Abstract
Epidemiological studies suggest that statins and non-steroidal anti-inflammatory drugs (NSAIDs) reduce the risk of prostate cancer. In the present study, LNCaP cells were cultured in regular medium containing fetal bovine serum or in medium supplemented with charcoal-stripped fetal bovine serum to mimic androgen-deprivation treatment. We found that atorvastatin (Lipitor) or celecoxib (Celebrex) treatment of LNCaP cells cultured in regular or androgen-depleted medium inhibited growth and stimulated apoptosis. A combination of atorvastatin and celecoxib was more effective than either agent alone. In animal studies, severe combined immunodeficient (SCID) mice were injected subcutaneously with LNCaP cells in Matrigel. After 4–6 weeks, mice with LNCaP tumors (about 0.6 cm wide and 0.6 cm long) were surgically castrated and received daily i.p. injections with vehicle, atorvastatin (10 μg/g body weight/day), celecoxib (10 μg/g/day) or a combination of atorvastatin (5 μg/g/day) and celecoxib (5 μg/g/day) for 42 days. In all groups, the androgen-dependent LNCaP tumors regressed initially in response to castration, but the tumors eventually progressed to androgen-independence and started to grow. Treatment of the mice with atorvastatin or celecoxib alone suppressed the re-growth of LNCaP tumors after castration. A combination of low doses of atorvastatin and celecoxib had a more potent effect for inhibiting the progression and growth of LNCaP tumors to androgen-independence than a higher dose of either agent alone. Our results indicate that administration of a combination of atorvastatin and celecoxib may be an effective strategy for the prevention of prostate cancer progression from androgen-dependence to androgen-independence.
Keywords: prostate cancer, androgen depletion, progression, apoptosis
INTRODUCTION
Prostate cancer is one of the leading causes of death among men in the United States (1). Despite aggressive efforts toward early detection and treatment, the mortality rate for prostate cancer continues at a high level (1, 2). Early stage prostate cancer requires androgen for growth and thus responds to androgen deprivation therapy (3, 4). However, eventually the disease progresses to an androgen-independent state that is unresponsive to androgen ablation. Treatment of these hormone-refractory prostate cancer patients with chemotherapeutic agents is generally unsatisfactory (3–5). Therefore, it is an important challenge to develop effective ways of preventing or slowing the formation of androgen-independent prostate cancer.
Atorvastatin (Lipitor) and other statins inhibit 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and are used clinically as a safe and effective approach for the control of hypercholesterolemia (6). Recent studies indicate that in addition to the cholesterol-lowering effect, statins have pleiotropic activities in modulating other biological processes such as cell proliferation and apoptosis (7). Although epidemiological studies investigating statin use and total cancer risk as the primary outcome have yielded conflicting results (8–10), a recent case-control study indicates that statins may reduce the risk of more aggressive prostate cancer (11). In another clinical study, Moyad and colleagues found that statins, especially atorvastatin, improved clinical presentations in prostate cancer patients (12). Furthermore, statin drugs were found to induce apoptosis in cultured prostate cancer cells and in acute myeloid leukemia cells (13, 14). In a recent study, atorvastatin in combination with aspirin or atorvastatin in combination with celecoxib (Celebrex) was found to inhibit azoxymethane (AOM)-induced colon carcinogenesis in male F344 rats to a greater extent than higher doses of atorvastatin, aspirin or celecoxib alone (15). Atorvastatin in combination with celecoxib also inhibited the formation and growth of androgen-independent prostate PC-3 xenograft tumors (16).
Celecoxib is a selective cyclooxygenase (Cox)-2 inhibitor. Earlier studies have shown that Cox-2 is overexpressed in humanprostate adenocarcinomas (17–20). Other studies showed that expression of Cox-2 in prostate cancer was not always observed suggesting that the chemopreventive effect of celecoxib on prostate cancer may be mediated by Cox-2-independent mechanisms (21, 22). In an earlier study, prostate cancer patients who had relapsed after radiation therapy or radical prostatectomy were treated with celecoxib 200 mg twice daily (23). Follow-up PSA levels were obtained at 3, 6 and 12 months after initiating treatment. Decreased serum PSA levels and increased PSA doubling time were found in some patients suggesting that celecoxib might have helped prevent or delay prostate cancer progression in these patients (23). Although recent clinical studies showed that long-term use of a high dose of celecoxib was associated with an increased cardiovascular risk (24, 25), the use of celecoxib to decrease mortality by delaying the progression of prostate cancer may have an overall favorable benefit/risk ratio. An effective strategy to reduce side effects is the use of a low dose of celecoxib in combination with other preventive agents such as atorvastatin.
In the present study, we assessed the preventive effect of atorvastatin and celecoxib alone or in combination on the progression of androgen-dependent LNCaP xenograft tumors to androgen independence in SCID mice. We found that a combination of low doses of atorvastatin and celecoxib had a more potent effect for inhibiting the androgen-independent growth of LNCaP tumors than a higher dose of either agent alone.
MATERIALS AND METHODS
Cell culture and reagents
LNCaP cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Atorvastatin and celecoxib were provided by the National Cancer Institute’s Repository. Propylene glycol, polysorbate 80, benzyl alcohol, ethanol and DMSO were purchased from Sigma (St. Louis, MO). Matrigel was obtained from BD Biosciences (Bedford, MA). RPMI-1640 tissue culture medium, penicillin-streptomycin, L-glutamine and fetal bovine serum (FBS) were from Gibco (Grand Island, NY). Charcoal-stripped FBS was purchased from HyClone Inc (Logan, UT). LNCaP cells were maintained in RPMI-1640 culture medium containing 10% FBS that was supplemented with penicillin (100 units/ml)-streptomycin (100 μg/ml) and L-glutamine (300 μg/ml). Cultured cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were passaged twice a week. LNCaP cells were initially seeded at a density of 0.5×105 cells/ml in 35-mm tissue culture dishes (2 ml/dish) for assays of proliferation and apoptosis, and seeded at a density of 1×105 cells/ml of medium in 100 mm culture dishes (10 ml/dish) for the Western blot analysis. Atorvastatin and celecoxib were dissolved in DMSO and the final concentration of DMSO in all experiments was 0.2%. In experiments with androgen-depleted medium, charcoal-stripped FBS was used to replace the regular FBS in cell culture medium.
Determination of the number of viable cells
The number of viable cells after each treatment was determined using a hemacytometer under a light microscope (Nikon Optiphot, Japan). Cell viability was determined by the trypan blue exclusion assay, which was done by mixing 80 μl of cell suspension and 20 μl of 0.4% trypan blue solution for 2 min. Blue cells were counted as dead cells and the cells that did not absorb dye were counted as live cells.
Morphological assessment of apoptotic cells
Apoptosis was determined by morphological assessment in cells stained with propidium iodide (26). Briefly, cytospin slides were prepared after each experiment and cells were fixed with acetone/methanol (1:1) for 10 min at room temperature, followed by 10 min with propidium iodide staining (1 μg/ml in PBS) and analyzed using a fluorescence microscope (Nikon Eclipse TE200, Japan). Apoptotic cells were identified by classical morphological features including nuclear condensation, cell shrinkage, and formation of apoptotic bodies (26). At least 200 cells were counted in each sample and the percentage of apoptotic cells was determined.
Progression of androgen-dependent prostate LNCaP tumors to androgen independence in immunodeficient mice
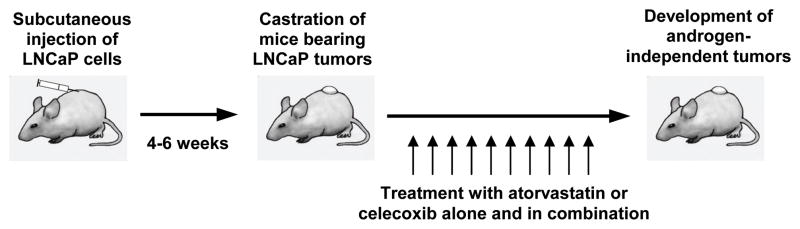
Male SCID mice were obtained from Taconic Farms Inc. (Germantown, NY). The animals were housed in sterile filter-capped microisolator cages and provided with sterilized 5010 rodent diet and water. As illustrated in Fig. 1, LNCaP cells (2.5×106 cells/0.1 ml/mouse) suspended in 50% Matrigel (Collaborative Research, Bedford, MA) in RPMI 1640 medium were injected subcutaneously into the right flank of the mice. After 4–6 weeks, mice with LNCaP tumors (0.6–1.0 cm wide and 0.6–1.0 cm long) were surgically castrated and injected with vehicle (5 μl/g body weight), atorvastatin (10 μg/g body weight), celecoxib (10 μg/g body weight) or atorvastatin (5 μg/g body weight) + celecoxib (5 μg/g body weight) once a day for 42 days. In all experiments, animals in the different experimental groups received the same amount of vehicle (5 μl/g body weight), which consisted of propylene glycol, polysorbate 80, benzyl alcohol, ethanol and water (40: 0.5: 1: 10: 48.5). Tumor size (length × width; cm2) and body weight were measured once every third day after surgical castration. The development of androgen independence was monitored by the growth of tumors. At the end of the study, mice were sacrificed, tumors were excised, weighed and placed in phosphate-buffered formalin at room temperature for 48 h and then placed in ethanol for 48 h before preparing paraffin sections as previously described (27). All animal experiments were carried out under an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Figure 1.
Schematic illustration of in vivo androgen-deprivation treatment by surgical castration and effects of atorvastatin or celecoxib alone or in combination on the development and growth of androgen-independent LNCaP tumors in SCID mice.
Plasma levels of atorvastatin and celecoxib
EDTA-treated plasma samples (100 μl each) were treated with 10 μl of 5% ascorbic acid before storage at −70°C. Extraction of atorvastatin and celecoxib from plasma samples was performed by treatment with 100 μl of 0.4 M sodium phosphate buffer (pH 6.8), followed by shaking with 1000 and 700 μl of ethyl acetate and centrifugation consecutively. The pooled upper ethyl acetate phase (1400 μl) was dried. The residue was reconstituted in 100 μl acetonitrile:water (1:1), and the sample was centrifuged. Ten μl of the resulting supernatant was applied to an LC MS/MS system. LC/MS was conducted on a Thermo LTQ linear ion trap mass detector (ThermoFisher Scientific, San Jose, CA) interfaced with an electrospray ionization (ESI) probe, with a Surveyor MS pump and a Surveyor refrigerated (4°C) autosampler. Chromatographic separation was performed on a Phenomenex Gemini C18 column (50 × 2.0-mm i.d., 3 μm particle size; Torrance, CA). The LC mobile phases consisted of acetonitrile/water (10:490, vol/vol, containing 0.2 mM HCOOH; solvent A) and acetonitrile/water (450:50, vol/vol, containing 0.2 mM HCOOH; solvent B). The mobile phase was delivered at 0.2 ml/min. The column was eluted with a linear gradient from 7% to 100% of B from 0 to 15 min and then with 100% of B from 15 – 16 min. The column was then re-equilibrated to 7% of B for 6 min prior to injection of the next sample. The LC eluent flow after 2 min was introduced to the mass spectrometer for data acquisition. The MS/MS parameters in the negative-ion ESI mode were tuned to maximize the generation of deprotonated drug molecules ([M-H]−, m/z557 for atorvastatin; m/z308 for celecoxib). All data acquired were processed by Finnigan Xcalibur software (version 2.0; ThermoFisher; Thermo Electron, San Jose, CA). The absolute solvent extraction recoveries of atorvastatin (1–4096 ng/ml) and celecoxib (1–4096 ng/ml) from plasma were 50–55 and 60–67 percent, respectively. Atorvastatin and celecoxib standards in control plasma were analyzed side-by-side with experimental samples and were used for the calculation of plasma levels.
Western Blotting
After treatment, LNCaP cells were washed with ice-cold PBS and lysed with 800 μl of lysis buffer (10 mM Tris-HCl, pH 7.4, 50 mM sodium chloride, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 100 μM sodium orthovanadate, 2 mM iodoacetic acid, 5 mM ZnCl2, 1 mM phenylmethylsulfonyl fluoride and 0.5% Triton X-100). The lysates were centrifuged at 12,000 g for 15 min at 4°C. The protein concentration of whole cell lysates was determined with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). Equal amounts (20 μg) of protein were then resolved on a 10% Criterion Precast Gel (Bio-Rad, Hercules, CA) and transferred to a PVDF membrane using a semi-dry transfer system. The membrane was then probed with anti-phosphorylated Akt (#9275, Cell Signaling Technology, Beverly, MA) or anti-phosphorylated Erk1/2 antibody (#4376, Cell Signaling Technology) primary antibody. After binding with primary antibody the membrane was washed with Tris-buffered saline three times, then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and washed with Tris-buffered saline three times. Final detection was performed with enhanced chemiluminescent reagents. The extent of protein loading was determined by blotting for β-actin. The membrane was incubated in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, and 62.5 mM Tris-HCl at pH 6.7) at 50°C for 30 min with occasional agitation before incubating in blocking buffer and re-probing using anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Immunostaining
An immunoperoxidase staining method was used to determine caspase-3 and NF-κB. Briefly, tumor sections were incubated with an antibody that detects the active form of caspase-3 (AF835, R&D Systems, San Diego) and cytospin slides were incubated with primary antibody against NF-κB (sc-114; Santa Cruz Biotechnology Inc) for 30 min at room temperature. The sections and cytospin slides were then incubated with a biotinylated secondary antibody for 30 min followed by incubation with conjugated-avidin solution (ABC ellite kit purchased from Vector Laboratories) for 30 min. Color development was achieved by incubation with 0.02% 3,3′-diaminobenzidine tetrahydrochloride containing 0.02% hydrogen peroxide for 10 min at room temperature. The slides were then counterstained with hematoxylin, dehydrated, and coverslips were added for permanent mounting. A positive reaction was shown as a brown precipitate in the cytoplasm and/or nucleus of the cells. The number of caspase 3-positive cells was determined in at least 1000 cells from each tumor. For NF-κB staining, photomicrographs were taken using a light microscope (Nikon Optiphot-2, Japan) linked to an Image System (Media Cybernetics, Silver Spring, MD).
NF-κB-dependent reporter genze expression assay
NF-κB transcriptional activity was measured by the NF-κB-luciferase reporter gene expression assay. An NF-κB-luciferase construct was kindly provided by Dr. Tony Kong (Department of Pharmaceutics at Rutgers). The NF-κB-luciferase construct was transiently transfected into LNCaP cells by using Lipofectamine 2000 (LF2000, Invitrogen Life Technology) following the manufacturer’s instructions. The cells were then treated with atorvastatin or celecoxib alone or in combination for 24 h, and the NF-κB-luciferase activities were measured using the luciferase assay kits (E1500, Promega Madison WI) according to the manufacture’s instruction. The luciferase activity was normalized against known protein concentrations and expressed as percent of luciferase activity in the control cells, which were treated with DMSO solvent. The protein level was determined by Bio-Rad protein assay kits (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions.
Statistical analyses
The analyses of changes in tumor size were based on a repeated measurement model with heterogeneous first order auto regressive correlation structure (28). Effects of the treatments were assessed by comparing the rates of change over time between treatment groups (i.e. comparing the slopes between treatment groups). The analysis of variance (ANOVA) method with the Tukey-Kramer test (29) was used for the comparison of tumor size, body weight, number of mitotic cells and number of caspase-3 positive cells among the different treatment groups at the end of the study.
RESULTS
Effects of atorvastatin and celecoxib on growth and apoptosis of prostate cancer LNCaP cells cultured in regular and androgen-depleted medium
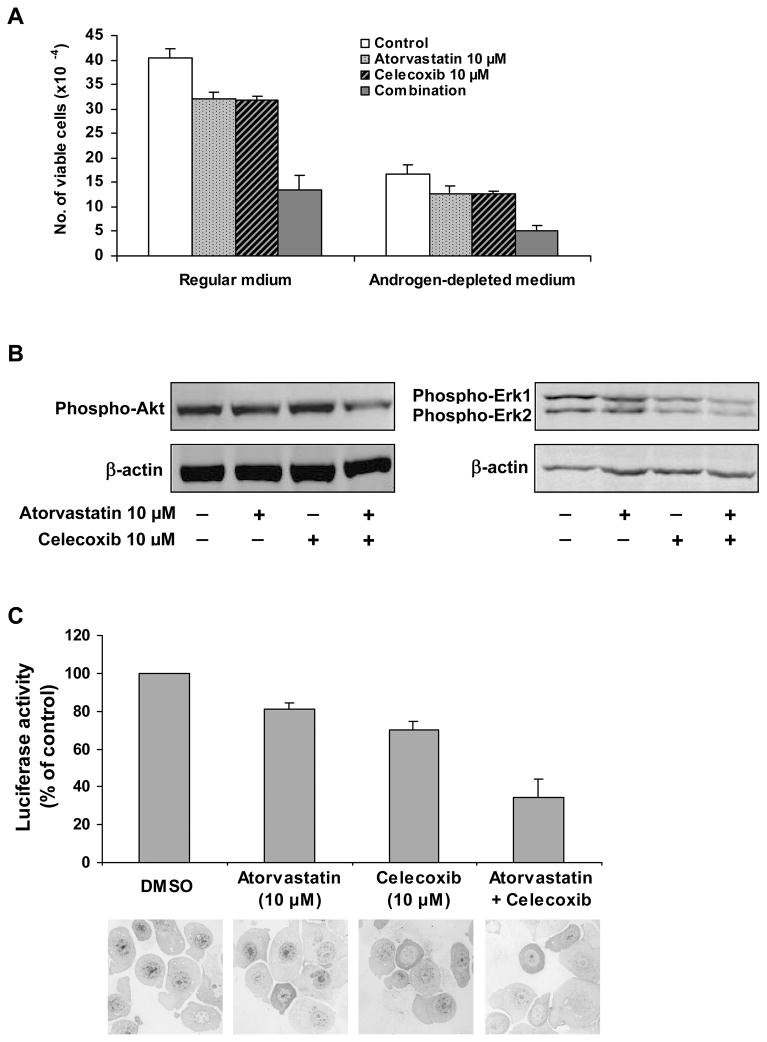
LNCaP cells cultured in regular (RPMI+10%FBS) or androgen-depleted (RPMI+10% charcoal-stripped FBS) medium were treated with atorvastatin (10 μM) or celecoxib (10 μM) alone or in combination for 96 h. As shown in Figure 2A, a combination of atorvastatin (10 μM) and celecoxib (10 μM) had a stronger inhibitory effect on the growth of LNCaP cells than either compound alone in both regular and androgen-depleted medium. As shown in Table 1, treatment with atorvastatin or celecoxib alone had a stimulatory effect on apoptosis in LNCaP cells cultured in regular medium or in androgen-depleted medium while treatment of the cells with a combination of these two agents resulted in a considerably greater increase in apoptosis. An increase in apoptosis was also found in LNCaP cells cultured in androgen-depleted medium when compared with LNCaP cells cultured in regular medium (Table 1). Treatment of LNCaP cells growing in androgen-depleted medium together with a combination of celecoxib and atorvastatin increased apoptosis by 33-fold when compared with untreated LNCaP cells grown in regular medium (Table 1).
Figure 2.
Effect of atorvastatin or celecoxib alone or in combination on the growth and activation of Akt, Erk1/2 and NF-κB in cultured LNCaP cells. (A) LNCaP cells were seeded at a density of 0.5×105 cells/ml in cell culture dishes and incubated in regular or androgen-depleted medium. The cells were treated with atorvastatin (10 μM) or celecoxib (10 μM) alone or in combination for 96 h. The number of viable cells was determined by using a trypan blue exclusion assay. (B) LNCaP cells were seeded at a density of 1×105 cells/ml of regular RPMI medium and incubated for 24 h. The regular medium was then changed to androgen-depleted medium and the cells were treated with atorvastatin (10 μM) or celecoxib (10 μM) alone or in combination for 24 h. Activated Akt and Erk1/2 were determined by using Western blotting with anti-phosphorylated-Akt (#9275, Cell Signaling Technology, MA) and anti-phosphorylated Erk1/2 (#4376, Cell Signaling Technology). (C) LNCaP cells were seeded at a density of 0.2×106 cells/ml of medium in 60 mm culture dishes (6 ml/dish) and incubated for 24 h. The cells were then transfected with a NF-κB-luciferase construct using Lipofectamine™ 2000 (LF2000, Invitrogen Life Technology). The cells were treated with atorvastatin (10 μM) or celecoxib (10 μM) alone or in combination for 24 h. The luciferase activity was determined by using a luciferase assay kits (E1500, Promega Madison). For immunostaining of NF-κB in LNCaP cells, cytospin slides were stained with anti-NF-κB antibody (sc-114; Santa Cruz Biotechnology Inc). Photomicrographs were taken using a light microscope (Nikon Optiphot-2, Japan) linked to an Image System (Media Cybernetics, Silver Spring, MD). Representative photomicrographs of NF-κB staining in cells treated with DMSO, atorvastatin, celecoxib or atorvastatin + celecoxib are shown.
Table 1.
Stimulatory effect of atorvastatin and celecoxib alone or in combination on apoptosis in LNCaP cells
| Treatment | % Apoptotic cells | Fold increase* |
|---|---|---|
| Regular medium | ||
| Control | 1.9±0.2 | – |
| Atorvastatin (10 μM) | 12.3±0.5 | 6.5 |
| Celecoxib (10 μM) | 15.1±0.6 | 7.9 |
| Atorvastatin (10 μM) + Celecoxib (10 μM) | 33.4±2.5 | 17.6 |
| Androgen-depleted medium | ||
| Control | 9.0±0.2 | 4.7 |
| Atorvastatin (10 μM) | 22.5±1.4 | 11.8 |
| Celecoxib (10 μM) | 27.8±2.0 | 14.6 |
| Atorvastatin (10 μM) + Celecoxib (10 μM) | 62.2±4.1 | 32.7 |
LNCaP cells were seeded at a density of 5×104 cells/ml in cell culture dishes and incubated in regular or androgen-depleted medium. The cells were treated with atorvastatin (10 μM) and celecoxib (10 μM) alone or in combination for 96 h. Apoptotic cells were determined by morphological assessment. Each value represents the mean ± S.E. from three experiments.
Fold-increase when compared with control cells grown in regular medium.
Effects of atorvastatin or celecoxib alone or in combination on the level of activated Akt, Erk1/2 and NF-κB in cultured LNCaP cells
In view of the important role of the Akt pathway in the survival of prostate cancer cells, the level of activated Akt in LNCaP cells cultured in androgen-depleted medium was evaluated by Western blot analysis using an anti-phosphorylated Akt antibody (#9275, Cell Signaling Technology, MA) that detects active, phosphorylated Akt. In these experiments, LNCaP cells were cultured in androgen-depleted medium and treated with atorvastatin (10 μM) or celecoxib (10 μM) alone or in combination for 24 h and analyzed by Western blotting. The level of phosphorylated Akt in the Western blot was quantified by absorbance measurement and normalized for actin. The level of phosphorylated Akt relative to control (1.00) was 0.94 in cells treated with atorvastatin, 0.98 in cells treated with celecoxib and 0.70 in cells treated with the combination of atorvastatin and celecoxib. We also determined the levels of phosphorylated Erk1/2 in LNCaP cells by Western blotting with an anti-phosphorylated Erk1/2 antibody (#4376, Cell Signaling Technology). Absorbance measurement showed that the level of phosphorylated Erk1 relative to control (1.00) was 0.85 in cells treated with atorvastatin, 0.75 in cells treated with celecoxib and 0.52 in cells treated with the combination of atorvastatin and celecoxib. The level of phosphorylated Erk2 relative to control (1.00) was 0.83 in cells treated with atorvastatin, 0.64 in cells treated with celecoxib and 0.43 in cells treated with the combination of atorvastatin and celecoxib. Representative Western blots from three separate experiments are shown in Figure 2B.
The effect of atorvastatin and celecoxib on the activation of NF-κB was determined by the luciferase reporter gene expression assay. As shown in Figure 2C, treatment of LNCaP cells cultured in androgen-depleted medium with atorvastatin or celecoxib alone caused some decrease in NF-κB activity and the combination of atorvastatin and celecoxib had a more potent inhibitory effect on NF-κB activity than either agent alone (Fig. 2C). NF-κB in LNCaP cells was also determined using immunostaining with an anti-NF-κB antibody (sc-114; Santa Cruz Biotechnology Inc). Representative photomicrographs of NF-κB staining in the cells treated with DMSO, atorvastatin, celecoxib or atorvastatin + celecoxib are shown (Fig. 2C). As shown in Figure 2C, treatment of LNCaP cells in androgen-depleted medium with either atorvastatin or celecoxib alone resulted in some decrease in nuclear staining of NF-κB. Treatment of LNCaP cells cultured in androgen-depleted medium with a combination of atorvastatin and celecoxib caused a stronger decrease in nuclear staining of NF-κB than either agent used alone (Fig. 2C).
Plasma levels of atorvastatin and celecoxib after i.p. injection in SCID mice
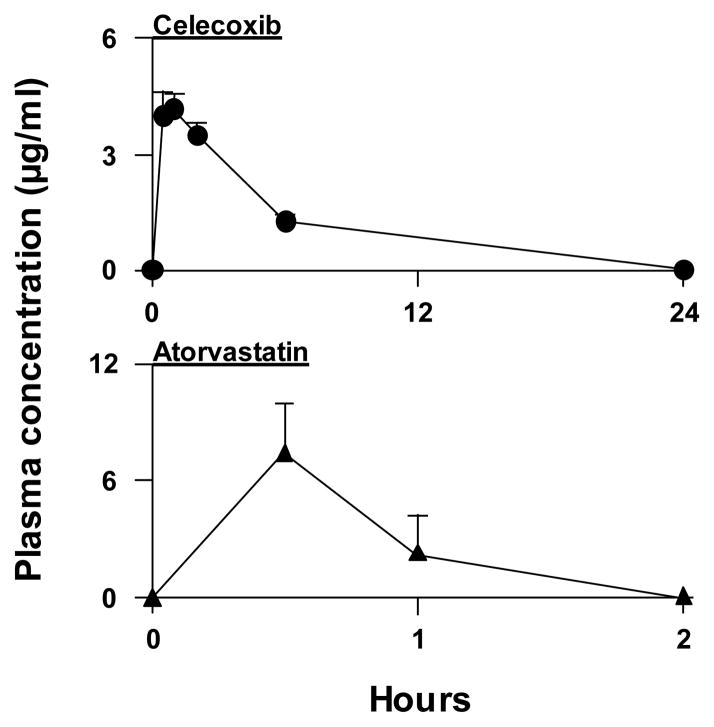
Plasma levels of atorvastatin and celecoxib were determined to show the levels associated with biological activity in our animal model. The plasma concentration of celecoxib at 0.5 h after an i.p. injection (10 μg/g body weight) in male SCID mice (7–8 weeks old) was 3.9 μg/ml (peak concentration), and a measurable plasma level could be detected for 24 h (Fig. 3). The plasma concentration of celecoxib at 24 h post-injection was 1.4 ng/ml. The area under the plasma concentration time curve (AUC0→24 h) for celecoxib was 25.6 μg•h/ml, and the half-life (t1/2) was ~2.0 h. The plasma concentration of atorvastatin at 0.5 h after an i.p. injection (10 μg/g) was 7.0μg/ml (peak plasma concentration), and the plasma level fell rapidly and could no longer be detected at 6 h post-injection (the limit of quantification was 1 ng/ml) (Fig. 3). The area under the plasma concentration time curve (AUC0→24 h) for atorvastatin was 7.0 μg•h/ml, and the t1/2 was ~0.6 h.
Figure 3.
Plasma concentration of celecoxib or atorvastatin at various times after an i.p. injection of celecoxib (10 mg/kg) or atorvastatin (10 mg/kg) in male SCID mice. Each value is the mean ± S.E. (N=3).
Effects of i.p injections of atorvastatin or celecoxib alone or in combination on the formation and growth of androgen-independent LNCaP tumors in castrated SCID mice
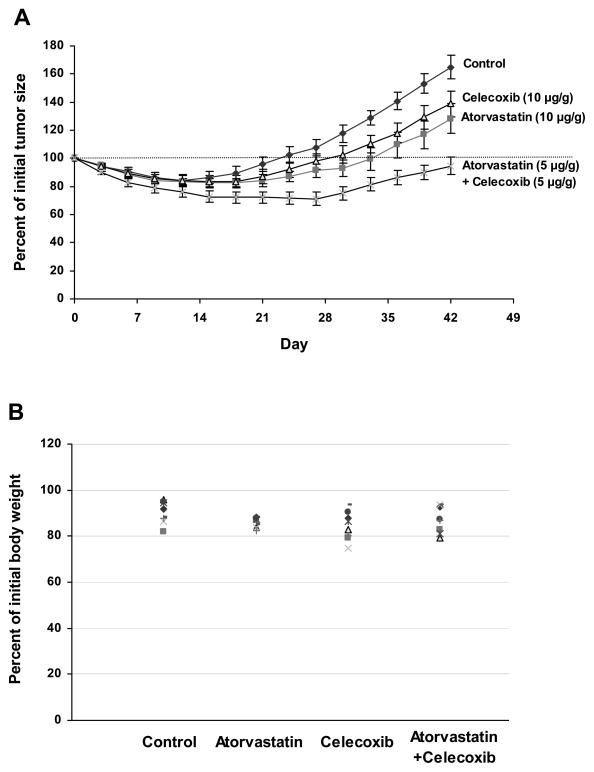
Male SCID mice (7–8 weeks old) were injected subcutaneously with LNCaP cells suspended in a 1:1 mixture of Matrigel and culture medium (2.0 × 106 cells/0.1 ml). When the tumors reached a moderate size (0.6–1.0 cm wide and 0.6–1.0 cm long), the mice were surgically castrated and then received daily i.p injections of vehicle, atorvastatin (10 μg/g body weight/day), celecoxib (10 μg/g body weight/day) or a combination of atorvastatin (5 μg/g body weight/day) and celecoxib (5 μg/g body weight/day) for 42 days. The average tumor size in each group was similar when the mice were castrated. In all groups, the LNCaP tumors regressed initially in response to castration, but the tumors then progressed to androgen-independence and started to grow at 2–4 weeks post-castration. Regrowth of the tumors started at 15, 21, 21 and 30 days post-castration in the control, atorvastatin, celecoxib and in the atorvastatin + celecoxib groups, respectively (Fig. 4A). The time that it took for the tumors to reach their original size at the time of castration was 24, 36, 33 and >42 days in the control, atorvastatin, celecoxib and atorvastatin + celecoxib groups, respectively (Fig. 4A). The growth curves of all groups showed significant quadratic trends. The growth rate (quadratic trend) for the control group was significantly higher than for any other group (p<0.001). The growth rate (quadratic trend) for the combination group was significantly lower than for the atorvastatin-treated group (p =0.003) or the celecoxib-treated group (p<0.001). The mean ± S.E for the percentage of initial tumor size after 42 days of treatment in the castrated mice was 164.9 ± 8.2 for the control group, 128.3 ± 9.0 for the atorvastatin (10 μg/g/day) group, 138.9 ± 10.6 for the celecoxib (10 μg/g/day) group and 94.6 ± 6.0 for the atorvastatin (5 μg/g/day) + celecoxib (5 μg/g/day) group. Statistical analysis using ANOVA with the Tukey-Kramer multiple comparison test showed that the percentage of initial tumor size was significantly lower in the combination group than in the atorvastatin group (p<0.05) or celecoxib group (p<0.01). The results indicate that treatment of the mice with a combination of atorvastatin and celecoxib had a stronger effect than treatment of the mice with twice the dose of either agent alone for inhibiting the formation and growth of androgen-independent prostate tumors. The effect of the various treatments on body weight is described in Figure 4B. The mean ± S.E. for the percent of initial body weight after 42 days of treatment was 90.9 ± 1.8% for the control group, 85.6 ± 0.8% for the atorvastatin group, 84.3 ± 2.2% for the celecoxib group and 89.5 ± 2.1% for the atorvastatin + celecoxib group. Statistical analysis with the Tukey-Kramer multiple comparison test showed that differences in the percent of initial body weight between any two groups were not statistically significant (p>0.05).
Figure 4.
Effect of i.p. injections of atorvastatin or celecoxib alone or in combination on the progression and growth of androgen-dependent LNCaP xenograft prostate tumors to androgen-independence. Male SCID mice were injected subcutaneously with LNCaP cells in 50% Matrigel (2.0 × 106 cells/0.1 ml). After 4–6 weeks, mice with LNCaP tumors (0.6–1.0 cm wide and 0.6–1.0 cm long) were surgically castrated. Castrated mice were injected i.p with atorvastatin (10 μg/g body weight/day), celecoxib (10 μg/g body weight/day) or a combination of atorvastatin (5 μg/g body weight/day) and celecoxib (5 μg/g body weight/day) for 42 days. Tumor size (length × width) and body weight were measured once every three days and expressed as percent of initial tumor size and percent of initial body weight, respectively. (A). Growth curve of LNCaP tumors in each group. Each value represents the mean ± S.E. from 8 mice (B). Individual body weight of mice after treatment for 42 days.
Effects of i.p. injections of atorvastatin or celecoxib alone or in combination on proliferation and apoptosis in LNCaP tumors
We determined the effects of daily i.p. injections of atorvastatin or celecoxib alone or in combination for 42 days on proliferation and apoptosis in the LNCaP tumors described in Figure 4. Tumor cell proliferation was determined by counting mitotic cells, and apoptosis was determined by immunostaining of caspase-3 (active form) positive cells. As shown in Table 2, the percent of mitotic cells was decreased significantly in tumors from mice treated with atorvastatin + celecoxib when compared to the control group. Apoptosis, as measured by the percentage of caspase 3 (active form) positive cells in tumors, was increased significantly in the atorvastatin + celecoxib group (Table 2). The ratio of the percent mitotic cells/percent caspase 3 (active form) positive cells which is an index of the balance between cell proliferation and cell death was also determined in the LNCaP tumors. We found that the ratio of the percent mitotic cells/percent caspase 3 (active form) positive cells ± S.E. in tumors was 1.62 ± 0.11 for the vehicle treated control group, 0.91 ± 0.07 for the atorvastatin group (10 μg/g), 1.03 ± 0.09 for the celecoxib group (10 μg/g), and 0.61 ±0.06 for the atorvastatin (5 μg/g) + celecoxib (5 μg/g) group (Table 2).
Table 2.
Effects of atorvastatin or celecoxib alone or in combination on mitotic and caspase-3 positive cells in LNCaP tumors
| Treatment | No. of animals | Percent mitotic cells | Percent caspase -3 positive cells | Ratio of percent mitotic cells/caspase-3 positive cells |
|---|---|---|---|---|
| Control | 8 | 0.4790.03 | 0.29±0.02 | 1.62±0.11 |
| Atorvastatin | 8 | 0.37±0.02* | 0.41±0.03* | 0.91±0.07** |
| Celecoxib | 8 | 0.40±0.03 | 0.39±0.02* | 1.03±0.09** |
| Atorvastatin + Celecoxib | 8 | 0.30±0.02** | 0.50±0.03** | 0.61±0.06*** |
Tumors from SCID mice in the experiment described in Fig. 1 were analyzed for mitotic cells and caspase-3 positive cells. Mitotic cells were identified and counted in H&E stained tissue sections using a light microscope. Caspase-3 positive cells were identified immunohistochemically. Mitotic cells and caspase 3 positive cells were determined in at least 1000 cells from each tumor. Each value represents the mean ± S.E. Differences between each treatment group and the control were analyzed by the Tukey-Kramer multiple comparison test:
p<0.05
p<0.01
p<0.001
DISCUSSION
In an earlier study, we demonstrated that a combination of atorvastatin and celecoxib was more effective than either drug alone for inhibiting the growth of cultured PC-3, Du145, LNCaP and CWR22Rv1 prostate cancer cells (androgen dependent and androgen independent cell lines) (16). In this earlier study, we found that atorvastatin and celecoxib decreased the level of phospho-Erk1/2 and the activity of NF-κB (16). Our earlier study also demonstrated that daily i.p. injections of a combination of atorvastatin (5 μg/g body weight) and celecoxib (5 μg/g body weight) was more effective at inhibiting the growth of androgen independent PC-3 xenograft tumors in SCID mice than daily i.p. injections of 10 μg/g body weight of either drug alone. Administration of the combination of drugs inhibited mitosis and stimulated apoptosis in PC-3 tumors (16).
In the present study, we determined whether administration of celecoxib and atorvastatin would inhibit the progression of androgen-dependent xenograft tumors to androgen independence. We found that administration of a combination of atorvastatin and celecoxib was more effective than either drug alone for inhibiting the progression of androgen-dependent xenograft LNCaP tumors to androgen independence in castrated SCID mice. Daily i.p injections of a combination of atorvastatin and celecoxib doubled the time that it took for the progression of androgen-dependent xenograft LNCaP tumors to androgen-independent growth (time for tumors to start regrowing after castration) (Fig. 4A). In cultured LNCaP cells, we found that a combination of atorvastatin, celecoxib and androgen-depletion strongly induced apoptosis in cultured LNCaP cells. Androgen depletion or treatment with celecoxib or atorvastatin alone resulted in a 5- to 8- fold increase in apoptosis in LNCaP cells, whereas a combination of all three treatments resulted in a 33- fold increase in apoptosis (Table 1). Although treatment of cultured LNCaP cells with a combination of atorvastatin and celecoxib in androgen-depleted medium resulted in 62% apoptotic cells (Table 1), the absolute number of apoptotic cells in tumors from castrated mice treated with atorvastatin and celecoxib was very low (Table 2). The low percentage of apoptotic cells in LNCaP tumors may be due to the removal of apoptotic cells by phagocytosis that prevents their accumulation. Although the absolute number of apoptotic cells in tumors was low, we found a significant increase in apoptotic cells and a significant decrease in mitotic cells in the tumors from mice treated with atorvastatin and celecoxib in combination. Our results indicate that the drug-induced delay in the progression of androgen-dependent LNCaP tumors to androgen independence was associated with a highly significant decrease in the ratio of proliferation/apoptosis in the tumors (Table 2).
The transition of prostate cancer cells to an androgen-independent phenotype is a complex process that involves the survival of prostate cancer cells during androgen deprivation treatment, adaptive changes in gene expression as well as alterations in growth/death signaling pathways (3, 4). Earlier studies have implicated activation of the Akt signaling pathway for the survival of prostatecancer cells treated with androgen ablation therapy (30, 31). Increased expression of Cox-2 and phosphorylated Erk1/2 was found in advanced prostate cancer (17, 32, 33). Increased androgen receptor (AR) signaling also plays an important role in the development of androgen independence (34–37). Another positive growth signal that is increased during androgen-independent progression is IGF-1 (38). In the present study, we found that atorvastatin and celecoxib in combination was more potent in suppressing the progression of androgen-dependent LNCaP tumors to androgen independence than either agent alone. We also found that the combination of these two drugs had a stronger inhibitory effect on the activation of Akt, Erk1/2 and NF-κB in cultured LNCaP cells than either compound used alone (Fig. 2B&C).
The mechanisms by which atorvastatin and celecoxib in combination inhibit the growth and induce apoptosis in LNCaP prostate tumors are not clear. Atorvastatin is an HMG-CoA reductase inhibitor that reduces the synthesis of isoprenoids, geranylgeranyl pyrophosphate (GGPP) and farnesylpyrophosphate (FPP) and their precursor mevalonate (39). Notably, GGPP and FPP are required for the function of Rho and Ras proteins, respectively (40). Because Ras and Rho are important signaling molecules in cell proliferation and survival, atorvastatin and other statin drugs may interfere with Ras/Rho activity and thus inhibit the growth and stimulate apoptosis in cancer cells. One of the downstream effecters of Ras activation is PI3K/Akt. Atorvastatin was shown to inhibit cytotoxic drug-induced activation of Akt in lung cancer cells (41). Although celecoxib is a selective Cox-2 inhibitor, this drug also inhibits prostate cancer growth by Cox-2-independent mechanisms (42). In an earlier study, it was shown that celecoxib inhibited Akt activation and stimulated apoptosis in prostate cancer cells (43). Celecoxib was also shown to inhibit the activation of NF-κB, Akt and Erk1/2 in lung cancer cells (44). A combination of atorvastatin and celecoxib strongly decreased the level of phosphorylated-Akt in colon cancer cells (45). In the present study, we found that atorvastatin and celecoxib in combination had a more potent inhibitory effect on the levels of activated Akt, Erk1/2 and NF-κB in LNCaP cells than either drug alone (Fig. 2B&C). Simultaneous inhibition of these pathways may lead to a strong inhibitory effect on proliferation and a strong stimulatory effect on apoptosis in prostate cancer cells.
Animal models were developed to mimic the formation and progression of prostate cancer in humans. Mouse models for prostate carcinogenesis include the TRAMP model (46), the Nkx3.1/Pten mutant mouse model (47), the c-myc transgenic mouse model (48) and the conditional Pten knockout mouse model (49). A mouse model for progression of an androgen-dependent prostate tumor to androgen independence was previously established (50). In this model, immunodeficient nude mice with human androgen dependent LNCaP tumors were surgically castrated to mimic androgen-ablation therapy in patients. Castration of mice with LNCaP tumors resulted in temporary tumor regression followed by androgen-independent growth of the tumors (50). In the present study, SCID mice with LNCaP tumors were surgically castrated, and tumor regression was observed for about 2 weeks after surgery. Then, as the tumors became androgen independent, they started to grow (See Fig. 4A). We found that this mouse model is very useful for studies on the prevention of progression of androgen-dependent prostate tumor to androgen independence. An appealing property of this model is that comparison of effects of different preventive agents alone or in combination on molecular events of androgen-independent progression can be made between the same type of human prostate cancer cells in vitro and in vivo.
In the present study, an i.p. injection of celecoxib (10 μg/g body weight) in male SCID mice resulted in a peak plasma concentration of 3.9 μg/ml, and the half life was ~2.0 h. It was reported that oral administration of celecoxib (200 mg) in humans resulted in a peak plasma level of 0.6–1.3 μg/ml, and the half-life was 7.6–15.2 h (51). In the present study, an i.p. injection of atorvastatin (10 μg/g body weight) in male SCID mice resulted in a peak plasma level of 7.0μg/ml and the half life was ~0.6 h. An earlier study showed that oral administration of atorvastatin (20 mg) in humans resulted in a peak plasma level of ~ 7 ng/ml (52). After oral administration of atorvastatin (20 mg) once a day for 14 days, the peak plasma level was 15 ng/ml (53). The half-life of atorvastatin in humans was 7–19.5 h (52, 53). The peak plasma levels of celecoxib and atorvastatin in the present study in male SCID mice were higher than that observed in humans. However, both drugs were eliminated from SCID mice much more rapidly (shorter t½) than in humans. Further studies are needed to determine whether a dosing regimen of celecoxib and atorvastatin that provide a blood level profile similar to humans (frequent dosing or minipump infusion) will have an inhibitory effect on the progression of androgen dependent LNCaP tumors to androgen independence.
In summary, we found that the combination of atorvastatin and celecoxib more strongly inhibited growth and the activation of Akt, Erk1/2 and NF-κB in cultured LNCaP cells than either agent alone. In addition, administration of a combination of celecoxib and atorvastatin had a strong inhibitory effect on the progression of androgen-dependent LNCaP prostate tumors to androgen-independence in castrated SCID mice. The delayed formation of androgen-independent LNCaP tumors was associated with decreased mitosis and increased apoptosis in the tumors.
Acknowledgments
Funding Support NIH R03 CA121391 and CA133902
The authors thank Ms. Florence Florek for her excellent help in the preparation of this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Morris MJ, Scher HI. Novel therapies for the treatment of prostate cancer: current clinical trials and development stratagies. Surg Oncol. 2002;11:13–23. doi: 10.1016/s0960-7404(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 3.So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol. 2005;23(1):1–9. doi: 10.1007/s00345-004-0473-1. [DOI] [PubMed] [Google Scholar]
- 4.Pilat MJ, Kamradt JM, Pienta KJ. Hormone resistance in prostate cancer. Cancer Metastasis Rev. 1998;17(4):373–81. doi: 10.1023/a:1006166511344. [DOI] [PubMed] [Google Scholar]
- 5.Raghavan D, Koczwara B, Javle M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur J Cancer. 1997;33(4):566–74. doi: 10.1016/s0959-8049(96)00510-2. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra HS, Goa KL. Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia. Drugs. 2001;61(12):1835–81. doi: 10.2165/00003495-200161120-00012. [DOI] [PubMed] [Google Scholar]
- 7.McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87(4):1451–8. doi: 10.1210/jcem.87.4.8412. [DOI] [PubMed] [Google Scholar]
- 8.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12(12):3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 9.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 10.Singal R, Khurana V, Caldito G, Fort C. Statins and prostate cancer risk: a large case-control study in veterans. Proceedings of the American Society for Clinical Oncology. 2005:23. [Google Scholar]
- 11.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162(4):318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 12.Moyad MA, Merrick GS, Butler WM, et al. Statins, especially atorvastatin, may favorably influence clinical presentation and biochemical progression-free survival after brachytherapy for clinically localized prostate cancer. Urology. 2005;66(6):1150–4. doi: 10.1016/j.urology.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64(18):6461–8. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 15.Reddy BS, Wang CX, Kong AN, et al. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66(8):4542–6. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Cui XX, Avila GE, et al. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res. 2007;13(18 Pt 1):5480–7. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42(1):73–8. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura R, Sano H, Masuda C, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000;89(3):589–96. [PubMed] [Google Scholar]
- 19.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56(4):671–6. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 20.Madaan S, Abel PD, Chaudhary KS, et al. Cytoplasmic induction and over-expression of cyclooxygenase-2 in human prostate cancer: implications for prevention and treatment. BJU Int. 2000;86:736–41. doi: 10.1046/j.1464-410x.2000.00867.x. [DOI] [PubMed] [Google Scholar]
- 21.Zha S, Gage WR, Sauvageot J, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61(24):8617–23. [PubMed] [Google Scholar]
- 22.Kohli M, Dennis RA, Mukunyadzi P, Johnson DE, Kaushal V. Cyclooxygenase-2 expression in prostate cancer: An inconsistent therapeutic target. Uro Oncology. 2004;4:113–8. [Google Scholar]
- 23.Pruthi RS, Derksen JE, Moore D. A pilot study of use of the cyclooxygenase-2 inhibitor celecoxib in recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. BJU Int. 2004;93(3):275–8. doi: 10.1111/j.1464-410x.2004.04601.x. [DOI] [PubMed] [Google Scholar]
- 24.Schneeweiss S, Solomon DH, Wang PS, Rassen J, Brookhart MA. Simultaneous assessment of short-term gastrointestinal benefits and cardiovascular risks of selective cyclooxygenase 2 inhibitors and nonselective nonsteroidal antiinflammatory drugs: an instrumental variable analysis. Arthritis Rheum. 2006;54(11):3390–8. doi: 10.1002/art.22219. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028–35. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Chang RL, Cui XX, et al. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans-retinoic acid on the growth of LNCaP prostate tumors in immunodeficient mice. Cancer Res. 2004;64(5):1811–20. doi: 10.1158/0008-5472.can-03-2848. [DOI] [PubMed] [Google Scholar]
- 27.Zheng X, Chang RL, Cui XX, et al. Effects of 12-O-tetradecanoylphorbol-13-acetate (TPA) in combination with paclitaxel (Taxol) on prostate Cancer LNCaP cells cultured in vitro or grown as xenograft tumors in immunodeficient mice. Clin Cancer Res. 2006;12(11 Pt 1):3444–51. doi: 10.1158/1078-0432.CCR-05-2823. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey JK. Models for Repeated Measurments. Oxford: Claredon Press; 1993. [Google Scholar]
- 29.Hsu JC. Comparisons: Tehory and Methods. New York: Chapman and Hall; 1996. [Google Scholar]
- 30.Graff JR, Konicek BW, McNulty AM, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275(32):24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 31.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142(11):4795–805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 32.Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res. 2001;29(1):23–8. doi: 10.1007/s002400000148. [DOI] [PubMed] [Google Scholar]
- 33.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59(2):279–84. [PubMed] [Google Scholar]
- 34.Koivisto P, Kononen J, Palmberg C, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57(2):314–9. [PubMed] [Google Scholar]
- 35.Marcelli M, Ittmann M, Mariani S, et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60(4):944–9. [PubMed] [Google Scholar]
- 36.Zhao XY, Malloy PJ, Krishnan AV, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 37.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 38.Krueckl SL, Sikes RA, Edlund NM, et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64(23):8620–9. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 40.Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–85. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- 41.Roudier E, Mistafa O, Stenius U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Molecular Cancer Therapeutics. 2006;5(11):2706–15. doi: 10.1158/1535-7163.MCT-06-0352. [DOI] [PubMed] [Google Scholar]
- 42.Patel MI, Subbaramaiah K, Du B, et al. Celecoxib inhibits prostate cancer growth: evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res. 2005 Mar 1;11(5):1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- 43.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275(15):11397–403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 44.Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004;173(3):2011–22. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Zhang Q, Lin Y, Reddy BS, Yang CS. Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. [see comment] Int J Cancer. 2008;122(9):2115–24. doi: 10.1002/ijc.23315. [DOI] [PubMed] [Google Scholar]
- 46.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92(8):3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim MJ, Cardiff RD, Desai N, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci U S A. 2002;99(5):2884–9. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellwood-Yen K, Graeber TG, Wongvipat J, et al. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4(3):223–38. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 50.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54(10):2577–81. [PubMed] [Google Scholar]
- 51.Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38(3):225–42. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- 52.Cilla DD, Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60(6):687–95. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- 53.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–60. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]