Abstract
We have optimized an immunohistochemical double-staining method combining immunohistochemical lymphocyte lineage marker detection and apoptosis detection with terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling. The method was used to trace Fas-mediated apoptosis in human reactive lymph nodes according to cell lineage and anatomical location. In addition to Fas, we also studied the expression of Fas ligand (FasL), CD3, CD20, CD19, CD23, and CD68 of apoptotic cells. The presence of simultaneous Fas and FasL positivity indicated involvement of activation-induced death in the induction of paracortical apoptosis. FasL expression in the high endothelial venules might be an inductor of apoptosis of Fas-positive lymphoid cells. In addition to B-lymphocyte apoptosis in the germinal centers, there was often a high apoptosis rate of CD23-expressing follicular dendritic cells. In summary, our double-staining method provides valuable new information about the occurrence and mechanisms of apoptosis of different immune cell types in the lymph node compartments. Among other things, we present support for the importance of Fas/FasL–mediated apoptosis in lymph node homeostasis. (J Histochem Cytochem 58:131–140, 2010)
Keywords: Fas, Fas ligand, lymph node, immunohistochemistry, double stain, germinal center, high endothelial venules, apoptosis, activation-induced cell death, lymphocytes
Lymph nodes are anatomically complicated structures with several functionally different compartments, such as the paracortex, cortex, medulla, and the sinusoidal system. They are also dynamic structures, reacting to various stimuli by changing their size and cellular composition by the influx of lymphocytes and other cells, and tightly regulated proliferation and apoptosis of these cells (van der Valk and Meijer 1987).
Fas/Fas ligand (Fas/FasL)–mediated apoptosis of the lymphatic cells is important in several aspects of lymph node physiology, including regulation of both T- and B-lymphocyte response (Lynch et al. 1995). Fas is a member of the tumor necrosis factor (TNF) receptor superfamily, and its ligand, FasL, is TNF-related. Fas is widely expressed in the lymphatic system, where it is found on activated B- and T-cells, natural killer (NK) cells, and myeloid cells (Itoh et al. 1991; Iwai et al. 1994). In contrast, in the peripheral blood, only a minority of resting lymphocytes express Fas (Yoshino et al. 1994). Among cells of the immune system, FasL is expressed by activated T-cells, NK cells (Nagata and Golstein 1995), activated macrophages (Badley et al. 1996), and neutrophils (Suda et al. 1993; Liles et al. 1996). The interaction of Fas on the surface of a target cell and FasL on the surface of an effector cell triggers target cell apoptosis through a complex cascade of caspases (Pinkoski et al. 2000; Wajant 2002; Lee and Ferguson 2003).
Lymphocyte homing to peripheral lymph nodes is a highly regulated process that occurs almost exclusively in high endothelial venules (HEVs) in the nodal paracortex, which express characteristic adhesion molecules recognized by l-selectin in the lymphocytes (von Andrian and M'Rini 1998). Only naïve T-lymphocytes are able to migrate through HEVs (Mackay 1991). Recently, we showed that lymph node HEVs express FasL and suggested that this provides a means to eliminate activated Fas-expressing lymphocytes from entering the lymph node parenchyma by inducing their apoptosis (Kokkonen et al. 2004). Another route to the lymph node is through afferent lymphatics, from where ∼10% of all lymphocytes reach the lymph node. These cells have migrated from the blood across non-lymphoid tissue and collect in lymph veins (Young 1999). Interestingly, the parenchymal wall of the marginal sinus shows FasL expression similar to that of the HEV endothelium, suggesting a similar protective role (Kokkonen et al. 2004).
In the lymph node parenchyma, Fas/FasL–induced apoptosis of T- and B-lymphocytes is an important regulatory mechanism. Activation-induced cell death (AICD) is a process through which the cells are predetermined to undergo apoptosis after being activated (Lynch et al. 1995). In this process, T-lymphocytes start to express both Fas and FasL. Such Fas-mediated apoptosis plays a crucial role in the downregulation of immunological reactions, which is actually started when the lymphocytes are activated. T-cells can regulate the extent of their populations by killing each other with the Fas/FasL–mediated system or even committing self-destruction when coexpressing both of these (Lynch et al. 1995). In the germinal centers, low-affinity or self-reacting B-cells are eliminated via Fas/FasL interaction, and high-affinity cells receive survival stimuli via CD40/CD40L, which blocks Fas-mediated apoptosis (van Eijk et al. 2001). The importance of FasL- and Fas-mediated homeostasis of lymphocytes is demonstrated in autoimmune lymphoproliferative syndrome, in which lymphocytes accumulate in lymph nodes owing to defective Fas-mediated apoptosis (Nagata and Suda 1995; Le Deist et al. 1996; Sneller et al. 1997; Lim et al. 1998).
Apoptosis mediated by Fas/FasL interaction is an important mechanism in the maintenance of lymph node homeostasis (Strasser et al. 2009). However, the commonly used methods for simultaneous characterization of apoptosis and cell markers in lymphatic tissues, such as flow cytometry, do not provide information about anatomical organization and therefore do not provide a complete picture of reactions going on in vivo. In the present study, we sought to survey Fas- and FasL-mediated apoptosis in different cell lineages and anatomical compartments of human reactive lymph nodes. We were also interested in the role of paracortical FasL-expressing HEVs in the homeostatic apoptosis of lymph nodes, on which there is no information at present. To determine how Fas expression and Fas-L expression are associated with apoptosis in lymph nodes, we optimized an immunohistochemical double-staining method to determine whether the apoptotic cells are activated (Fas+ or FasL+) and to categorize their cell lineage. The results indicate that each anatomical compartment of the lymph node shows a characteristic pattern of cell lineage–specific apoptosis. Furthermore, the observation that in the vicinity of HEVs in the paracortical area, 70% of apoptotic cells express Fas and the same proportion express FasL supports our hypothesis that HEV FasL plays an important role in the elimination of activated (FasL-positive) lymphocytes entering the lymph node parenchyma.
Materials and Methods
Lymph node samples from 13 patients (ages 1 to 73 years, mean age 35.6 years; 9 males) representing different types of reactive alterations were selected from the files of the Department of Pathology, University of Oulu. Most of the samples were used in our previous study (Kokkonen et al. 2004). Lymph nodes had all been enlarged, and had been removed for diagnostic purposes. Based on histology and a review of the clinical history, there were no cases with specific infection, immunological deficiency, or neoplastic disease. The regions of their anatomical distribution varied, including neck, axilla, abdominal cavity, and inguinal areas. Three of the patients were under 18 years old, seven were 19–55 years old, and three were over 55 years old. The material included five paracortical hyperplasias, one sinus histiocytosis, four follicular hyperplasias, and three cases with a mixed reaction.
Single Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections were cut into 4-μm sections and stained using the Dako Envision DAB+ detection system kit (Dako A/S; Glostrup, Denmark) with 3,3′-diaminobenzidine (DAB) as a chromogen. Sections were pretreated in a microwave oven (150 W) for 15 min in Tris-EDTA (pH 9.0). Table 1 summarizes the primary antibodies used. After the primary antibody was applied, staining was performed according to the manufacturer's instructions. Counterstaining was performed using Mayer's hematoxylin (Reagena Oy; Helsinki, Finland) blued in 2% NH3-water. For negative control staining, the primary antibody was omitted. As internal positive controls, lymphocytes in B- and T-lymphocyte regions of lymph nodes, germinal centers, and HEV vessels in the lymph nodes (Kokkonen et al. 2004) served as positive controls for CD20, CD3, Fas, and FasL staining, respectively.
Table 1.
Summary of antibodies and pretreatment conditions used in the immunohistochemical stainings
| Antibody (clone or code) | Dilution (diluent) | Incubation time (temperature) | Manufacturer |
|---|---|---|---|
| Fas ligand (mouse monoclonal G247-7) | 1:1000 (PBS) | Overnight (4C) | Pharmingen; San Diego, CA |
| Fas (rabbit polyclonal C-20) | 1:500 (Envision Ab diluent) | 60 min (room temperature) | Santa Cruz Biotechnology; Santa Cruz, CA |
| CD3 (monoclonal F7.2.38) | 1:100 (Envision Ab diluent) | 60 min (room temperature) | Dako |
| CD20 (monoclonal L26) | 1:1000 (Envision Ab diluent) | 60 min (room temperature) | Dako |
| CD19 (mouse monoclonal LE-CD19) | 1:200 (Envision Ab diluent) | 60 min (room temperature) | Dako |
| CD23 (mouse monoclonal 1B12) | 1:50 (Envision Ab diluent) | 60 min (room temperature) | Novocastra; Newcastle upon Tyne, UK |
| CD68 (mouse monoclonal KP-1) | 1:10,000 (Envision Ab diluent) | 60 min (room temperature) | Dako |
| s-100α (rabbit polyclonal) | 1:2000 (Envision Ab diluent) | 60 min (room temperature) | Dako |
Terminal Deoxyribonucleotidyl Transferase–mediated dUTP Nick End Labeling (TUNEL)/Lymphocyte Marker Double Staining
Problems are often encountered when applying double staining, including crossreactivity, common false-positive background staining, and many methodological problems in the selection of pretreatment and chromogens. The manufacturer of the TUNEL kit (ApopTag Peroxidase In Situ, catalog number S7100; Chemicon, Temecula, CA) recommends using proteinase K or microwave cooking in citrate buffer (pH 6.0) for 15 min as pretreatment. We compared these two pretreatment methods to microwave cooking in Tris-EDTA (pH 9.0) for 15 min. Our results showed that proteinase K and Tris-EDTA provide similar positive labeling, but with citrate, the result is almost negative. For chromogens, we selected DAB with and without cobalt chloride (see below), producing either a black or a brown color, respectively. DAB-based compounds were used as chromogens because they are both permanent, in contrast to easily soluble alkaline phosphatase (AP)-based chromogens. Our self-made DAB-black did not work with the Envision detection system, decreasing the chance of false positivity. While testing the TUNEL kit with DAB-black, we noticed that DAB-black is actually dark-bluish black. Therefore, we changed the background staining from the commonly used hematoxylin to Nuclear Fast Red, which gives a better color contrast to both brown and black.
For double staining, the 4-μm sections in slides were first incubated at 70C for 10 min and then deparafinized and rehydrated. Pretreatment was performed with Tris-EDTA as in single staining. After cooling at room temperature, slides were moved to 3% H2O2 (diluted with PBS) for 5 min. TUNEL staining continued according to the manufacturer's instructions until addition of chromogen. The chromogen used was DAB (catalog number D8001; Sigma-Aldrich Finland Oy, Helsinki, Finland), 3 g/l in 0.5 M Tris buffer (pH 7.6) with 2.5% CoCl2 added (Hsu and Soban 1982) after filtering and incubated for 10 min at room temperature. Then 0.8 μl/ml 30% H2O2 was added to the DAB-black solution and incubated for a further 10 min. This gives TUNEL-positive cells a black color. Sections were then stained as with Envision in single staining. For counterstaining, Nuclear Fast Red (0.05% Karcherot, E. Merck Ag; Darmstadt, Germany), diluted in 5% aluminium sulfate-18 hydrate, was used (incubated for 2 min) to give a good contrast to both the black and brown color (Figures 1 and 2).
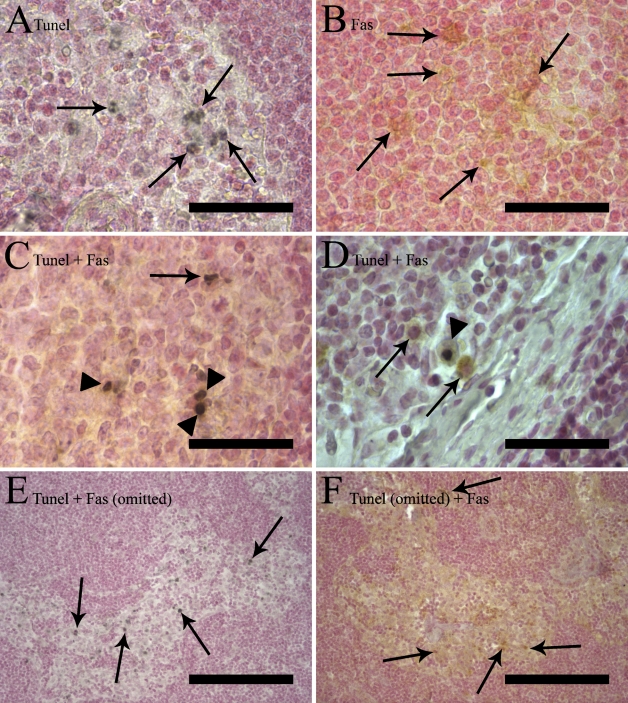
Figure 1.
Five different combinations of terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) and immunostaining of the same sample. Counterstaining was performed with Nuclear Fast Red. (A,B) Single staining. (A) A single TUNEL staining labeling apoptotic nuclei in a germinal center black diaminobenzidine (DAB-black) chromogen. Arrows indicate single TUNEL stained cells. (B) Fas single staining with brown label (DAB chromogen) in the cytoplasm (arrows), leaving the nucleus unstained. (C,D) Double staining with TUNEL and Fas showing many double-stained cells with a black nucleus and brown cytoplasm (arrowheads). There are also single-positive cells (arrows) in both images, TUNEL-positive in C and Fas-positive in D (arrows). (E,F) Control staining in which either the primary antibody (anti-Fas) or terminal deoxynuleotidyl transferase (TdT) enzyme has been omitted. Arrows indicate single-positive cells. (E) After omission of the Fas primary antibody in a double staining (sinus), there is no brown coloration of the immunoreaction at all in the section, whereas the TUNEL staining still shows a staining pattern similar to that seen in A, C, and D. (F) After omission of the TdT enzyme in a double staining (sinus), only Fas-positive brown cells are visible. Bars: A–D = 50 μm; E–F = 200 μm.
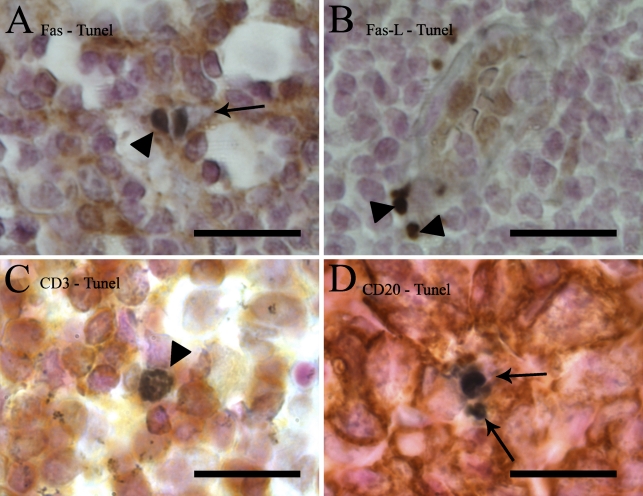
Figure 2.
Details of TUNEL and lymphocyte marker double staining of lymph nodes. TUNEL is stained with DAB-black (black) and Fas (A), FasL (B), CD3 (C), or CD20 (D) with DAB (brown). (A) A view of the paracortical region showing the difference between a TUNEL+/Fas+ cell (arrowhead) with brown cytoplasm around the black nucleus, and a TUNEL+/Fas− cell (arrow). In the latter, the cytoplasm is clear. (B) In the paracortical area, FasL was seen in high endothelial venules (HEVs) and in 70% of TUNEL-positive cells (arrowheads). (C) Apoptotic T-lymphocytes (TUNEL+/CD3+ double-positive cells) were only seen in the paracortex (arrowhead). (D) Within the germinal centers, TUNEL+/CD20− cells were frequently seen (arrows). Bar = 20 μm.
For TUNEL-Fas, TUNEL-CD3, TUNEL-CD20, TUNEL-CD19, TUNEL-CD23, and TUNEL-CD68 double staining, the sections were stained as described above. For the TUNEL-FasL combination, FasL was first stained with Envision, then staining was continued with the TUNEL kit. FasL staining requires overnight incubation with the primary antibodies, and it showed no positivity at all when it was stained after TUNEL.
For controlling staining specificity, double stainings were compared with five separate controls: (1) single staining for TUNEL; (2) single staining with the primary antibody; (3) omission of terminal deoxynuleotidyl transferase (Tdt) enzyme (TUNEL); (4) omission of primary antibody, and (5) omission of both Tdt enzyme and the primary antibody. Figure 1 shows the results of control staining.
Evaluation of Staining
For evaluation of TUNEL and the lymphocyte marker double-positive cells in different regions and reaction types of lymph nodes, TUNEL-positive cells were assessed using an oil-immersion objective (×1000 magnification). A cell was classified as a double-positive (TUNEL and CD marker) if it had a black nucleus and brown rim of cytoplasm or cell membrane. In some cells without visible cytoplasm, some brown color was seen within the black nucleus, which was often fragmented. Such a brownish staining pattern was seen in single immunostaining in some morphologically apoptotic cells, but it was absent from single TUNEL-stained sections (Figure 2C). This staining was considered positive. In cases in which a TUNEL-positive cell was surrounded by a group of cells positive to some antibody (e.g., CD20-positive cells in germinal centers), the TUNEL cell was classified as single-positive if the black nucleus was surrounded by a clear halo separating it from the brown-positive cells. Cells with no halo surrounding the black nucleus and brown staining in contact with the nucleus were classified as double-positive. However, the S-100α antibody used in our experiment stained the nucleus of cells more intensively than the cytoplasm and gave the nucleus a very dark brown color. Reliable evaluation with black TUNEL was impossible, and it was discarded from further evaluation. The proportion of TUNEL double-positive cells was assessed for each double stain combination and each anatomical region by analyzing a minimum of 10 TUNEL+ cells. For each lymph node specimen, several anatomical regions of each type were analyzed.
To semiquantitatively estimate the numerical density of apoptotic cells in each anatomical region of lymph nodes, high magnification (×1000) and the following scores were used. Score 1 was only used if one or less TUNEL+ cell per visual field was detected. Score 2 was used if there were 2–3 TUNEL+ cells per visual field, and score 3 was used if the TUNEL+ cell count was more than three. To evaluate reproducibility of apoptosis counts with double stainings, we used the Friedman test for related samples. Absence of any significant differences between different double stainings indicates that various double stainings did not affect the TUNEL positivity rate (Figure 3).
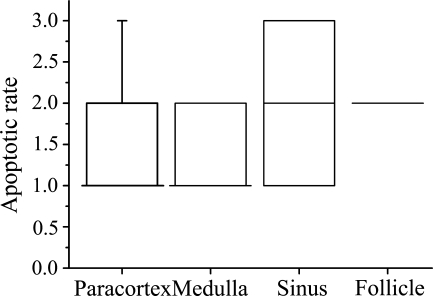
Figure 3.
Box plot chart showing the density score of TUNEL-positive cells in different anatomical compartments of human reactive lymph nodes. Numerical density was based on different double stainings combining TUNEL with either Fas, FasL, or cell lineage markers CD3 or CD20. The number of TUNEL-positive cells in a high-power field (100× objective) including both single and double positives was calculated in each area and converted to a density score (1–3). Box plots include median, quartiles, 5–95% interval. See Materials and Methods for details.
Statistics and Software Used
Statistical analyses were performed using non-parametric tests due to skewness of distributions. Statistical evaluations were made using SPSS v. 12.0 (SPSS, Inc.; Chicago, IL). Box plot charts were made using Origin 7.0 (OriginLab Corp.; Northampton, MA). Microphotographs were taken with Leica DFC320 (Leica Microsystems Ltd.; Wetzlar, Germany), and composed with Adobe Photoshop 8.0 (Adobe Systems, Inc.; San Jose, CA).
Results
First, we confirmed with extensive control staining that the double-staining protocols for apoptosis, Fas, FasL, and lymphocyte lineage markers did not compromise specificity or sensitivity of detection of either DNA degradation by TUNEL, or the protein markers (Figure 1). Omission of TdT in double staining resulted in a staining pattern similar to that of the single immunostaining (Figure 1F). Similarly, omission of the primary antibody did not affect TUNEL staining in terms of intensity or the number of positive cells (Figure 1E). Because TUNEL stained only the nucleus of the cells, leaving both cytoplasm and the cell membrane unlabeled, and all selected lymphocyte lineage markers, Fas, and FasL were expressed in the cytoplasm or the cell membrane (Figures 1B and 1F), the recognition of single- and double-positive cells was straightforward (Figure 2). Finally, comparison of apoptotic rates detected with different double stainings indicated that the rates did not differ significantly, further confirming that double staining does not affect TUNEL positivity.
As reported previously, FasL was expressed in the endothelial cells of HEVs and in the parenchymal wall of marginal sinus tissue (not shown) (Kokkonen et al. 2004). FasL was expressed in occasional lymphocytes in the paracortical area and germinal centers. In the germinal centers, FasL expression was not present in all cases, but when present, was more intensive at the light zone. Morphologically, FasL-positive cells looked like macrophages, but some had the features of reticular dendritic cells, inasmuch as the positive reaction was seen at the membrane of a “hollow”-like structure.
Using the double-staining method, it was possible to evaluate the density of apoptotic cells in the different lymph node compartments and to determine the cell lineage and Fas or FasL expression of the apoptotic cells. Figure 3 summarizes the overall apoptotic rates in the different lymph node compartments. Figure 4 shows which proportions of apoptotic cells in each anatomical compartment express cell lineage markers Fas or FasL, based on double staining of 13 reactive human lymph node samples.
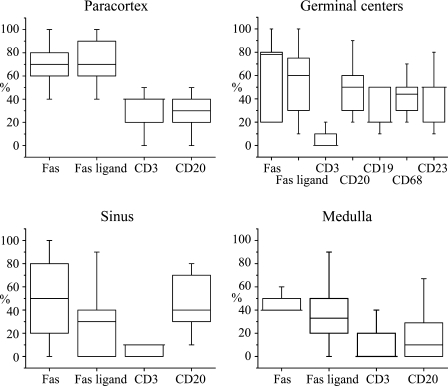
Figure 4.
Box plot charts showing distribution of the proportions of TUNEL-positive cells expressing different cell lineage and activation markers of all TUNEL-positive cells in the different anatomical areas of 13 lymph node samples. For counting the proportions, double-stained sections were used, and a representative number of TUNEL-positive cells in different anatomical regions were classified as marker positive or negative. The mean proportion of double-positive cells from TUNEL+ cells for each region and sample was calculated. The plots show median (horizontal line), quartiles, 5–95% interval. If the median is the same as either of the quartiles, it is shown as a longer line.
In the paracortical area, TUNEL-positive cells were relatively rarely seen (Figure 3). The majority (∼70%) of these apoptotic cells expressed Fas and/or FasL (Figure 4). This high figure suggest that a significant proportion of apoptotic cells express both Fas and FasL. About 40% and 30% of TUNEL-positive cells were positive for CD3 and CD20, respectively, suggesting that the majority (in total ∼70%) of paracortical apoptotic cells are lymphocytes of either B- or T-cell lineage.
Germinal centers (follicles) were the second most abundant in TUNEL+ cells after the sinuses (Figure 3). About half of TUNEL+ cells showed Fas expression, but the double positivity showed a wide variation (Figure 4). This is probably because the reaction types and the state of activation of the studied lymph nodes were variable in different cases. FasL was also found in ∼50% of apoptotic cells in the germinal centers. Apoptotic T-lymphocytes were practically absent in the germinal centers (Figure 3). About half of the TUNEL-positive cells showed CD20 expression, indicating a B-lymphocyte lineage (Figure 3). Because about half of the TUNEL-positive germinal center cells were negative for both CD3 and CD20, leaving the lineage of the negative population unresolved, we further investigated germinal centers by combining TUNEL with CD19, CD23, and CD68. The proportion of TUNEL+/CD19+ cells was lower than that of TUNEL+/CD20+, speaking against the hypothesis that those B-cells that are apoptotic would not be mature enough to express CD20. TUNEL-CD68 staining indicated that the other half of the apoptotic cells are macrophages, and a similar proportion of TUNEL+/CD23+ cells indicated that a major portion of the apoptotic cells of the macrophage lineage are follicular dendritic cells (Figure 4).
The sinuses were the compartment with the highest density of TUNEL+ cells (Figure 3). Here, Fas expression among apoptotic cells showed the most variation between cases (Figure 4); almost all TUNEL+ cells were positive for Fas in some samples and none in others. FasL and CD3 were found only in a few TUNEL+ cells, and CD20 was found in less than half of the cells.
The medullary zone showed only a few TUNEL+ cells (Figure 3), of which only a minority were T- or B-cells (Figure 4). Fas and FasL were also found in the medulla, involved in apoptosis, but less than in the other regions of the lymph nodes (Figure 4).
To gain insight into the functional relationships of apoptotic activities in the different anatomical compartments of lymph nodes, correlation analyses were performed. Spearman's rank correlation test was used due to the skewness of the distributions. There were strong positive correlations between paracortical apoptotic CD3+ and CD20+ cell densities (0.854, p<0.001), and they were both correlated with medullary apoptotic CD3+ and CD20+ cell densities (CD3 0.804, p<0.05; CD20 0.883, p<0.001). In germinal centers, apoptotic Fas+ cell densities were positively correlated with paracortical apoptotic Fas+ cells (0.748, p<0.05) and negatively correlated with apoptotic CD20+ densities in the germinal centers (−0.770, p<0.001). In contrast to CD20+, CD19+ does not have these same correlations; instead, germinal center apoptotic CD19+ cells are correlated with apoptotic CD3+ cells in germinal centers (0.776, p=0.023) and to apoptotic Fas+ cells within sinuses (0.811, p=0.027).
Discussion
Until now, simultaneous detection of apoptosis and cell markers in lymphatic tissues has been possible by flow cytometry (Bang et al. 2003), immunofluorescently (Mita et al. 2005), or immunohistochemically using AP-based chromogens (Guan et al. 2000). However, all of these have their disadvantages. Flow cytometry does not allow consideration of functionally different anatomical compartments of the lymph node; immunofluoresence and AP-based chromogens tend to disolve over time. Here, we have optimized an immunohistochemical double-staining method combining the detection of apoptotic cells by the TUNEL method and immunohistochemical detection of the lymphocyte lineage or Fas/FasL expression in human lymph nodes, using DAB as chromogen for both stainings. We used this method for analyzing anatomical location and mechanisms of apoptosis of the cell societies of lymph nodes. Fas-mediated apoptosis seems to be dominant in both T- and B-lymphocyte regions of the lymph nodes, but in the sinuses and medulla, other mechanisms of apoptosis have important roles as well. The method provides interesting new information about lymph node physiology.
Optimization of the double-staining method involved selection of the pretreatment and chromogens, and modification of the sequence of the components of the double stain. For detection of apoptosis, the TUNEL method was modified by boiling in Tris-EDTA (pH 9.0) for pretreatment instead of proteinase K or boiling in citrate buffer. This proved to provide results comparable to proteinase K pretreatment. The rationale for using Tris-EDTA was that it is more suitable for the immunohistochemistry step of the double staining than is either proteinase K or citrate buffer treatments. Further, Tris-EDTA is superior to citrate buffer for antigen retrieval for most antibodies (Yamashita 2007). An additional benefit of using the Tris-EDTA pretreatment was a clearly more-intensive and consistent labeling of apoptotic cells than with the citrate buffer pretreatment. To provide contrast with the immunohistochemistry step, DAB chromogen was used with cobalt chloride solution for labeling apoptotic cells (Hsu and Soban 1982). The DAB-black chromogen results in an intense black reaction, easy to differentiate from the brown immunolabel of protein markers (Figures 1 and 2).
As expected, apoptosis of T-lymphocytes was largely restricted to the paracortical area, where almost half of the apoptotic cells were T-lymphocytes (Figure 4). In this region, the number of apoptotic B-lymphocytes was nearly as high as that of T-lymphocytes, suggesting that considering the low overall numbers of B-lymphocytes in the paracortex (van der Valk and Meijer 1987), the proportion of apoptotic cells among the paracortical B-lymphocytes is much higher than that of T-lymphocytes. Such abundant apoptosis of B-lymphocytes outside the germinal centers has not been described in humans to date. In murine models, extrafollicular B-lymphocyte response has been described, and involves apoptotic elimination of autoreactive cells in the T-cell regions (Shlomchik 2008). We speculate that apoptotic paracortical B-lymphocytes represent an analogous extrafollicular selection process of B-lymphocytes.
What are the mechanisms for apoptosis in the lymph node paracortex? A majority of apoptotic cells in the paracortex were Fas positive (∼70%; Figure 4), suggesting that apoptosis of these cells was induced by Fas/FasL interaction. However, apoptosis even in the Fas-expressing cells may be induced by FasL-independent mechanisms, such as lymphocyte cytotoxicity or intrinsic mechanisms (Russell and Ley 2002; Fan and Zhang 2005). About 70% of apoptotic cells in the paracortex showed expression of Fas and FasL (Figure 4), which indicates that ∼40% of apoptotic cells are positive for both Fas and FasL. Simultaneous expression of Fas and FasL in apoptotic cells supports AICD-like induction of apoptosis (Lynch et al. 1995; Zhang et al. 2004).
A second potential mechanism of apoptosis induction in the lymph node paracortex is based on our previous finding that paracortical HEVs express FasL (Kokkonen et al. 2004). These vessels are the main route through which lymphocytes enter lymph node parenchyma. We suggest that if the entering lymphocytes are activated and therefore express Fas (Lynch et al. 1995; Zhang et al. 2004), their apoptosis is induced by FasL in the HEV endothelium. The observed high Fas positivity rate of apoptotic lymphocytes in the paracortical region is in agreement with this idea. In addition, there is experimental evidence that anatomically non-specified endothelial FasL is an important inductor of lymphocyte apoptosis (Walsh and Sata 1999; Ferguson and Griffith 2006). Furthermore, the presence of much more intensive FasL expression in the HEV endothelium than in other endothelial cells (Kokkonen et al. 2004) supports the concept of a specific functional significance of the FasL molecules in lymph node HEVs. However, because lymph node paracortex contains other possible sources of FasL signaling in addition to HEV endothelium, this concept requires experimental evidence.
There are now five different pieces of evidence favoring the idea that FasL expression in lymph node HEVs induces apoptosis in activated lymphocytes entering the lymph node parenchyma: (1) Activated lymphocytes are not able to migrate into lymph node parenchyma (Mackay 1991; Tanaka et al. 2004); (2) In inactivating mutations of the Fas gene, both in mouse and in humans, lymph nodes are enlarged with paracortical expansion (Le Deist et al. 1996; Sneller et al. 1997); (3) HEVs express Fas ligand in the lymph node paracortex (Kokkonen et al. 2004); (4) Most paracortical (near-HEV) apoptotic cells express Fas, as shown here; (5) Similarly, in immune-privileged sites, FasL in epithelium provides protection against the inflammatory process (Abbas 1996; Ferguson and Griffith 2006).
About 50% of the germinal center apoptotic cells showed expression of CD20, a marker of mature B-lymphocytes, and ∼20% expressed CD19, a marker of immature B-lymphocytes, indicating that a majority of apoptotic cells in germinal centers are of B-lymphocyte lineage. Our finding that 80% of apoptotic cells in the germinal centers express Fas is consistent with the role of Fas/FasL-mediated apoptosis in the negative selection of low-affinity or self-reacting B-lymphocytes via Fas/FasL interaction (Hur et al. 2000; van Eijk et al. 2001). The observed negative correlation between the proportion of Fas-expressing apoptotic cells and the proportion of CD20-expressing apoptotic cells (−0.770, p<0.001) indicates, however, that Fas expression is not the main regulator of B-lymphocyte apoptosis, and is consistent with the important role of survival stimuli in the selection process. It has been shown that high-affinity B-lymphocytes receive survival stimuli that block Fas-mediated apoptosis (van Eijk et al. 2001).
About a third of the apoptotic cells in the germinal centers were composed of CD68-positive macrophages and CD23-positive follicular dendritic cells (Figure 4). This is the first evidence for such abundant apoptotic activity in the follicular dendritic reticulum cells. Because dendritic reticulum cells are important regulators of the germinal center reaction (Or-Guil et al. 2007; Mueller and Ahmed 2008; Batista and Harwood 2009) and also play a role in the pathogenesis of B-cell lymphomas (Carbone et al. 2009), dissection of the mechanisms and disorders of their apoptosis may provide useful information for understanding B-cell immunity and B-cell neoplasia. Disturbed apoptosis may also be important in the pathogenesis of rare sarcomas of follicular dendritic cells.
In the lymph node sinuses and medulla, TUNEL positivity rates showed extensive variation among patients, probably due to different reaction types of lymph nodes and their different states of activation. Because sinuses are connected with both the efferent and afferent lymphatics and the cell trafficking both in and out of them is active, it is not surprising that both the apoptotic rate and Fas expression are so variable in them (Figure 4). Interestingly, almost none of the apoptotic cells in the sinuses were T-lymphocytes and only a few were B-lymphocytes, indicating that sinusoidal Fas-positive cells are from a cell lineage different from that of lymphocytes. From the standpoint of AICD, it would be futile if freshly activated lymphocytes would undergo apoptosis at the sinuses to which they have just migrated from the lymph node parenchyma.
In summary, we have used a new double-staining method to analyze apoptosis and its mechanisms in different functional compartments of human lymph nodes. Because our material consisted of reactively enlarged lymph nodes with an active phase of immunological response, the observations may not be directly applicable to non-enlarged, resting lymph nodes. Fas was expressed in apoptotic cells in all four anatomical areas of lymph nodes and in both B- and T–lymphocytes, suggesting the importance of Fas-mediated apoptosis in lymph node homeostasis. We show for the first time that the paracortex is an important location for B-lymphocyte apoptosis in humans and that follicular dendritic cells often show a high apoptosis rate. Most paracortical apoptotic cells were lymphocytes bearing Fas on their surface. This finding is consistent with the hypothesis that strong FasL expression in the endothelium of HEVs is crucial for inducing apoptosis of activated Fas-positive lymphocytes, leaving only naïve lymphocytes to enter the lymph node via the circulatory system. Our double-staining approach provides a useful tool for the analysis of mechanisms of apoptosis of specific cell types in different anatomical compartments of lymph nodes and in other lymphatic tissues.
Acknowledgments
This study has been supported by the Alma and K.A. Snellman Foundation.
We thank Manu Tuovinen, Erja Tomperi, and Mirja Vahera.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Abbas AK (1996) Die and let live: eliminating dangerous lymphocytes. Cell 84:655–657 [DOI] [PubMed] [Google Scholar]
- Badley AD, McElhinny JA, Leibson PJ, Lynch DH, Alderson MR, Paya CV (1996) Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol 70:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang B, Gniadecki R, Larsen JK, Baadsgaard O, Skov L (2003) In vivo UVB irradiation induces clustering of Fas (CD95) on human epidermal cells. Exp Dermatol 12:791–798 [DOI] [PubMed] [Google Scholar]
- Batista FD, Harwood NE (2009) The who, how and where of antigen presentation to B cells. Nat Rev Immunol 9:15–27 [DOI] [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Cabras A, Elia G (2009) The germinal centre-derived lymphomas seen through their cellular microenvironment. Br J Haematol 145:468–480 [DOI] [PubMed] [Google Scholar]
- Fan Z, Zhang Q (2005) Molecular mechanisms of lymphocyte-mediated cytotoxicity. Cell Mol Immunol 2:259–264 [PubMed] [Google Scholar]
- Ferguson TA, Griffith TS (2006) A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev 213:228–238 [DOI] [PubMed] [Google Scholar]
- Guan DW, Ohshima T, Kondo T (2000) Immunohistochemical study on Fas and Fas ligand in skin wound healing. Histochem J 32:85–91 [DOI] [PubMed] [Google Scholar]
- Hsu SM, Soban E (1982) Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem 30:1079–1082 [DOI] [PubMed] [Google Scholar]
- Hur DY, Kim DJ, Kim S, Kim YI, Cho D, Lee DS, Hwang Y, et al. (2000) Role of follicular dendritic cells in the apoptosis of germinal center B cells. Immunol Lett 72:107–111 [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, et al. (1991) The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233–243 [DOI] [PubMed] [Google Scholar]
- Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, et al. (1994) Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood 84:1201–1208 [PubMed] [Google Scholar]
- Kokkonen TS, Augustin MT, Makinen JM, Kokkonen J, Karttunen TJ (2004) High endothelial venules of the lymph nodes express Fas ligand. J Histochem Cytochem 52:693–699 [DOI] [PubMed] [Google Scholar]
- Le Deist F, Emile JF, Rieux-Laucat F, Benkerrou M, Roberts I, Brousse N, Fischer A (1996) Clinical, immunological, and pathological consequences of Fas-deficient conditions. Lancet 348:719–723 [DOI] [PubMed] [Google Scholar]
- Lee HO, Ferguson TA (2003) Biology of FasL. Cytokine Growth Factor Rev 14:325–335 [DOI] [PubMed] [Google Scholar]
- Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ (1996) Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med 184:429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, Sneller MC, et al. (1998) Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol 153:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DH, Ramsdell F, Alderson MR (1995) Fas and FasL in the homeostatic regulation of immune responses. Immunol Today 16:569–574 [DOI] [PubMed] [Google Scholar]
- Mackay CR (1991) T-cell memory: the connection between function, phenotype and migration pathways. Immunol Today 12:189–192 [DOI] [PubMed] [Google Scholar]
- Mita A, Hashikura Y, Tagawa Y, Nakayama J, Kawakubo M, Miyagawa S (2005) Expression of Fas ligand by hepatic macrophages in patients with fulminant hepatic failure. Am J Gastroenterol 100:2551–2559 [DOI] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R (2008) Lymphoid stroma in the initiation and control of immune responses. Immunol Rev 224:284–294 [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456 [DOI] [PubMed] [Google Scholar]
- Nagata S, Suda T (1995) Fas and Fas ligand: lpr and gld mutations. Immunol Today 16:39–43 [DOI] [PubMed] [Google Scholar]
- Or-Guil M, Wittenbrink N, Weiser AA, Schuchhardt J (2007) Recirculation of germinal center B cells: a multilevel selection strategy for antibody maturation. Immunol Rev 216:130–141 [DOI] [PubMed] [Google Scholar]
- Pinkoski MJ, Brunner T, Green DR, Lin T (2000) Fas and Fas ligand in gut and liver. Am J Physiol Gastrointest Liver Physiol 278:G354–366 [DOI] [PubMed] [Google Scholar]
- Russell JH, Ley TJ (2002) Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 20:323–370 [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ (2008) Sites and stages of autoreactive B cell activation and regulation. Immunity 28:18–28 [DOI] [PubMed] [Google Scholar]
- Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, et al. (1997) Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood 89:1341–1348 [PubMed] [Google Scholar]
- Strasser A, Jost PJ, Nagata S (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ebisuno Y, Kanemitsu N, Umemoto E, Yang BG, Jang MH, Miyasaka M (2004) Molecular determinants controlling homeostatic recirculation and tissue-specific trafficking of lymphocytes. Int Arch Allergy Immunol 134:120–134 [DOI] [PubMed] [Google Scholar]
- van der Valk P, Meijer CJ (1987) The histology of reactive lymph nodes. Am J Surg Pathol 11:866–882 [DOI] [PubMed] [Google Scholar]
- van Eijk M, Defrance T, Hennino A, de Groot C (2001) Death-receptor contribution to the germinal-center reaction. Trends Immunol 22:677–682 [DOI] [PubMed] [Google Scholar]
- von Andrian UH, M'Rini C (1998) In situ analysis of lymphocyte migration to lymph nodes. Cell Adhes Commun 6:85–96 [DOI] [PubMed] [Google Scholar]
- Wajant H (2002) The Fas signaling pathway: more than a paradigm. Science 296:1635–1636 [DOI] [PubMed] [Google Scholar]
- Walsh K, Sata M (1999) Is extravasation a Fas-regulated process? Mol Med Today 5:61–67 [DOI] [PubMed] [Google Scholar]
- Yamashita S (2007) Heat-induced antigen retrieval: mechanisms and application to histochemistry. Prog Histochem Cytochem 41:141–200 [DOI] [PubMed] [Google Scholar]
- Yoshino T, Kondo E, Cao L, Takahashi K, Hayashi K, Nomura S, Akagi T (1994) Inverse expression of bcl-2 protein and Fas antigen in lymphoblasts in peripheral lymph nodes and activated peripheral blood T and B lymphocytes. Blood 83:1856–1861 [PubMed] [Google Scholar]
- Young AJ (1999) The physiology of lymphocyte migration through the single lymph node in vivo. Semin Immunol 11:73–83 [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu X, Liu Y (2004) Activation-induced cell death in T cells and autoimmunity. Cell Mol Immunol 1:186–192 [PubMed] [Google Scholar]