entire mesoderm becomes Spemann organizer (Kao et al., 1986; Heasman, 2006). This screen gave us chordin (Sasai et al., 1994) (Fig. 7). Tewis Bouwmeester then screened the dorsal lip library with probes made from isolated dorsal or ventral regions subtracted with cDNA from ventral fragments (Bouwmeester et al., 1996). Because chordin is a very abundant and long mRNA, this method greatly enriched in its transcripts; the first 70 clones sequenced corresponded to chordin. Fortunately, after this the screen also gave us cerberus, Frzb-1, and Paraxial Protocadherin (Bouwmeester et al., 1996), all of which proved to have interesting developmental functions.
Fig. 7.
Secreted proteins that have been cloned from the dorsal lip or the ventral center of the Xenopus gastrula. Many laboratories contributed to this effort; genes first isolated by our group are shown in red. See text for further description.
As technology evolved, we made macroarrays that were screened with RT-PCR probes from embryos in which various signaling pathways had been activated (Wessely et al., 2004). This screen identified a dorsally-enriched intracellular protein called x-BTGx (B-cell Translocation Gene, first discovered as a chromosomal translocation in B-cell chronic lymphocytic leukemia), which has the remarkable property of inducing complete Wnt-like twinning when microinjected into embryos (Wessely et al., 2005). Many ventral-specific genes were also identified in this screen (Wessely et al., 2004). Finally, Edgar Pera used an unbiased screening method for proteins secreted in the Xenopus gastrula. Pools of 16 cDNA plasmids were transfected into mammalian cultured cells, labeled with S[35] Methionine, the culture medium electrophoresed in SDS polyacrylamide gels, and samples showing a radioactive band sib-selected (Pera et al., 2005). This secretion-cloning approach gave us IGFBP5 (Insulin-like growth factor binding protein 5, which led to the finding that IGF has neural-inducing activity, Pera et al., 2001), and the Xenopus organizer-specific Crescent and sFRP2 Wnt inhibitors (Pera and De Robertis, 2000). One advantage of this method is that it generates full-length functional mRNAs. Secretion-cloning produced a long list of Xenopus secreted protein expression constructs, which are available to the community (Pera et al., 2005).
At this point in time it seems possible that most of the genes expressed in Spemann’s organizer may have been isolated. The current challenge is to discover their biochemical functions and how they interact with each other to construct a harmonious embryonic morphogenetic field. In the case of ventral patterning genes, probably many still remain to be discovered. In addition, the function of many of the ventral genes that have been already identified (Wessely et al., 2005) has not been studied in depth yet. There is a practical reason for this, which is that dorsal organizer genes can induce the formation of new structures such as heads or trunks, while overexpression of ventral genes in general causes defects in dorsal or head structures. Loss of structures can also be triggered by non-specific effects, and therefore ventral genes have received less attention than they deserve. Their investigation may prove a productive area in the future.
In conclusion, the Spemann organizer of Xenopus provided a very productive fishing ground for novel genes. Cells in the early embryo are dedicated to establishing their positions with respect to each other rather than to histotypic differentiation, which takes place later in development. For this reason, many of the genes isolated were specifically involved in the control of embryonic patterning.
3.2 Goosecoid
Initially, organizer-specific homeobox genes were isolated (reviewed in De Robertis, 2006). The homeobox gene goosecoid was particularly important because it allowed the visualization of Spemann’s organizer for the first time (Cho et al., 1991). When Herbert Steinbeisser called me to the dissecting microscope to observe the still-developing staining of an in situ hybridization using antisense goosecoid RNA as a probe, this was a great and exciting moment. The goosecoid expression domain comprised about 60 degrees of the dorsal marginal zone of the gastrula, the same region that possessed Spemann’s embryonic inductive activity. Previously, one was forced to follow the organizer indirectly through its inductive activity after transplantation. Goosecoid helped developmental biologists understand the comparative anatomy of zebrafish, chick, mammalian and Xenopus gastrulation (De Robertis, 2004). In addition, goosecoid mRNA injection induced secondary axes, mimicking in part Spemann’s organizer activity (Cho et al., 1991; Sander et al., 2007). However, the agents that mediate cell-cell induction are secreted proteins, while goosecoid was a DNA-binding protein. We still needed to isolate the secreted factors that were turned on by goosecoid (Niehrs et al., 1992). During the following years, a great many novel secreted factors were cloned from the Xenopus laevis organizer (Fig. 7).
3.3 A plethora of secreted growth factor antagonists
The first surprise was that many of the secreted factors isolated from the organizer turned out to function as antagonists of growth factors. We were expecting to find novel growth factors, but discovered instead that embryonic patterning was mediated to a large degree by novel secreted inhibitors (Fig. 7). Thus, Chordin, Noggin and Follistatin are BMP inhibitors, while Frzb-1, Crescent, sFRP2 (De Robertis and Kuroda, 2004) and Dkk (Glinka et al., 1998) are Wnt antagonists. Cerberus is an inhibitor mainly of Nodal (a growth factor of the TGFβ, Transforming Growth Factor beta, family) and also of BMP and Wnt signaling (Bouwmeester et al., 1996; Piccolo et al., 1999), while IGFBP-5 is a modulator of IGF (Insulin-like growth factor) signaling (Pera et al., 2001). ADMP (Anti Dorsalizing Morphogenetic Protein; Moss et al., 1995) is a growth factor of the BMP (Bone Morphogenetic Factor) family which, together with BMP2 (Inomata et al., 2008), is expressed in Spemann’s organizer. The expression of BMP2 and ADMP on the dorsal side was paradoxical, for this is the side of lowest BMP signaling.
The second surprise was that a number of secreted molecules were expressed in the ventral side of the gastrula. These included BMP4 (Fainsod et al., 1994), BMP7 (Reversade et al., 2005), Twisted gastrulation (Oelgeschläger et al., 2000), the zinc metalloproteinase Xolloid-related (Dale et al., 2002) and Crossveinless-2 (Coffinier et al., 2002; Rentzsch et al., 2006; Ambrosio et al., 2008). CV2 is a protein containing Chordin-like Cysteine-rich (CR) repeats, first identified in a Drosophila mutant lacking crossveins (Conley et al., 2000).
This ventral signaling center establishes a dialogue with the dorsal Spemann organizer, as explained below. The tissue which we call the ventral center (De Robertis and Kuroda, 2004) had been described previously by Niehrs and Pollet (1999) under the name of the BMP4 synexpression group. They discovered that some groups of genes that function in common biological processes are coordinately expressed in embryos. The ventral center forms because all the genes in this synexpression group are transcriptionally activated by BMP4. Importantly, the expression of genes expressed in Spemann’s organizer is under the opposite regulation and is repressed by BMP signaling.
Of this cornucopia of genes (Fig. 7) the one that is closest to the heart of the Spemann organizer morphogenetic field is Chordin, for reasons we analyze next.
3.4 Chordin
Yoshiki Sasai isolated Chordin only a few weeks after arriving at UCLA; he already was an expert molecular biologist before arriving in the lab. By in situ hybridization chordin mRNA was expressed exactly in the regions that have organizer activity after transplantation and its expression was activated by goosecoid mRNA injection (Sasai et al., 1994). The hybridization signal was much stronger than anything we had seen previously. We later learned that Chordin protein is secreted in prodigious amounts. If distributed uniformly in the extracellular space, Chordin protein would reach concentrations of 33 nM during gastrulation (Lee et al., 2006). Chordin must reach much higher extracellular concentrations in the dorsal side of the gastrula embryo.
With Sasai we found that microinjection of chordin mRNA induced CNS formation in animal cap ectodermal explants (Sasai et al., 1995). Neuralization by Chordin could be reversed by overexpressing BMP4. Intriguingly, in the same study we found that neuralization by noggin or follistatin mRNA could also be reversed by BMP4 (Sasai et al., 1995). This was the first indication that these three molecules worked by a common molecular mechanism; we now know that all three antagonize BMP signaling extracellularly, lowering levels of active Smad1/5/8 transcription factor.
When Stefano Piccolo, a new postdoc from Italy, arrived at the lab on our very first conversation I said to him: “Purify Chordin protein and you will be fine”. This once, my wishful thinking proved true. Piccolo purified large amounts of Xenopus Chordin protein from baculovirus vectors in no time at all and this opened many doors for biochemical exploration. Soon we knew that Chordin could bind I125 BMP4 protein with an affinity (KD) of about 200 picomolar (Piccolo et al., 1996). Subsequent measurements using Biacore (surface plasmon resonance) gave higher affinities in the nanomolar range, but I think that the iodinated BMP4 measurements still remain the most accurate.
Chordin functioned in the simplest possible way – by binding directly to BMP4 and preventing its binding to BMP receptors on the cell surface (Piccolo et al., 1996). In parallel work, Richard Harland’s laboratory showed that Noggin also bound to BMP4 (Zimmerman et al., 1996), and using the Drosophila embryo Chip Ferguson (in collaboration with us) showed that Xenopus noggin mRNA blocked Dpp signaling upstream of its receptor in Drosophila epistatic experiments (Holley et al., 1996). I arranged with Benjamin Lewin, the editor of Cell, that publication of the Chordin paper (Piccolo et al., 1996) be delayed so that the three papers could be published back-to-back. The extracellular antagonists of BMP signaling had an auspicious start.
3.5 Chordin is required for induction by organizer
As mentioned, Chordin is secreted in large amounts by Spemann’s organizer. What is important about Chordin is not the amount, but rather that it is absolutely required for the inductive activity of Spemann organizer grafts. Morpholino oligos are antisense reagents that efficiently block protein translation in Xenopus embryos (Heasman, 2002). Michael Oelgeschläger found that, because Xenopus laevis is a subtetraploid species, it was necessary to inject a mixture of two antisense morpholinos directed against each paralogue in order to deplete Chordin (Oelgeschläger et al., 2003).
Chordin depletion increases the amount of ventral tissues and decreases dorso-anterior ones. Chordin-depleted embryos still generate an axis and have a small brain, presumably through the action of other organizer anti-BMP factors (Bachiller et al., 2000; Khoka et al. 2005). Yet, as shown in Fig. 8, when a Chordin-depleted organizer is transplanted, it loses completely the ability to induce a second axis or dorsal tissues, with the depleted organizer remaining as a patch of epidermis in the surface of the embryo (Oelgeschläger et al., 2003). This experiment demonstrates that the dorsal blastopore lip exerts its organizing effect through the secretion of Chordin, a BMP antagonist.
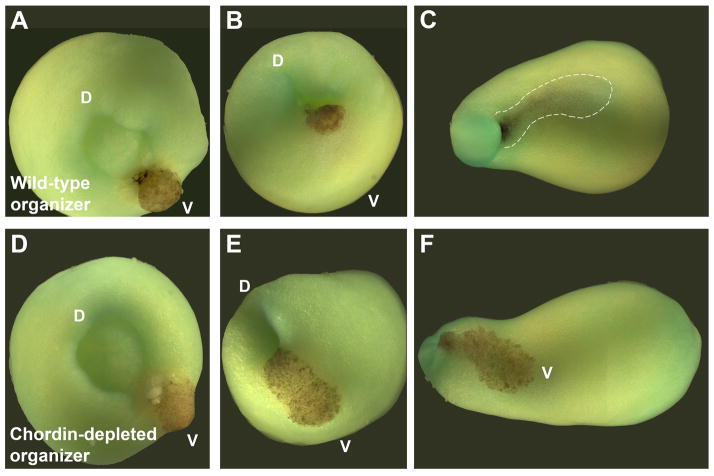
Fig. 8.
Chordin is required for the activity of Spemann organizer grafts. Shown here are transplants of pigmented organizers into albino hosts. (A–C) Transplant of a wild-type organizer followed for a few hours, showing how it involutes through the ventral blastopore until it is barely seen by transparency below the ectoderm (dotted line). (D–F) Depletion of Chordin in the organizer graft (Oelgeschläger et al., 2003) prevents all inductive activity, and the transplanted cells remain in the surface of the embryo, becoming epidermis. D, dorsal: V, ventral. Transplantation experiment by E.M.D.R., photographs by J.L. Plouhinec
Another experiment that revealed an essential role for Chordin was to treat Xenopus ectodermal explants with Activin protein, a TGF-β-like growth factor. As demonstrated by the work of Jim Smith, Activin is a morphogen that can induce increasing thresholds of dorsal mesoderm differentiation markers (Green et al., 1992). We found that in Chordin-depleted ectodermal explants Activin could only induce ventral mesoderm (Oelgeschläger et al., 2003). This showed that, remarkably, all the dorsal differentiation caused by Activin is mediated through the transcriptional activation of Chordin in Xenopus ectodermal explants. Although Chordin-depleted whole embryos do form some dorsal tissues, when animal cap cells are challenged experimentally with Activin all dorsal differentiation is eliminated. These experiments suggest that Chordin protein emanating from the organizer determines histotypic differentiation along the D-V axis of the embryo.
There are three main techniques in experimental biology: genetics, biochemistry and transplantation. Transplantation is the least used one. When a cell is challenged in new surroundings its full biological capacity is rendered more evident. Now that transplantation can be combined with inactivation of gene products, transplantation has become an even more powerful technique in the hands of the developmental biologist.
4. The Chordin Biochemical Pathway
4.1 The ventral center reacts to actions of the dorsal center
In principle the dorsal expression of the BMP antagonist Chordin should suffice to generate a dorso-ventral (D-V) gradient of BMP activity, minimal in the dorsal and maximal in the ventral side. However, what we found when analyzing the system in depth is that Chordin is part of a biochemical network of extracellular proteins that comprises the entire embryo. In particular, what has become clear is that the organizer effect is not due only to the action of the dorsal side, but also to the reaction of the ventral center. As indicated in Fig. 9, the Chordin biochemical pathway is composed of Chordin, ADMP and BMP2 in the dorsal side, and Tolloid (three tolloid enzymes exist in vertebrates, of which Xolloid-related is expressed ventrally; Dale et al., 2002), Sizzled (Szl), Crossveinless-2 (CV2), BMP4 and BMP7 in the ventral side. We shall examine the reactions of this biochemical cycle below.
Fig. 9.
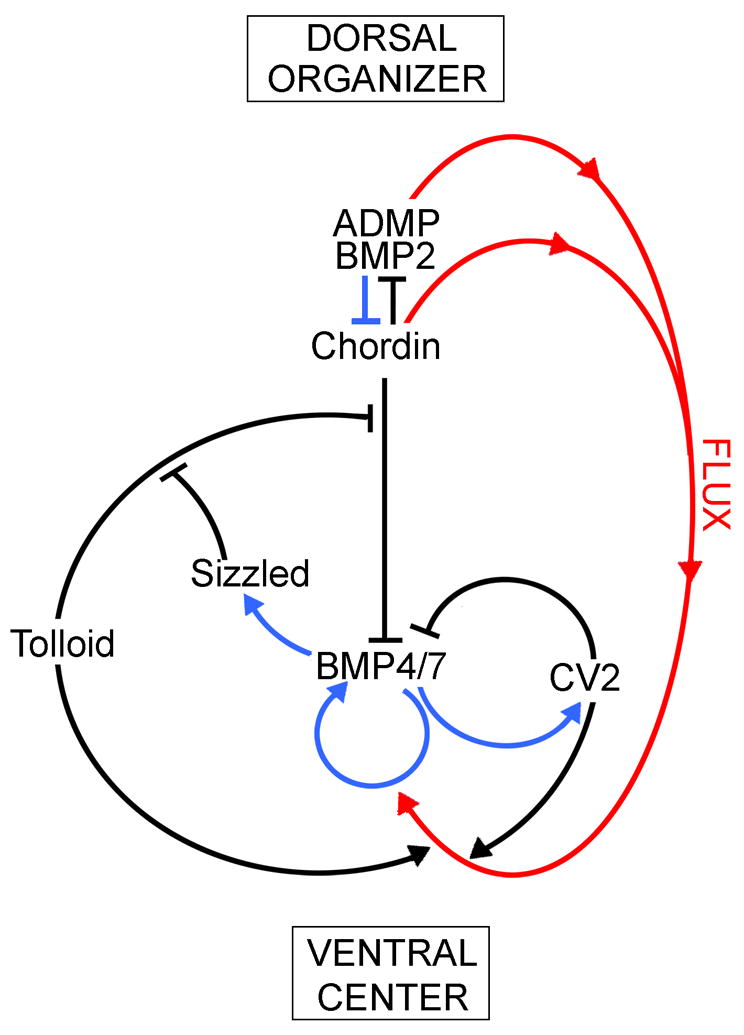
An extracellular biochemical network of interacting proteins explains self-regulation of the Xenopus embryonic field. The dorsal organizer and the ventral center communicate with each other through secreted proteins that bind to each other, inhibiting or activating the BMP gradient. All these protein-protein interactions (shown in black) were determined in our laboratory using biochemical measurements of affinity constants. Blue arrows indicate transcriptional regulation by Smad1/5/8 signaling, which activates ventral center genes and represses dorsal center genes. The two centers self-adjust to signaling changes in one another because of this opposite transcriptional control by BMPs. For example, when BMP levels increase, this causes an increase in Sizzled expression, which is an inhibitor of the Tolloid metalloproteinase that degrades Chordin. Thus, when Sizzled increases, Chordin levels increase, inhibiting BMP signaling and restoring the gradient. Red arrows denote the flux of Chordin/ADMP/BMP2 complexes from dorsal to more ventral regions. Mathematical modeling suggests that this Chordin-mediated flow of BMP is essential for the resilience of the gradient.
Although not shown in the Fig. 9 diagram, Twisted gastrulation (Tsg) plays an essential role as well. Tsg was identified in the original Nüsslein-Volhard and Wieschaus screens in Drosophila (Jürgens et al., 1984; Mason et al., 1994). We cloned the vertebrate homologue from Xenopus and showed that Tsg is both a BMP-binding and a Chordin-binding protein (Oelgeschläger et al., 2000). Tsg makes Chordin a better antagonist, forming a ternary complex that is able to diffuse in the extracellular space of the embryo (Fig. 10). Tsg is expressed ventrally and functions to keep BMP in a soluble, active state. Therefore Tsg also has pro-BMP effects. In zebrafish, depletion of Tsg with morpholinos results in a dorsalized phenotype, showing that the overall pro-BMP effect of Tsg predominates (Little and Mullins, 2006; Xie and Fisher, 2005).
Fig. 10.
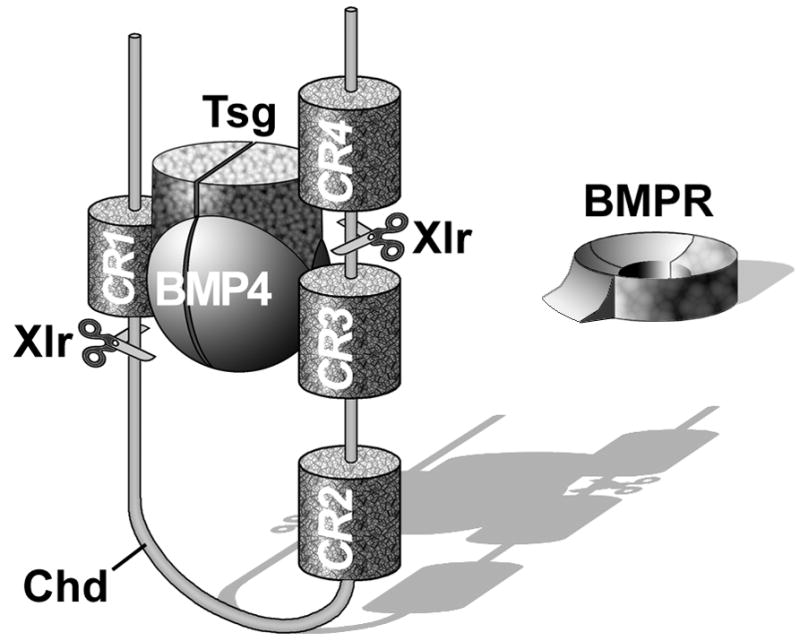
Chordin (Chd) forms a ternary complex with BMP4 and Twisted gastrulation (Tsg), which prevents binding to BMPR and allows the complex to diffuse within the embryo. The inhibition of BMP signaling is reversed by a ventral enzyme called Xolloid-related (Xlr) which is able to cleave Chordin at two specific sites (indicated by scissors), releasing BMPs for signaling through BMPR. Note that Chordin has four BMP-binding Cysteine-rich modules called CRs. CR domains function as regulators of BMP or TGF-β signaling in many extracellular proteins (De Robertis and Kuroda, 2004).
4.2 Tolloid protease provides the rate-limiting step in D-V patterning
With Stefano Piccolo and Eric Agius we found that Tolloid metalloproteinases promote BMP signaling by cleaving Chordin at two specific sites, indicated by scissors in Fig. 10. When this occurs, the affinity of Chordin for BMP greatly decreases and the growth factor, which was previously inactive, is reactivated and able to signal through BMP receptors (Piccolo et al., 1997). This enzymatic cleavage is crucial to the Chordin cycle, and constitutes the rate-limiting step in dorsal-ventral (D-V) pattern. Tolloid had been identified in the original Nobel prize-winning Nüsslein-Volhard and Wieschaus Drosophila genetic screens, but its biochemical mechanism of action was unknown.
Working in Drosophila, the group of Michael O’Connor found in parallel studies that the Drosophila homologue of Chordin, called Short gastrulation (Sog; François et al., 1994), is cleaved by Tolloid (Marqués et al., 1997). In a very satisfying convergence, the D-V mutations that had been isolated in exhaustive zebrafish genetic screens also were found to belong to the Chordin pathway. These mutations identified genes such as chordino (chordin), swirl (BMP2), snailhouse (BMP7), lost-a-fin (Alk2 type I BMP receptor), mini-fin (tolloid) and ogon/mercedes (sizzled) (reviewed by Little and Mullins, 2006). Therefore, the Chordin pathway, which was worked out by embryological and biochemical methods in Xenopus, is strongly supported by Drosophila and zebrafish genetics.
4.3 Sizzled is an inhibitor of tolloid enzymes
The sizzled gene is probably the best marker of the ventral center (Collavin and Kirschner, 2003). A long-time paradox was that Sizzled depletion (or mutation of the ogon/mercedes gene in zebrafish) caused ventralized (high-BMP) phenotypes similar to those of Chordin loss-of-function (Collavin and Kirschner, 2003; Yabe et al., 2003; Little and Mullins, 2006; Lee et al., 2006). This was intriguing, because Sizzled is a secreted Frizzled-related protein (sFRP) containing a Frizzled domain and a netrin domain. sFRPs were thought to act exclusively as inhibitors of the Wnt pathway. After several years of research, we discovered that Sizzled functions biochemically as a competitive inhibitor of Tolloid enzymes (Lee et al., 2006). In the embryo, tolloid enzymes are faced with the choice of binding to Chordin and cleaving it, or of binding to Sizzled, which cannot be cleaved and therefore behaves as a competitive inhibitor (Fig. 9). Interestingly, the inhibition of Tolloid is mediated by the Frizzled domain, previously thought to function exclusively as a Wnt-binding domain. These findings were quickly corroborated for Ogon/Mercedes, the zebrafish homologue of Sizzled (Muraoka et al., 2006).
We measured the levels of Sizzled produced by the Xenopus gastrula, and found them to be about as high as those of Chordin (Lee et al., 2006). When Sizzled is expressed, it acts as a feedback inhibitor of BMP signaling in the ventral center. This is done indirectly, through the inhibition of the chordinase Tolloid, resulting in elevated levels of the BMP antagonist Chordin (Fig. 9). The activity of Tolloid is highly regulated, for it is the rate-limiting step in maintaining a self-regulating D-V gradient of BMP activity.
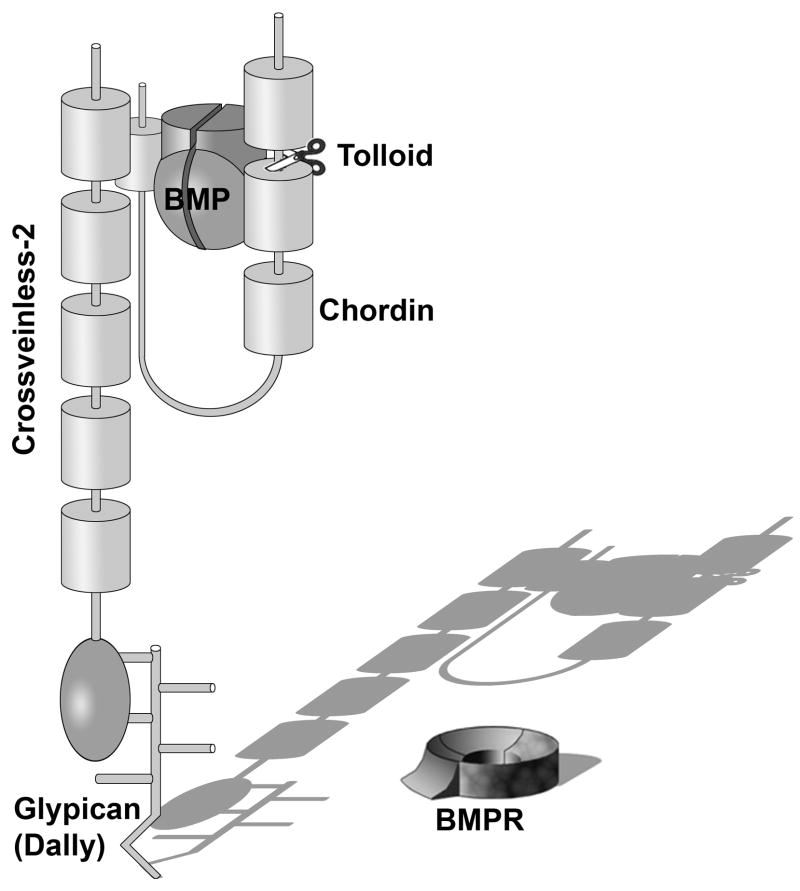
4.4 Crossveinless-2 binds Chordin providing a ventral BMP sink
CV-2 functions in Drosophila increase BMP/Dpp signals in the wing crossveins (Conley et al., 2000; Ralston and Blair, 2005). In a number of systems CV2 has both anti-BMP and pro-BMP activities. The anti-BMP activity results from the direct binding of BMP to the Chordin-like Cysteine-rich CR domains in CV2 (Zhang et al., 2008; Serpe et al., 2008), and is enhanced by the cofactor Tsg (Ambrosio et al., 2008). CV2 inhibits signaling by binding to BMPs and mediating their endocytosis and clearance from the extracellular space (Kelley et al., 2009). Although CV2 is a secreted protein, it remains tethered to the surface of the cells in which it is synthesized by binding to the glypican Dally via its von Willebrand factor D (vWF-d) domain (Rentszch et al., 2006; Serpe et al., 2008). Glypicans are Heparan Sulphate proteoglycans (HSPGs) that have a Glycosyl Phosphatidyl Inositol (GPI) modification that anchors them to the cell membrane and in particular to cholesterol-rich lipid rafts. Thus, BMP binding followed by endocytosis explains the anti-BMP effects of CV2. But what about its pro-BMP effects?
With Andrea Ambrosio we found that CV2 binds with high affinity (KD of about 1–2 nM) to Chordin protein (Fig. 11). CV2 binds with even higher affinity to Chordin bound to BMP or to Chordin fragments resulting from Tolloid digestion (Ambrosio et al., 2008). CV2 acts as a molecular sink, concentrating Chordin/BMP/Tsg complexes in the ventral side, where Tolloid activity can cleave Chordin allowing BMP to signal through its cognate receptors (Fig. 11). Thus, the pro-BMP effects of CV2 result from the facilitation of the flow of BMPs produced in more dorsal regions of the embryo (Fig. 9, flux indicated by red arrows). Serpe et al. (2008) have shown that CV2 can also interact directly with the Drosophila type I BMPR Thickvein, providing a second mechanism by which CV2, by recruiting Chordin/BMP to the vicinity of type I BMP receptor, can facilitate BMP signaling.
Fig. 11.
Crossveinless-2 (CV2) serves as a molecular sink that concentrates Chordin/Tsg/BMP complexes on the ventral side of the embryo. Once there, BMPs secreted by more dorsal regions of the embryo can be released by Tolloid enzymes and signal through BMP receptors (BMPR). CV2 is a secreted protein but does not diffuse far from the cells that secrete it, because it remains anchored to the cell surface by GPI-anchored glypicans, such as Dally in Drosophila, via its COOH-terminal vWF-d (von Willebrand Factor-D) domain.
In conclusion, D-V patterning does not result from a simple gradient of BMP antagonists from Spemann’s organizer diffusing to the ventral side. The ventral center - through the production of Xolloid-related, Sizzled and CV2 - plays a crucial role in the self-adjusting communication between the dorsal and ventral centers. The Chordin/BMP pathway (Fig. 9) was assembled by identifying new nodes of protein-protein interactions through biochemical studies: Chordin binds BMP and ADMP, Tsg binds BMP and Chordin, Tolloid cleaves Chordin, Sizzled is a competitive inhibitor of Tolloid, and CV2 binds Chordin/BMP complexes.
5. The molecular mechanism of self-regulation
5.1 Opposite transcriptional regulation of dorsal and ventral secreted proteins
The key to understanding self-regulation is the opposite transcriptional control of dorsal and ventral genes (Reversade and De Robertis, 2005). Spemann organizer genes are turned on by low BMP levels, while ventral genes are activated by high BMP levels (Fig. 9, blue arrows indicate transcriptional regulation). The dorsal and ventral centers express molecules of similar biochemical activities but under opposite transcriptional control. For example, BMP2 and ADMP are expressed dorsally, while BMP4 and BMP7 are produced ventrally. Chordin is secreted by Spemann’s organizer, while in the ventral center another BMP antagonist containing CR modules, Crossveinless-2, is produced (Fig. 11). Similarly, in the ventral center Sizzled is secreted, while in the dorsal side a closely related molecule, Crescent, is produced (Pera and De Robertis., 2000).
Molecules of the ventral side compensate for the loss of dorsal products. For example, knockdown of Chordin or CV2 with antisense morpholinos cause similar high-BMP phenotypes (Ambrosio et al., 2008). However, when CV2 and Chordin are depleted simultaneously, the pro-BMP phenotypes is much greater. This indicates that when Chordin is knocked down in the dorsal side the elevation of CV2, a BMP-binding protein of similar molecular structure, in the ventral center is able to compensate for the loss of Chordin (Ambrosio et al., 2008). For each action in Spemann’s organizer there is a reaction in the ventral side of the embryo.
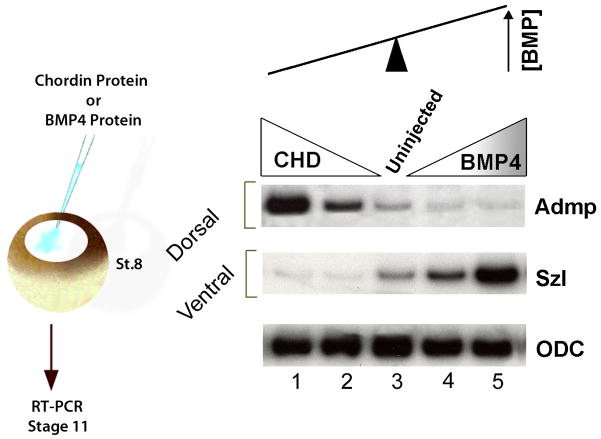
The consequences of the opposite transcriptional regulation in the gastrula morphogenetic field are illustrated in Figure 12, in which BMP levels were decreased or increased by injection of Chordin or BMP4 protein into the blastula cavity (Reversade and De Robertis, 2005). The embryo field self-regulates because it behaves as a molecular see-saw. When BMP levels are decreased, the transcription of ADMP goes up, so that BMP signaling levels are restored. When BMP4 levels are increased, Sizzled transcription goes up, inhibiting BMP levels (through the inhibition of Tolloid, which causes Chordin to increase). This opposite transcriptional regulation generates a self-adjusting BMP signaling gradient (Fig. 12).
Fig. 12.
The self-adjusting nature of the D-V BMP gradient can be revealed by lowering or increasing BMP signaling. In this experiment, BMP signaling was lowered by Chordin or increased by BMP proteins microinjected into the blastula cavity at stage 8. A molecular see-saw explains self-regulation of the gradient. When BMP signaling is inhibited, transcription of ADMP (a BMP) increases and restores the gradient. When BMP4 signaling is increased, Sizzled transcription is elevated and Sizzled protein inhibits tolloid proteinases, indirectly increasing levels of the BMP antagonist Chordin. ODC, Ornithine Decarboxylase, serves as an mRNA loading control in these RT-PCR reactions. From Reversade and De Robertis, 2005, reproduced with permission.
5.2 Flow of Chordin and BMP within the gastrula
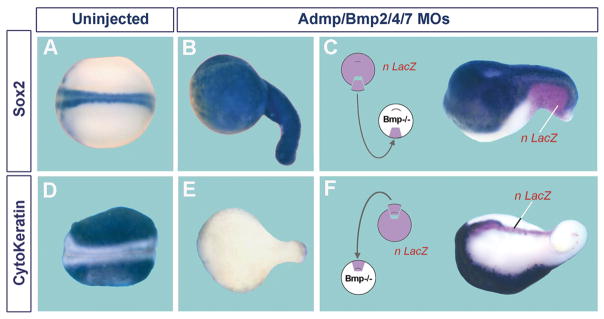
When BMP2, ADMP, BMP4 and BMP7 are knocked down simultaneously, self-regulation collapses, causing a spectacular dorsalization: the embryo becomes covered in CNS marked by Sox2 (Fig. 13A and B). Conversely, the epidermal marker cytokeratin is lost from the ectoderm (Fig. 13D and E). This represents a major change in cell differentiation, in which the entire embryo becomes covered in brain tissue, with a small region close to the blastopore taking spinal cord identity (Reversade and De Robertis, 2005). If any one of these four BMPs is not depleted, the embryo retains D-V pattern (Reversade et al., 2005).
Fig. 13.
Simultaneous depletion of four BMPs causes ubiquitous CNS differentiation, which can be restored by transplantation of either a wild-type ventral center or a dorsal organizer. (A) Control Xenopus embryo showing normal Sox2 mRNA expression in the CNS. (B) Sibling depleted of ADMP, BMP2, 4 and 7 with antisense morpholinos; note that the entire embryonic surface is covered by CNS tissue. (C) Transplantation of a wild-type ventral center (labeled with nuclear LacZ lineage tracer) into BMP-depleted embryos restores formation of a neural plate with epidermis ventrally to it. (D) Cytokeratin mRNA is abundantly expressed in epidermis. (E) Cytokeratin expression is eliminated in BMP-depleted embryos (because epidermis is replaced by CNS). (F) Transplantation of a wild-type dorsal organizer rescues BMP depletion. Epidermis is induced, but at a considerable distance from the transplanted tissue (which gives rise to notochord). BMP does not signal close to the graft because it is inhibited by Chordin. These experiments show, first, that BMP inhibition causes ubiquitous neural induction and, second, that the embryo has dorsal and ventral sources of BMP signals. Experiments from Reversade and De Robertis (2005), reproduced with permission.
By transplanting wild type tissue into BMP-depleted embryos we were able to show that both the dorsal and ventral centers serve as sources of BMPs that diffuse over long distances in the embryo (Reversade and De Robertis, 2005). Ventral tissue is able to rescue epidermal differentiation at a distance from the graft (compare Fig. 13B and C), making the important point that it indeed functions as a ventral signaling center. Transplantation of dorsal organizer also rescues epidermis, but at a distance from the graft (compare Fig. 13E and F). Near the dorsal graft, signaling by ADMP and BMP2 is inhibited by the large amounts of Chordin present in the organizer, and is then reactivated ventrally after tolloid cleavage (Reversade and De Robertis, 2005). Although Spemann’s organizer is the tissue with the lowest levels of BMP signaling, it secretes BMP2 and ADMP, which can signal in the opposite side of the embryo after Chordin is cleaved by tolloid. These experiments suggest that a double gradient of BMP signals flowing from opposite poles of the embryo helps explain the resilience of the embryo, which generates a perfect organism time after time. It also illustrates the power of combining classical transplantation with modern loss-of-function techniques.
The D-V flow of BMP within the Xenopus embryo has been recently directly demonstrated by microinjection of tagged BMP4 into dorsal blastomeres. It was found that BMP4 can flow from the dorsal to the ventral center in a process that requires Chordin (Ben-Zvi et al., 2008). We have corroborated these results with ADMP-GFP fusions (J.L. Plouhinec and E.M.D.R., unpublished observations). Mathematical modeling has suggested that the flux of BMP (Fig. 9) mediated by Chordin/Sog is crucial to ensure the robustness of the BMP gradient in Drosophila (Eldar et al., 2002) and in Xenopus (Ben-Zvi et al., 2008; J.L. Plouhinec and E.M.D.R., unpublished observations). Long-range communication between the dorsal and ventral sides of the gastrula requires the regulated flow of BMPs transported by Chordin, which are concentrated and then released by tolloid metalloproteinases at a distance from where they were produced (Plouhinec and De Robertis, 2009).
It will be interesting to ask whether this self-adjusting flow of growth factors occurs in later, secondary morphogenetic fields. One intriguing case is provided by the morphogenetic field of the developing vertebrae of the mouse. We have shown that knockout of CV2 in embryonic vertebral bodies decreases BMP signaling in intervertebral discs at a considerable distance from where CV2 protein is localized (Zakin et al., 2008). This action at a distance might be explained by the disrupted flow of Chordin in the vertebral field of CV2−/− embryos, and is currently being investigated.
5.3 Why is self-regulation required?
The need for self-regulation in developing embryos remains an enigma. The embryo is exposed to environmental challenges during its development that might need frequent adjustments. For example, the developing frog embryo must adjust to variations in the temperature of the pond water. In addition, throughout gastrulation the size of the blastopore decreases as it envelops the vegetal yolky cells in the process known as epiboly, while the germ layers involute and move with respect to each other. Despite all these morphogenetic movements, the D-V gradient of BMP activity remains constant, explaining why cells at opposite poles of the embryo need to communicate with each other.
Maintaining the D-V Chordin/BMP gradient is essential for the embryo because it determines histotypic differentiation. In the ectoderm, the BMP gradient dictates cell differentiation into neural plate, neural crest and epidermis (Little and Mullins, 2006). In the mesoderm, increasing BMP levels control the choice between prechordal plate, notochord, kidney, lateral plate mesoderm and blood islands (Heasman, 2006). The regulation of the Chordin/BMP gradient plays a central role in allocating the proper amounts of embryonic tissues, not only in Xenopus, but also in many other organisms.
6 Evo-Devo: the ancestral Chordin/BMP and Hox gene patterning pathways
6.1 An inversion of the D-V axis was predicted by Etienne Geoffroy St. Hilaire
The Drosophila homologue of Chordin is Short-gastrulation (Sog), a gene that is expressed ventrally (François et al., 1994). The homologue of BMP4, Dpp, is expressed dorsally in Drosophila (O’Connor et al., 2006). In collaboration with Chip Ferguson we showed that Sog can induce neural tissue in Xenopus and that Chordin can do the same in Drosophila (Holley et al., 1995). This indicated that the same patterning system is used by both species. The main difference is that the D-V axis has been inverted during the course of evolution, as first suggested by French zoologist Etienne Geoffroy Saint-Hilaire in 1822 (Appel, 1987; De Robertis and Sasai, 1996).
The evolutionary conservation applies not only to Chordin/Sog and BMP/Dpp, but also to many other components of the Chordin biochemical pathway, such as Tolloid, Tsg and CV2 (De Robertis, 2008a). The entire network has been conserved in other organisms, such as hemichordates (Lowe et al., 2006), spiders (Akiyama-Oda and Oda, 2006) and amphioxus (Yu et al., 2007). In all these animals there is evidence that the Chordin/BMP/Tsg/Tolloid/CV2 pathway mediates communication between the dorsal and ventral sides of the early embryo much in the same way as in Xenopus and Drosophila.
Such an intricate biochemical system of extracellular cell signaling would be most unlikely to have evolved independently twice in evolution. The inescapable conclusion is that the Chordin pathway patterned the D-V axis in the last common ancestor of the protostome and deuterostome animals (Fig. 14). This ancestor, called Urbilateria, gave rise to all 30 phyla of bilateral animals (De Robertis and Sasai, 1996; De Robertis, 2008a). Since there are only four or five non-bilaterian animal phyla, most of the body plans of the animals that surround us in daily life had their D-V axis shaped by the Chordin/BMP/Tsg/Tolloid/CV2 network of extracellular proteins.
Fig. 14.
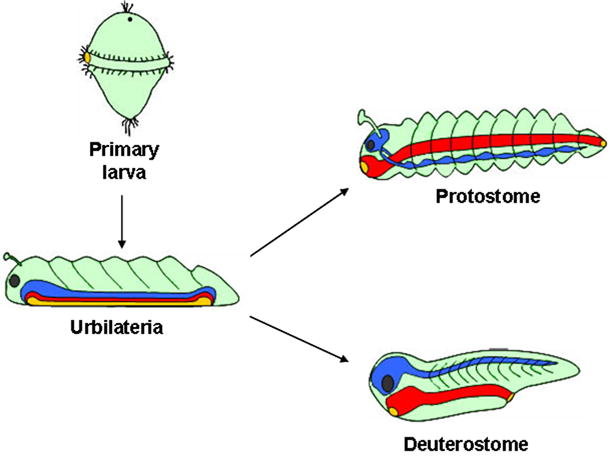
The common ancestor of bilateral animals had a D-V axis patterned by the Chd/BMP/Tsg/Tolloid/CV2 pathway. Shown here are the two branches of bilateral animals, which underwent a D-V inversion of the CNS. The protostomes (proto, first; stomo, mouth) have the nerve cord ventral to the gut. The deuterostomes (deutero, second) have the CNS dorsal to the gut. Urbilateria is the last common ancestor of all bilateral animals. Evo-Devo studies suggest that Urbilateria was a highly complex animal (De Robertis, 2008a). The blastopore of Urbilateria is shown as an elongated slit that gives rise both to the mouth and anus (a situation called amphistomy); recent findings showing that hemichordates (acorn worms) have not yet undergone D-V inversion of the CNS (Benito-Gutiérrez and Arendt, 2009) imply that the urbilaterian CNS likely resembled that of protostomes. In the diagram, Urbilateria is depicted as a segmented bottom-dwelling (benthic) animal. While a common ancestry of animal segmentation mechanisms is the subject of debate, two recent studies favor this idea: in the cockroach Notch pathway genes cycle rhythmically as in vertebrate segmentation (Pueyo et al., 2008), and Smad1/5/8 and Mad are required for segmentation in Xenopus and Drosophila (Eivers et al., 2009). Urbilateria probably had a life-cycle including a marine free-swimming (pelagic) primary larval stage, shown here with trochophore-like beating cilia. Many extant phyla have such larvae – annelids, mollusks, hemichordates and echinoderms – although this phase of the life-cycle has been repeatedly lost during evolution (Jägersten, 1972; Nielsen, 1998). Both the D-V (Chd/BMP/Tsg/Tld/CV2) and A-P (Hox genes) patterning systems were utilized by the urbilaterian ancestor to generate pattern. The use of these ancestral gene networks must have placed important developmental constraints in the evolution of animal body plans. Ectoderm is shown in green, CNS in blue, eye in black, and endoderm in red, with its openings in yellow. Reproduced, with permission, from De Robertis 2008b.
6.2 Hox genes define the A-P axis
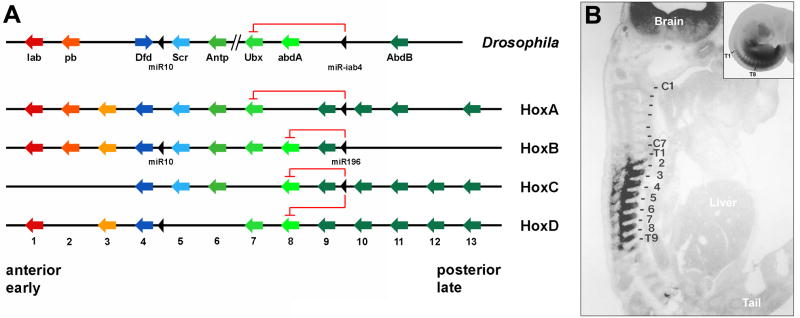
Before UCLA, I was a professor of Cell Biology at the Biozentrum of the University of Basel, Switzerland. In collaboration with Walter Gehring we cloned the first vertebrate Hox gene, Hox-C6, from a Xenopus laevis genomic library (Carrasco et al., 1984). This was an important finding, which marked the molecular beginnings of the young discipline of Evolution and Development, Evo-Devo. The last sentence of the abstract of the landmark paper by Carrasco et al. (1984) read: “If the frog gene cloned here eventually turns out to have functions similar to those of the fruit fly [homeotic] genes, it would represent the first development-controlling gene identified in vertebrates.” It was eventually found out, through the work of others, that entire Hox complexes have indeed been conserved between Drosophila and the vertebrates (Fig. 15) (reviewed in Lemons and McGinnis, 2006; Duboule, 2007). The degree of conservation is so extraordinary that even a micro RNA, called miR196 in vertebrates and infra-abdominal 4 (miRiab-4) in Drosophila, which inhibits translation of more anterior Hox genes, has been conserved (Fig. 15) (Yetka et al., 2004; Ronshaugen et al, 2005). Such a complex gene network could not have evolved twice independently and therefore was already present in Urbilateria (Fig. 14).
Fig. 15.
The Hox complexes have been conserved between Drosophila and mammals, down to level of micro RNAs (miRs) that repress the translation of more anterior genes. (A) Vertebrates have four Hox complexes and Drosophila only one (which became separated into two segments). Vertebrates underwent two rounds of whole-genome duplication when they evolved from a simpler chordate ancestor. These whole-genome duplications may explain the evolutionary success of the vertebrates. The ancestral chordate Hox complex had 13 genes, but some paralogues have been lost in mammals. (B) Hox-C6 protein is detected in eight thoracic segments of mouse embryos. The inset shows that Hox-C6 mRNA is expressed all the way to the tail. Hox-C6 protein is not detected posteriorly probably because of translational repression by miR196 inhibition. Redrawn from De Robertis, 2008a, reproduced with permission.
The body plans of bilateria were constructed using Hox complexes for antero-posterior (A-P) patterning and the Chordin/BMP pathway for the D-V axis. These deep homologies discovered by Evo-Devo must have channeled, or constrained, the possible outcomes of animal evolution (De Robertis, 2008a). The use of conserved developmental gene networks to pattern body plans channeled the responses to the strictures of the guiding hand of Natural Selection. The study of embryonic development has contributed greatly to our current view of Evolution. On the sesquicentennial of the publication of The Origin of the Species (Darwin, 1859), embryologists have reason to celebrate in Evo-devo the marriage between developmental biology and Darwinian theory.
In closing, it has been wonderful to see how the beauty of the experimental embryology legacy of Harrison and Spemann has found chemical explanations. We hope that cut-and-paste embryology will continue to provide insights into the molecular mechanisms by which cells communicate with each other within self-regulating morphogenetic fields for a long time into the future.
Supplementary Material
Acknowledgments
The author wishes to thank Sir John Gurdon for teaching him the powerful art of working with Xenopus, and the group of talented graduate students and postdoctoral fellows that made possible these explorations on the chemical nature of Spemann’s organizer; many of them are now leaders in the field of developmental biology. Our work has received generous long-term support from the Norman Sprague Jr. Endowed Chair, NIH grant HD21502-23, and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel TA. The Cuvier-Geoffroy Debate. Oxford University Press; Oxford: 1987. [Google Scholar]
- Bachiller D, et al. The organizer secreted factors Chordin and Noggin are required for forebrain development in the mouse. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Barth LG. Neural diffferentiation without organizer. J Exp Zool. 1941;87:371–384. [Google Scholar]
- Ben-Zvi D, Shilo B, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- Benito-Gutiérrez E, Arendt A. CNS evolution: new insight from the mud. Curr Biol. 2009;19:R640–R642. doi: 10.1016/j.cub.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim SH, Sasai Y, Lu B, De Robertis EM. Cerberus, a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s Organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Carrasco AE, McGinnis W, Gehring WJ, De Robertis EM. Cloning of a Xenopus laevis gene expressed during early embryogenesis that codes for a peptide region homologous to Drosophila homeotic genes: implications for vertebrate development. Cell. 1984;37:409–414. doi: 10.1016/0092-8674(84)90371-4. [DOI] [PubMed] [Google Scholar]
- Cho KWY, Morita EA, Wright CVE, De Robertis EM. Overexpression of a homeodomain protein confers axis-forming activity to uncommitted Xenopus embryonic cells. Cell. 1991;65:55–64. doi: 10.1016/0092-8674(91)90407-p. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Ketpura N, Tran U, Geissert D, De Robertis EM. Mouse Crossveinless-2 is the vertebrate homolog of a Drosophila extracellular regulator of BMP signaling. Mech Dev. 2002;119:179–184. doi: 10.1016/s0925-4773(03)00113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection, or Preservation of Favored Races in the Struggle for Life. Murray; London: 1859. [Google Scholar]
- De Robertis EM. Goosecoid. In: Stern C, editor. Gastrulation. Cold Spring Harbor Laboratory Press; New York: 2004. pp. 581–589. [Google Scholar]
- De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nature Rev Mol Cell Biol. 2006;4:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Evo-Devo: Variations on Ancestral themes. Cell. 2008a;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Evolutionary Biology: The molecular ancestry of segmentation mechanisms. Proc Natl Acad Sci USA. 2008b;105:16411–16412. doi: 10.1073/pnas.0808774105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Morita EA, Cho KWY. Gradient fields and homeobox genes. Development. 1991;112:669–678. doi: 10.1242/dev.112.3.669. [DOI] [PubMed] [Google Scholar]
- Dirksen ML, Jamrich M. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992;6:599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- Eivers E, Fuentealba LC, Sander V, Clemens J, Hartnett L, De Robertis EM. Mad is required for Wingless signaling in wing development and segment patterning in Drosophila. PLoS ONE. 2009;4:e6543. doi: 10.1371/journal.pone.0006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, De Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford University press; Oxford: 1988. [Google Scholar]
- Harrison RG. Experimentelle Untersuchungen über die Entwicklung der Sinnesorgane der Seitenlinie bei den Amphibien. Arch f mikr Anat. 1903;63:35–149. [Google Scholar]
- Harrison RG. Experiments on the development of the fore-limb of Amblystoma, a self-differentiating equipotential system. J Exp Zool. 1918;25:413–461. [Google Scholar]
- Heasman J. Morpholino oligos: making sense or antisense? Dev Biol. 2002;15:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffman FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving short gastrulation and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. Neural differentiation of ectoderm through exposure to saline solution. J Exp Zool. 1944;95:307–343. [Google Scholar]
- Hörstadius S. Experimental embryology of echinoderms. Clarendon Press; Oxford: 1973. [Google Scholar]
- Hurtado C, De Robertis EM. Neural induction in the absence of organizer in salamanders is mediated by MAPK. Dev Biol. 2007;307:282–289. doi: 10.1016/j.ydbio.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley JS, de Beer GR. The Elements of Experimental Embryology. Cambridge University Press; Cambridge: 1934. [Google Scholar]
- Inomata H, Haraguchi T, Sasai Y. Robust stability of the embryonic axial pattern requires a secreted scaffold for Chordin degradation. Cell. 2008;134:854–865. doi: 10.1016/j.cell.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Jägersten G. Evolution of the Metazoan Life Cycle: A Comprehensive theory. Academic Press; London and New York: 1972. [Google Scholar]
- Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux’ Arch Dev Biol. 1984;193:283–293. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- Kao KR, Masui Y, Elinson RP. Lithium-induced respecification of pattern in Xenopus laevis embryos. Nature. 1986;322:371–373. doi: 10.1038/322371a0. [DOI] [PubMed] [Google Scholar]
- Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted Frizzled-related proteins as inhibitors of Tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty five years later. Nobel lecture . 1986 doi: 10.1007/BF02623703. http://nobelprize.org/nobel_prizes/medicine/laureates/1986/levi-montalcini-lecture.html. [DOI] [PubMed]
- Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Def Res. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- Lowe CJ, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biology. 2006;4:1603–1619. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G, Musacchio M, Shimell MJ, Wünnenberg-Stapleton K, Cho KWY, O’Connor MB. Production of DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- Moos M, Wang S, Krinks M. Anti-dorsalizing morphogenetic protein is a novel TGF-beta homolog expressed in the Spemann organizer. Development. 1995;121:4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M. Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol. 2006;8:329–338. doi: 10.1038/ncb1379. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Pollet N. Synexpression groups in eukaryotes. Nature. 1999;402:483–487. doi: 10.1038/990025. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Keller R, Cho KWY, De Robertis EM. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993;72:491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- Nielsen C. Origin and evolution of animal life cycles. Biol Reviews. 1998;73:125–155. [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Pera E, De Robertis EM. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech Dev. 2000;96:183–195. doi: 10.1016/s0925-4773(00)00394-4. [DOI] [PubMed] [Google Scholar]
- Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by Insulin-like Growth Factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Hou S, Strate I, Wessely O, De Robertis EM. Exploration of the extracellular space by a large-scale secretion screen in the early Xenopus embryo. Int J Dev Biol. 2005;49:781–796. doi: 10.1387/ijdb.052003ep. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Battacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouhinec JL, De Robertis EM. Systems biology of the self-regulating morphogenetic gradient of the Xenopus gastrula. In: Briscoe J, Lawrence P, Vincent JP, editors. Reading and Interpreting Gradients during Development. Cold Spring Harb. Perspect. Biol; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo JI, Lanfear R, Couso JP. Ancestral notch-mediated segmentation revealed in the cockroach P. americana. Proc Natl Acad Sci USA. 105:16614–16619. doi: 10.1073/pnas.0804093105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Blair SS. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev Biol. 2005;280:187–200. doi: 10.1016/j.ydbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronshaugen M, Biemar F, Piel J, Levine M, Lai EC. The Drosophila microRNA iab-4 causes a dominant homeotic transformation of hateres to wings. Genes Dev. 2005;19:2947–2952. doi: 10.1101/gad.1372505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Alvarado A. The shredding of a caricature. Cell. 2008;135:991–992. [Google Scholar]
- Sander K. Specification of the basic body pattern in insect embryogenesis. Adv Insect Physiol. 1976;12:125–238. [Google Scholar]
- Sander V, Reversade B, De Robertis EM. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J. 2007;26:2955–2965. doi: 10.1038/sj.emboj.7601705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-Binding Protein Crossveinless 2 Is a Short-Range, Concentration-Dependent, Biphasic Modulator of BMP Signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Spemann H. Embryonic Development and Induction. Yale University Press; New Haven, Conn: 1938. reprinted by Hafner publishing company, 1962. [Google Scholar]
- Spemann H, Mangold H. Induction of embryonic primordial by implantation of organizers from a different species. Roux’s Arch Entw Mech. 1924;100:599–638.Reprinted and translated in Int J Dev Biol. 2001;45:13–31.
- Taira M, Jamrich M, Good PJ, Dawid IB. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992;6:356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Wessely O, Kim JI, Geissert D, Tran U, De Robertis EM. Analysis of Spemann Organizer formation in Xenopus embryos by cDNA macroarrays. Dev Biol. 2004;269:552–566. doi: 10.1016/j.ydbio.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Wessely O, Kim JI, Tran U, Fuentealba L, De Robertis EM. xBtg-x regulates Wnt/-Catenin signaling during early Xenopus development. Dev Biol. 2005;283:17–28. doi: 10.1016/j.ydbio.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M. Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development. 2003;130:2705–2716. doi: 10.1242/dev.00506. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih I, Bartel DP. Micro RNA-directed cleavage of HoxB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Yu JK, Satou Y, Holland ND, Shin-I T, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted gastrulation. Dev Biol. 2008;323:6–18. doi: 10.1016/j.ydbio.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Qiu L, Kotzsch A, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.