Abstract
Nine commercially available vascular endothelial growth factor (VEGF) antibodies were investigated for their ability to immunostain vascular malformations (VMs) with or without immature capillary proliferation. First, all antibodies were optimized for their performance in IHC, with placenta and colon adenocarcinoma as positive control tissues. Five antibodies were regarded as unfit for VEGF immunostaining based on poor immunostaining criteria. Subsequently, Western blot analysis using VEGF rabbit polyclonal antibody (Thermo RB-9031) revealed a clear 45-kDa band in tissue extracts from VMs with immature capillary proliferation and a high Ki67-labeling index, whereas tissue extracts from mature VMs without microvascular proliferation and no Ki67-labeling index demonstrated only a very weak 45-kDa band. In contrast, two VEGF antibodies, including the popular Santa Cruz A-20, revealed bands at 45 kDa of similar intensity in tissue extracts from both types of VMs. Staining characteristics of the 45-kDa band were reflected in the results obtained in IHC. (J Histochem Cytochem 58:109–118, 2010)
Keywords: placenta, colon cancer, endothelium, VEGF, immunohistochemistry, angiogenesis
Vascular endothelial growth factor A (VEFG-A), the most prominent member of the VEGF family, is one of the key regulators of angiogenesis in general, including the promotion of tumor progression and metastasis (Kim et al. 1993; Ferrara et al. 2003). The important role of this growth factor in different areas of biological sciences makes it therefore an interesting target in many immunohistochemical studies. At present, at least nine different primary anti-VEGF antibodies are commercially available that can be applied to formalin-fixed and paraffin-embedded tissue samples (Table 1). Considering the literature on VEGF IHC applications, there is surprisingly little discussion about the selection of the applied VEGF antibody, and no consensus on which VEGF antibody is most reliable. In an attempt to validate five VEGF antibodies, Zhang et al. (1998) reported the R and D Systems mouse antibody (MAB293) to be most specific on the basis of immunostaining of VEGF and mock-transfected cell lines. In addition, these authors also showed that Santa Cruz VEGF polyclonal antibody A-20 was found unsuitable, although this antibody has been applied in the vast majority of IHC studies (Table 1). No standardization was obtained with the VG1 mouse monoclonal antibody created especially for staining formalin-fixed and paraffin-embedded tissue samples (Turley et al. 1998).
Table 1.
Anti-VEGF antibodies
| Clone | Type or species | Vendor code | Reactive with | Number of articlesa | Western blottingb |
|---|---|---|---|---|---|
| JH121c | Mouse IgG1 | Thermo MS-350d | VEGF-121 | 76 | + |
| Rabbit | Thermo RB-9031d | N-term | 4 | + | |
| VG1c | Mouse IgG1 | Thermo MS-1467d | VEGF-121, -165, -189 | 110 | + |
| 5C3.F8c | Mouse IgG1 | Thermo MS-290d | VEGF-165 | 2 | + |
| Rabbit | Thermo RB-222d | VEGF-165 | 11 | + | |
| SP28 | Rabbit monoclonal | Thermo RM-9028d | N-term | 4 | + |
| C-1 | Mouse IgG2a | Santa Cruz sc-7269e | VEGF-121, -165, -189 | 95 | + |
| A-20 | Rabbit | Santa Cruz sc-152e | VEGF-121, -165, -189 | 314 | + |
| G153-694 | Mouse IgG2b | BD Pharmingenf | VEGF-121, -165, -189 | 27 | + |
Number of articles applying this antibody for immunohistochemical visualization on formalin-fixed and paraffin-embedded tissue specimens (numbers obtained from Google search; all individual hits were checked for reliability).
Application of antibody for Western blotting; + indicated by vendor.
Clones JH121, VG1, and 5C3.F8 were also available from different vendors.
Thermo/LabVision.
Santa Cruz Biotechnology.
BD Pharmingen (Erembodegem, Belgium).
VEGF, vascular endothelial growth factor.
Considering positive control tissues, there is clear evidence from cultured trophoblast cell lines (Taylor et al. 1997) and RNA in situ hybridization (Clark et al. 1996; Ghosh et al. 2000) that trophoblast and decidual cells in normal human placenta and other mammalian species are truly VEGF positive (Ahmed et al. 2000). In colon adenocarcinoma, VEGF is upregulated in the tumor cells, whereas adjacent normal epithelium is weakly positive or negative (Junquera et al. 1999; Lee et al. 2000; George et al. 2001). In an attempt to immunostain VEGF in blood vessels of diseased human tissue with presumed high angiogenic potential, using colon adenocarcinoma and placenta as positive control tissues, we found major differences in immunostaining patterns using the VEGF antibodies MAB293, A-20, and VG1. These observations provided the background for the present study, to verify the specificity and practical usefulness of a panel of nine commercially available anti-VEGF polyclonal and monoclonal antibodies tested on placenta, colorectal carcinoma, vascular malformations (VMs) consisting only of mature vessels, and arteriovenous VMs of skin and soft tissue complicated by an immature angioproliferative process. These anomalies are of interest because they can contain both preexisting mature vessels and immature newly formed vessels (Meijer-Jorna et al. 2007).
VEGF is seen with the amino acid splice variants VEGF-121, -165, -189, and -206 (Ferrara et al. 2003; Olsson et al. 2006). The selected antibodies (Table 1) are at least reactive with the most commonly present isoforms, VEGF-121 and/or VEGF-165 (Neufeld et al. 1996), or with a domain near the N terminus of the VEGF protein that is shared by all isoforms. Furthermore, the selection of the nine VEGF antibodies was based on the applicability on formalin-fixed and paraffin-embedded tissue material as provided by the vendors.
Materials and Methods
Tissue Samples
Included in this study were: 20th week of gestation placenta tissue (n=2); colon adenocarcinoma with adjacent normal intestinal mucosa (n=2); VM complicated with immature capillary proliferation (n=5); and VM composed of mature vessels without proliferation (n=5). Tissue samples were obtained from the archives of the Department of Pathology, Academic Medical Center (Amsterdam, The Netherlands).
Traditionally, VMs in skin and soft tissue are congenital vascular anomalies that are slowly progressive mass-forming lesions, composed of dysplastic but mature vessels. By contrast, immature capillary proliferation can occur in VMs, especially in those with a significant arteriovenous component (Meijer-Jorna et al. 2007). The presence of proliferating vessels amid the mature vessels of the VM was established by means of morphological parameters (solid sheets of closely packed vessels, inconspicuous-to-small lumina, plump endothelial lining) and increased Ki67-labeling index. All tissues were routinely fixed in buffered formalin and embedded in paraffin. For immunostaining, 4-μm sections were cut and mounted on silanized glass slides and dried at 37C. In addition, fresh placenta (n=2), VMs with immature capillary proliferations (n=2), and VMs composed of mature vessels only (n=2) tissue blocks were snap-frozen in liquid nitrogen and stored at −80C for Western blot studies.
IHC
Paraffin tissue sections were dewaxed in xylene and re-hydrated via graded alcohols. Endogenous peroxidase activity was blocked with methanol + 0.3% peroxide (20 min, room temperature). Tissue pretreatment was performed with either 0.25% pepsin (Sigma P5000; Sigma, St. Louis, MO) in 10 mM HCl (10 min, 37C), or heat-induced epitope retrieval using Tris-EDTA, pH 9.0, or citrate, pH 6.0, for 20 min at 98C in a pretreatment module (Thermo/LabVision; Fremont, CA). A two-step polymer detection system was applied, using appropriate anti-mouse or anti-rabbit horseradish peroxidase (HRP)-conjugated polymers (ImmunoLogic; Duiven, The Netherlands). Diaminobenzidine+ was used as chromogen (Dako; Glostrup, Denmark).
Nine different anti-VEGF polyclonal and monoclonal antibodies, as listed in Table 1, were employed. All antibodies were advertised by the vendors as being applicable for IHC on paraffin tissue sections. Each antibody was initially tested overnight at 4C at 0.5 and 2.5 μg/ml using four tissue pretreatments (none; pepsin; citrate, pH 6.0; Tris-EDTA, pH 9.0) (Van der Loos 2007). After selection of the best pretreatment on the basis of IHC staining criteria (intensity, localization, nonspecific background staining), the optimal antibody concentration was established by a dilution series. Tris-HCl buffered saline (TBS) was used throughout for washing.
Because VEGF is capable of inducing endothelial cell proliferation (Ferrara et al. 2003), VEGF/Ki67 double staining was tested on VMs with immature capillary proliferation and mature VMs without proliferation. The sequential alkaline phosphatase double immunostaining method was performed as previously described (Van der Loos and Teeling 2008), combining the proliferation marker Ki67 rabbit monoclonal clone SP6 antibody (Thermo/LabVision) in red with a rabbit VEGF antibody in blue.
Western Blot
According to information provided by the vendors, all VEGF antibodies were applicable for both IHC and immunoblot analysis (Table 1). The specificity of the VEGF antibodies was characterized by immunoblot analysis using tissue extracts of placenta, VM with immature capillary proliferation, and mature VM without proliferation. Standard hematoxylin and eosin stains were employed on each first and last section (before and after a series of thick sections cut for immunoblot analysis) to determine whether the material was appropriate to use; i.e., whether the material consisted of immature capillaries or mature large vessels. Fifty 20-μm tissue sections were cut, treated with lysis buffer [10 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 10.4 mg/ml Na-orthovanadate, 4 mM EDTA (pH 8.0), and 5 mM NaF], and separated by SDS-PAGE in 10% gels (190 μg/lane) and semi-dry–transferred to nitrocellulose membranes (Boer et al. 2008). Blots were blocked for nonspecific binding with TBS with 0.1% Tween-20 (TBST) plus 5% non-fat dry milk (1 hr) and incubated overnight with the primary antibodies diluted in TBST/5% non-fat dry milk (JH121, RB-9031, VG1, 5C3.F8, SP28, C-1, A-20 1:200; RB-222 1:1000, and G153-694 1:500). After several TBST washes, the membranes were incubated for 1 hr with appropriate swine anti-rabbit immunoglobulin/HRP or rabbit anti-mouse immunoglobulin/HRP (both Dako) 1:1000 diluted in TBST/5% non-fat dry milk. After TBST washing, HRP activity was visualized using an enhanced chemiluminescence kit (Roche/Pierce; Rockford, IL). After stripping the membranes, expression of β-actin (mouse monoclonal 1:50,000; Sigma, St. Louis, MO) was visualized as reference protein.
Results
IHC
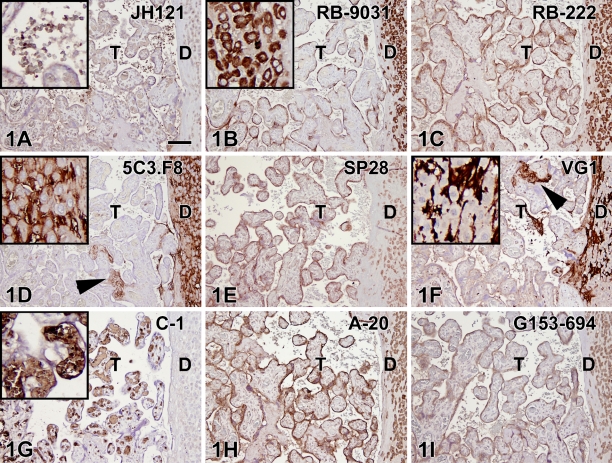
Optimal IHC staining results with all nine VEGF antibodies revealed different staining patterns with both placenta and colon tissue (containing adenocarcinoma and adjacent normal epithelium). The optimal tissue pretreatment, antibody concentration, and immunoreactivity in different tissue compartments are summarized in Table 2. In the placenta (Figure 1), five of nine VEGF antibodies demonstrated a clear positive trophoblast lining (RB-9031, RB-222, A-20, G153-694, and SP28), two stained very weakly (VG1, JH121), and two were fully negative (C-1, 5C3.F8). Decidual cells were found to be positive with six VEGF antibodies (JH121, RB-9031, RB-222, A-20, G153-694, and SP28), two showed an opposite staining pattern with positive stroma and negative decidual cells (5C3.F8, VG1), and one showed no staining at all (C-1). In addition, the 5C3.F8 and VG1 VEGF antibodies showed immunostaining of fibrin deposits (Figures 1D and 1F). In C-1 and JH121, erythrocytes were positive (Figures 1A and 1G).
Table 2.
Immunohistochemical staining results with anti-VEGF antibodies on placenta and colon tissue
| Placenta |
Colon |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decidua |
||||||||||||
| Antibody | Final concentration (μg/ml) | Pretreatment | Trophoblasta | Cellsa | Stroma | Erythrocytes | Fibrin | Carcinomaa | Normal | Stroma | Erythrocytes | Muscularis propria |
| JH121 | 0.5 | TE9.0 | ± | ± | − | ++ | − | + | + | ± | ++ | − |
| RB-9031 | 0.2 | citr6.0 | ++ | + | − | − | − | + | ± | − | − | − |
| VG1 | 0.5 | TE9.0 | ± | − | ++ | − | + | ± | ± | − | − | − |
| 5C3.F8 | 1.0 | citr6.0 | − | − | ++ | − | + | − | − | ± | − | ± |
| RB-222 | 0.5 | TE9.0 | ++ | ++ | − | − | − | + | ++ | + | − | ++ |
| SP28 | 0.5 | TE9.0 | + | + | − | − | − | + | ++ | ± | − | ++ |
| C-1 | 0.5 | pepsin | − | − | − | + | − | − | − | − | + | − |
| Rb A-20 | 0.2 | TE9.0 | ++ | ++ | − | − | − | + | ++ | ± | − | + |
| G153-694 | 5.0 | TE9.0 | + | + | − | − | − | + | + | − | − | ± |
Cell types considered to be VEGF-positive.
TE9.0, Tris-EDTA pH 9.0; citr6.0, citrate pH 6.0; immunoreactivity: − negative, ± weak, + moderate, ++ strong.
Figure 1.
IHC staining results in nine vascular endothelial growth factor (VEGF) antibodies on paraffin placenta tissue sections. Left, trophoblast (T); right, decidual plate (D). Positive staining of the trophoblast lining is observed in B,C,E,H,I, whereas staining in A,D,F,G was very weak or negative. Positive staining of the decidual cells is found in A–C,E,H,I (inset in B), whereas G is negative. (D,F) Decidua (insets) is stained oppositely, showing negative cells and positive surrounding stroma. D and F also show positive staining of fibrin deposits (arrowheads). In A and G, positive staining of erythrocytes (insets) is demonstrated. Bar = 0.1 mm.
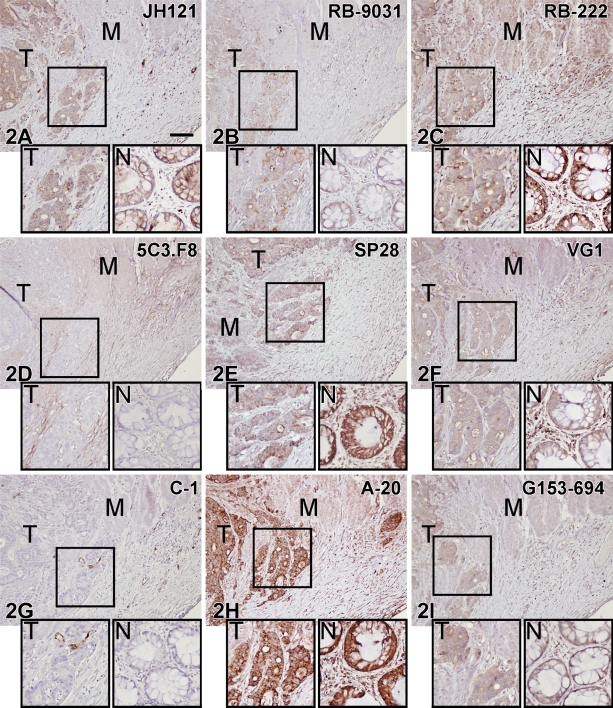
Variations in immunostaining were also seen in the colon tissue samples, as illustrated in Figure 2. VEGF antibodies RB-222, SP28, and A-20 showed strong staining of both tumor cells and adjacent normal epithelium. JH121, VG1, and G153-694 revealed moderate/weak staining of positive tumor cells and adjacent epithelium, and 5C3.F8 and C-1 were negative. RB-9031 antibody stained the colon adenocarcinoma moderately positive, whereas the normal adjacent epithelium was only weakly positive.
Figure 2.
IHC staining results in nine VEGF antibodies on paraffin colon adenocarcinoma tissue sections. Boxed area of the tumor part is magnified in the left inset (T); in the right inset (N) is a detail of the adjacent normal colon. M, muscularis propria. (D,G) Note negative staining of tumor (T) and normal epithelium (N). All other VEGF antibodies showed variable staining patterns. Bar = 0.1 mm.
Western Blot
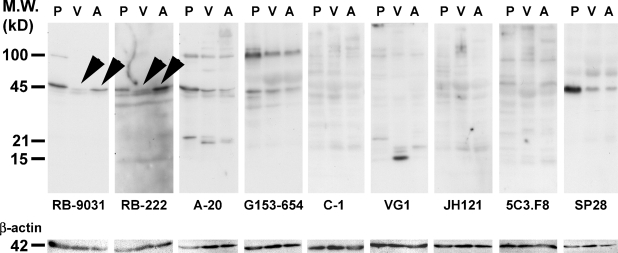
Immunoblot analysis of placenta (Lane 1, P), mature VM (Lane 2, V), and VM with immature capillary proliferation (Lane 3, A) with nine VEGF antibodies resulted in different staining patterns. Prominent bands were found at 15, 21, 45, and 100 kDa, distributed in different combinations and different staining intensities over the different lanes (Figure 3). VEGF antibodies RB-9031 and RB-222 showed a prominent band at 45 kDa with placenta and the VM with immature capillary proliferation, whereas a weak 45-kDa band was found with the mature VM (Figure 3, arrowheads). Membranes stained with VEGF antibody RB-222 showed more nonspecific background staining than RB-9031. All other VEGF antibodies did not reveal any difference at 45 kDa between VM with immature capillary proliferation and mature VM. After removing VEGF immunostaining from the nitrocellulose membrane, restaining with anti-β-actin antibody showed a single band at 42 kDa of similar staining intensity in all lanes.
Figure 3.
Western blot staining results of placenta (P), vascular malformation (VM) without vascular proliferation (V), and VM with immature capillary proliferation (A) in nine VEGF antibodies. Arrowheads indicate that RB-9031 demonstrated a distinct band at 45 kDa in Lane A, whereas only a weak band is found in Lane V. Nearly the same observation is seen in RB-222, but more nonspecific background staining is included here. After stripping, the membranes were restained with β-actin antibody.
IHC on VMs
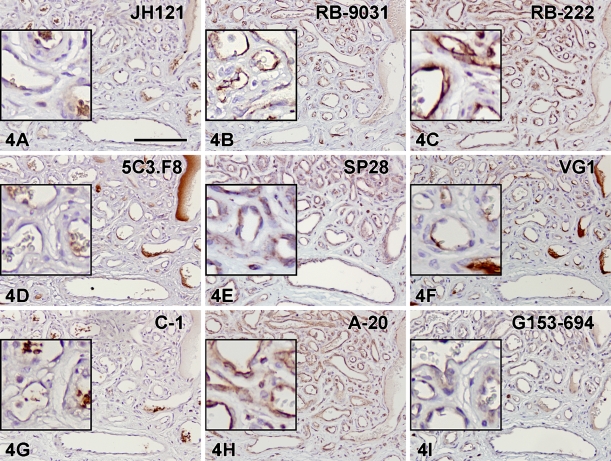
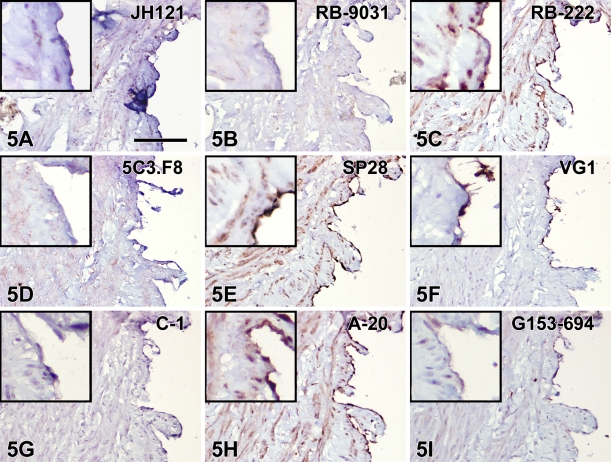
Immunostaining with VEGF antibodies RB-222 and RB-9031 on VMs revealed that only RB-9031 showed focal areas of distinctly positive endothelial cells of microvessels in VMs with immature capillary proliferation and no staining of endothelium in VMs without vascular proliferation (Figures 4B and 5B). RB-222 showed positive endothelium in both types of VM (Figures 4C and 5C). All other VEGF antibodies demonstrated variable staining patterns, as shown in Figures 4 and 5 and summarized in Table 3. In VMs with and without vascular proliferation, RB-9031 demonstrated some moderate staining of pericytes and smooth-muscle cells.
Figure 4.
IHC staining results in nine VEGF antibodies on paraffin tissue sections from VMs with capillary proliferation. Note the alternating staining pattern with RB-9031 (B) of areas with strongly VEGF-positive endothelium of microvessels, whereas larger vessels are weakly stained or negative. Positive VEGF-staining of endothelial cells (B,C,E,H,I), erythrocytes (A,G), and fibrin deposits (D,F). Bar = 0.1 mm.
Figure 5.
IHC staining results in nine VEGF antibodies on paraffin tissue sections from mature VMs without vascular proliferation. Positive VEGF-staining of endothelial cells is observed in C, E, H, and I. Note the absence of staining of endothelial cells with RB-9031 (B). Bar = 0.1 mm.
Table 3.
Immunohistochemical staining results with anti-VEGF antibodies on vessel malformations with immature capillary proliferation and mature vessel malformations without proliferation
| VM with immature capillary proliferation |
Mature VM, no vascular proliferation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibody proliferation | Final concentration (μg/ml) | Pretreatment | Endothelial cells | Pericytes | Erythrocytes | Fibrin | Endothelial cells | Pericytes | Erythrocytes | Fibrin |
| JH121 | 0.5 | TE9.0 | − | + | ++ | − | − | + | ++ | − |
| RB-9031 | 0.2 | citr6.0 | ++a | ± | − | − | −a | ± | − | − |
| VG1 | 0.5 | TE9.0 | − | ± | − | ++ | − | ± | − | + |
| 5C3.F8 | 1.0 | citr6.0 | − | ± | − | ++ | − | ± | − | − |
| RB-222 | 0.5 | TE9.0 | ++ | ++ | − | − | ++ | ++ | − | − |
| SP28 | 0.5 | TE9.0 | + | + | − | − | ++ | ++ | − | − |
| C-1 | 0.5 | pepsin | − | − | ++ | − | − | − | ++ | − |
| Rb A-20 | 0.2 | TE9.0 | ++ | ++ | − | − | ++ | ++ | − | − |
| G153-694 | 5.0 | TE9.0 | ± | ± | − | − | ± | ± | − | − |
Note that RB-9031 is the only antibody that demonstrates positive endothelium in the VM with immature capillary proliferation and absence of staining of endothelium in mature VM without vascular proliferation.
TE9.0, Tris-EDTA pH 9.0; citr6.0, citrate pH 6.0; immunoreactivity: − negative, ± weak, + moderate, ++ strong. VM, vessel malformation.
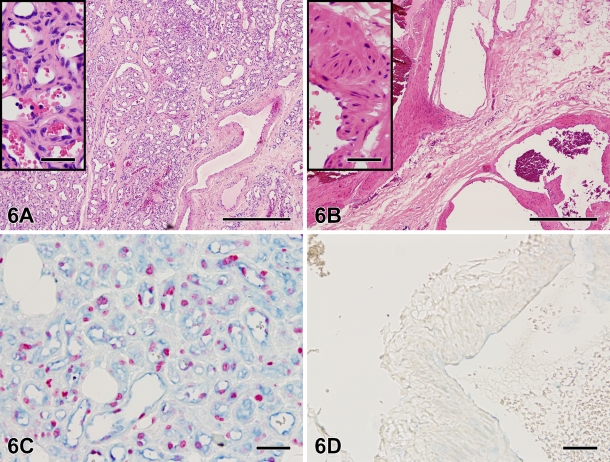
Double staining combining VEGF RB-9031 (blue) with Ki67 antibody (red) on a case with immature vascular proliferation (Figure 6A) demonstrated that areas of VEGF+ endothelial cells were associated with Ki67+ nuclei (Figure 6C). Both VEGF and Ki67 were absent in mature VMs (Figures 6B and 6D.
Figure 6.
Hematoxylin and eosin staining of a VM with immature capillary proliferation (A) and a VM without vascular proliferation (B). Note the difference in vessel diameter. The inset in A shows immature capillaries with small lumina and a plump endothelial lining. (C) Immunohistochemical double staining combining VEGF antibody RB-9031 in blue with proliferation marker Ki67 in red. A detail of the VM with immature capillary proliferation (same case as A) showing many proliferating (Ki67+) nuclei and extensive VEGF-positive endothelium. (D) Both markers are absent in a detail of the vessel wall of a large VM without vascular proliferation (same case as in B). Bar = 0.05 mm.
Discussion
In this study, we applied an elimination procedure to select the best VEGF antibody out of nine candidates for immunohistochemical staining of proliferating microvessels. At first, dilution and optimal tissue pretreatment procedure were established on placenta and colon adenocarcinoma-positive tissue controls. Of the nine candidates, five VEGF antibodies were regarded as unfit for VEGF immunostaining:
VEGF antibodies JH121 and C-1, because of a weak-to-negative immunostaining of trophoblast lining in the placenta, which is generally accepted as a reliable positive control (Ahmed et al. 2000). Moreover, in both placenta and colon adenocarcinoma tissue sections, these antibodies showed a positive staining of erythrocytes inside vessels and capillaries and, although we have no information about the specificity of this staining pattern, it obviously obscures the immunohistochemical observation of positive endothelium and pericytes.
VEGF antibodies 5C3.F8 and VG1, because of an opposite non-reliable staining of the placenta decidua (cells are negative, surrounding stroma is positive), a negative (5C3.F8) or very weak (VG1) trophoblast lining, and a strong positive nonspecific immunostaining of fibrin deposits (Figures 1D and 1F).
VEGF rabbit monoclonal antibody SP28 was eliminated despite a reliable staining of the trophoblast lining. However, in colon adenocarcinoma as well as adjacent normal colon epithelium, smooth-muscle cells and many more stromal cells were intensely immunostained, suggestive of a nonspecific staining. Higher dilutions did not increase the specificity.
After the first selection procedure, immunoblot analysis was performed. In tissue extracts from placenta and VMs with and without immature capillary proliferation, a prominent band near 45 kDa was observed with five of nine VEGF antibodies, concordant with VEGF immunoblotting results described by others (Boer et al. 2008). Some VEGF antibodies also revealed other bands at higher and lower molecular mass. Remarkable was that of five VEGF antibodies that were eliminated (as mentioned before) because of their aberrant immunostaining profile, four also did not reveal this typical 45-kDa band at all, or only very weakly among many other bands. Only VEGF SP28 antibody showed this typical band in the placenta lane very strongly, but much weaker in the lanes with both types of VM tissue extracts.
Based on immunoblot analysis, two more antibodies were regarded as unfit for VEGF immunostaining. VEGF antibodies A-20 and G153-694 did not demonstrate a difference between VMs with angiogenesis or mature VMs without angiogenesis. In contrast, VEGF antibodies RB-9031 and RB-222 showed a significant 45-kDa band in extracts from VMs with immature capillary proliferation and a weak one in mature VMs (Figure 3, arrowheads). The angiogenesis process in areas of VMs with immature capillary proliferation, as marked by a high Ki67-labeling index (Meijer-Jorna et al. 2007), is the result of a VEGF-driven process through signaling via its receptors at the surface of the endothelial cells (Ferrara et al. 2003; Brieger et al. 2004). Therefore, one may also assume that VM lesions with immature capillary proliferation contain relatively high amounts of VEGF, compared with mature VMs without proliferation. This difference is reflected in immunoblotting patterns of RB-9031 and RB-222, but not of A-20 and G153-694.
Testing of RB-9031 and RB-222 in IHC showed that only RB-9031 gave a reliable immunostaining pattern: positive staining of capillary endothelial cells in VMs with immature vascular proliferation (Figure 4B) and negative staining of endothelial cells in mature VMs without immature vascular proliferation (Figure 5B; Table 3). In contrast, RB-222 showed more nonspecific background staining in the immunoblot (Figure 3), as well as in IHC (Figures 1C and 2C; Table 2). But of more importance, RB-222 clearly stained endothelial cells in both types of VM lesions (Figures 4C and 5C; Table 3). Immunostaining of both types of VM with all other VEGF antibodies did not reveal other antibodies with staining characteristics similar to those of RB-9031 (Table 3). Earlier elimination of antibodies on the basis of poor immunostaining criteria of placenta and colon therefore seems justified. Finally, from this study, it was concluded that rabbit polyclonal RB-9031 antibody, in combination with a pH 6.0 citrate tissue pretreatment, most reliably immunostained VEGF.
One may speculate as to whether the observation of different immunostaining patterns using different VEGF antibodies is caused by a different reactivity with VEGF splice variants. Particularly at the level of endothelial cell staining, some authors have described differences between immunostaining patterns when using VEGF antibodies of different splice variant specificity (Boulton et al. 1998; Nishikawa et al. 1999; Chaudhry et al. 2001). In this study, we could not confirm these observations. As shown in Figures 1F, 1H,1I, 2F, 2H, 2I, 4F, 4H, 4I, 5F, 5H, and 5I and Table 2, VEGF antibodies VG1, A-20, C-1, and G153-694 clearly showed different immunostaining patterns, although these VEGF antibodies are all reactive with the splice variants VEGF-121, -145, and -189 (Table 1). The same holds true for different immunostaining patterns with VEGF antibodies RB-222 and 5C3.F8, which are both reactive with VEGF-165 (Figures 1C, 1D, 2C, 2D, 4C, 4D, 5C, and 5D). Moreover, RB-9031 and SP28 are both reactive with the VEGF N-term but also show different immunostaining results (Figures 2B, 2E, 5B, and 5E).
The immunohistochemical visualization of VEGF in VMs with proliferating microvessels includes the immunostaining of VEGF bound to its receptor at the membrane of proliferating endothelial cells (Ferrara et al. 2003; Olsson et al. 2006), and might be different from the visualization of VEGF in neoplasms, dealing with locally produced VEGF by tumor and/or stromal cells. It is unknown whether receptor-bound or free VEGF behaves differently in immunostaining. Nevertheless, the presently selected VEGF antibody, RB-9031, seems to perform well in immunostaining of both proliferating immature capillaries in VMs (Figure 4B) and tumor cells of colon adenocarcinoma (Figure 2B).
Combining VEGF RB-9031 (blue) with Ki67 (red) in double immunostaining demonstrated an excellent correlation between these markers. Both were clearly positive in VMs with immature capillary proliferation, whereas in more-mature VMs without proliferation, both VEGF and Ki67 were fully absent (Figures 6C and 6D). This observation clearly fits with the concept that VEGF acts as a powerful mitogen for endothelial cells (Ferrara et al. 2003; Brieger et al. 2004). A good correlation between VEGF and cell proliferation has also been described in neoplasms by Takahashi et al. (1995) and Brieger et al. (2004).
We have demonstrated here that VEGF RB-9031 rabbit polyclonal antibody is the only antibody that showed the ability to demonstrate clearly different staining of the endothelial cells in VMs with and without proliferation of blood vessels. All other VEGF antibodies in this study were not capable of showing this difference. On the basis of these findings, we concluded that RB-9031 antibody in combination with a pH 6.0 citrate tissue pretreatment appears to be the most appropriate antibody for immunostaining of VEGF in sections from formalin-fixed and paraffin-embedded tissue samples. Our study illustrates the importance of testing more primary antibodies for one antigen. It also emphasizes that interpretation of positive and negative immunostaining results from both positive and negative control tissues and target tissue is important, and that other criteria, such as Western blotting, may be needed to prove or disprove immunostaining reliability.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Ahmed A, Dunk C, Ahmad S, Khaliq A (2000) Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PlGF) and soluble Flt-1 by oxygen: a review. Placenta 21(suppl A):16–24 [DOI] [PubMed] [Google Scholar]
- Boer K, Troost D, Spliet WGM, Van Rijen PC, Gorter JA, Aronica E (2008) Cellular distribution of vascular endothelial growth factor A (VEGFA) and B (VEGFB) and VEGF receptors 1 and 2 in focal cortical dysplasia type IIB. Acta Neuropathol 115:683–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Foreman D, Williams G, McLeod D (1998) VEGF localization in diabetic retinopathy. Br J Ophthalmol 82:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieger J, Wierzbicka M, Sokolov M, Roth Y, Szyfter W, Mann WJ (2004) Vessel density, proliferation and immunolocalization of vascular endothelial growth factor in juvenile nasopharyngeal angiofibromas. Arch Otolaryngol Head Neck Surg 130:727–731 [DOI] [PubMed] [Google Scholar]
- Chaudhry IH, O'Donovan DG, Brenchley PEC, Reid H, Roberts ISD (2001) Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology 91:409–415 [DOI] [PubMed] [Google Scholar]
- Clark ED, Smith SK, Sharkey AM, Charnock-Jones DS (1996) Localization of VEGF and expression of its receptors Flt and KDR in human placenta throughout pregnancy. Hum Reprod 11:1090–1098 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H-P, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- George ML, Tutton MG, Janssen F, Amaout A, Abulafi AM, Eccles SA, Swift RI (2001) VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia 3:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Sharkey AM, Charnock-Jones DS, Dhawan L, Dhara S, Smith SK, Sengupta J (2000) Expression of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) in conceptus and endometrium during implantation in the rhesus monkey. Mol Hum Reprod 6:935–941 [DOI] [PubMed] [Google Scholar]
- Junquera F, Saperas E, De Torres I, Vidal MT, Malagelada J-R (1999) Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol 94:1070–1076 [DOI] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844 [DOI] [PubMed] [Google Scholar]
- Lee L-C, Chow N-H, Wang S-T, Huang S-M (2000) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36:748–753 [DOI] [PubMed] [Google Scholar]
- Meijer-Jorna LB, Van der Loos CM, De Boer OJ, Van der Horst CMAM, Van der Wal AC (2007) Microvascular proliferation in congenital vascular malformations of skin and soft tissues. J Clin Pathol 60:798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gitay-Goren H, Poltorak Z, Tessler S, Sharon R, Gengrinovitch S, et al. (1996) Similarities and differences between vascular endothelial growth factor (VEGF) splice variants. Cancer Metastasis Rev 15:153–158 [DOI] [PubMed] [Google Scholar]
- Nishikawa R, Matsutani M, Cheng S-Y, Su Huang HJ, Cavenee WC (1999) Reply to Christov and Gheradi (letter to the editor). Acta Neuropathol 97:431–432 [Google Scholar]
- Olsson A-K, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signaling: in control of vascular function. Nat Rev Mol Cell Biol 7:359–371 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM (1995) Expression of vascular endothelial growth factor and its receptor KDR, correlates with vascularity, metastasis and proliferation of human colon cancer. Cancer Res 55:3964–3968 [PubMed] [Google Scholar]
- Taylor CM, Stevens H, Anthony FW, Wheeler T (1997) Influence of hypoxia on vascular endothelial growth factor and chorionic gonadotrophin production in the trophoblast-derived cell lines: JEG, Jar and BeWo. Placenta 18:451–458 [DOI] [PubMed] [Google Scholar]
- Turley H, Scott PAE, Watts VM, Bicknell R, Harris A, Gatter KC (1998) Expression of VEGF in routinely fixed material using a new monoclonal antibody VG1. J Pathol 186:313–318 [DOI] [PubMed] [Google Scholar]
- Van der Loos CM (2007) A focus on fixation. Biotech Histochem 82:141–154 [DOI] [PubMed] [Google Scholar]
- Van der Loos CM, Teeling P (2008) A generally applicable sequential alkaline phosphatase immunohistochemical double staining. J Histotechnol 31:119–127 [Google Scholar]
- Zhang L, Scott PAE, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, et al. (1998) Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol 185:402–408 [DOI] [PubMed] [Google Scholar]