Abstract
Experiments were performed to measure the dynamical response of individual SonoVue® microbubbles subjected to pulsed ultrasound. Three commonly used bubble dynamic models (i.e., Hoff’s, Sarkar’s, and linearized Marmottant’s models) were compared to determine the most appropriate model for fitting to the experimental data. The models were evaluated against published optical microscopy data. The comparison suggests that it is difficult to rank these models for lipid-shelled microbubbles undergoing small-amplitude oscillations, because under these conditions the shell parameters in these models are closely related. A linearized version of the Marmottant model was used to estimate the shell parameters (i.e., shear modulus and shear viscosity) of SonoVue® microbubbles from the experimental light scattering data, as a function of ambient microbubble radius. The SonoVue® microbubble shell elasticity and dilatational viscosity increase with ambient bubble radius, in agreement with previously published data. The results suggest that light scattering, used in conjunction with one of several popular bubble dynamics models, is effective at characterizing microbubble response and evaluating shell parameters.
INTRODUCTION
The sensitivity and specificity of diagnostic ultrasound (US) imaging can be improved by IV injection of gas microbubbles as contrast agents.1 These agents can enhance the acoustic backscattering from blood and produce enhancement of both Doppler flow signals and gray-scale B-mode images, due to the large acoustic impedance difference between the gas and the surrounding blood∕tissue. Most US contrast agents (UCAs) are comprised of suspensions of micron-sized bubbles (“microbubbles”), with a stabilizing shell (e.g., albumin or lipid) and a relatively insoluble gas (e.g., perfluorocarbons). The UCA studied in this paper is SonoVue® (Bracco Diagnostics Inc., Geneva, Switzerland), one of the more commonly used new generation UCAs.2, 3 SonoVue® microbubbles, coated with a very thin lipid monolayer membrane shell encapsulating the gas SF6, have a mean radius of around 1.5 μm and a concentration of (2–5)×108 bubbles∕ml.
The encapsulating shell, which is the additional material present at the gas-liquid interface to stabilize UCA microbubbles against dissolution and coalescence,4 affects the dynamical response of microbubbles. A better understanding of the physical interaction of US with UCAs will provide new insights and enable new approaches in both diagnostic and therapeutic US applications; e.g., the nonlinear behavior of oscillating microbubbles has led to the development of contrast harmonic imaging, whereas observations of microbubble destruction have led to the development of high mechanical index imaging techniques, and novel therapeutic applications such as high intensity focused ultrasound (HIFU) and targeted UCA drug∕gene delivery.5, 6 Understanding shell properties is thus an important area of current investigations.
May et al.7 measured the shell thickness of MRX552 microbubbles (ImaRx, Co., Tucson, AZ), a therapeutic US agent with triacetin shell, based on SEM fracture measurements. The precondition of their scanning electron microscopy (SEM) study is that MRX552 microbubble has a thick measurable fluid shell (∼500 nm) with known viscosity. However, for most diagnostic UCAs with thin-shell materials (e.g., albumin or lipid), it is impossible to employ SEM analysis. In fact, there is no method to directly measure UCA shell properties. Thus, the shell properties of UCAs can only be investigated by comparing experimental dynamical responses [e.g., R(t) curve or scattering cross-section] to simulation results obtained from numerical models. This approach, measurements coupled to numerical simulations, is the focus of this paper.
To date, about a half dozen numerical models have been proposed to describe the dynamical response of shelled UCA microbubbles, with parameters for shell characteristics such as shell thickness, viscosity, stiffness, friction parameter, and surface tension.8, 9, 10, 11, 12, 13 These numerical models are usually based on approximations and scaling arguments from Rayleigh–Plesset (RP)-like equations that describe the nonlinear spherical oscillations of bubbles exposed to an external acoustic field.14, 15 The early version of these models was based on semi-empirical observations8 by treating the shell as surface layers of elastic solids and introducing elasticity and shell friction parameters. The model was validated and the shell parameters were estimated by fitting the acoustic transmission measurements of the scattering and extinction cross-section of Albunex® microbubbles with the numerical model.9 Church10 regarded the shell as a continuous layer of incompressible solid elastic material by accounting for shell elasticity, in terms of a shear modulus and viscous dissipation of the shell. His theoretical derivations agreed with the experimental work of de Jong and Hoff16 on the scattering cross-section and attenuation of Albunex®. According to Hoff’s report,11 Albunex® microbubbles have an albumin shell with the thickness of ∼15 nm. However, SonoVue® microbubbles are encapsulated with a lipid monolayer membrane (∼4 nm, in Hoff’s report11), which is thinner than the albumin shell. In order to study the dynamical motion of lipid-shelled UCA microbubbles, thin-shell assumptions have been incorporated by Hoff11 and Chatterjee and Sarkar,12 and Morgan, et al.17 All the above models regard the microbubble shell as infinitesimal or constant in thickness with a fixed surface tension coefficient. However, an oscillating UCA microbubble may express variations in surface tension during its oscillation. Marmottant et al.13 proposed an improved model that described the shell elasticity with a radius-dependent surface tension over a certain range of microbubble radii (elastic range), below which the microbubble would buckle and above which the microbubble shell would break. They claimed that this model should be more precise and suitable for UCA microbubbles with thin lipid monolayers or elastic solid shells. However, the question remains whether or not the various models can be ranked in order of accuracy. Such a ranking is attempted in this paper in order to determine which model is most appropriate for fitting to the data.
Since the shell parameters cannot be measured directly, some indirect experimental approaches, including backscatter detection18, 19, 20 and high-speed photography,21, 22, 23 have been applied to characterize UCA shell properties and quantify the responses of UCA microbubbles. However, backscattering signals are often machine or concentration dependent;24 and high-speed cameras are expensive and the amount of data collection is small. Furthermore, even though high-speed cameras offer a “direct” measurement of bubble size via visual inspection of the images, identifying the indistinct bubble edge generates inaccuracies in the measurement. In the present work, light scattering was used to measure the instantaneous dynamical response of individual SonoVue® microbubbles exposed to pulsed US from an M-mode diagnostic US system. Compared to other modalities, light scattering offers some advantages such as collecting real-time data over nearly unlimited successive acoustic pulses to an individual microbubble (to study, e.g., microbubble evolution), and to a nearly unlimited number of microbubbles (e.g., to characterize a large sample of microbubbles), and low-cost implementation. It can also be incorporated easily into an experimental setup. For example, it should be possible to analyze thousands of microbubbles in a few minutes (or even seconds), whereas a high-speed camera can take days to image and analyze just a few microbubbles.
Two major studies were preformed here: (1) A comparative study was performed to evaluate three typical shelled microbubble dynamics models by fitting the models to two types of experimental data: In one case, the experimental data were performed on BR14 microbubbles using high-speed microscopy, and come from previously published work;23 the other case is a SonoVue® microbubble, whose dynamical response [i.e., R(t) curve] was measured with light scattering. (2) The shell parameters of SonoVue® microbubbles were quantified as a function of initial ambient radius using a best-fit approach to the measured light-scattered data. The results suggest that (1) the models generate similar results, and, thus, when comparing a single data set, cannot be ranked; (2) under assumptions of thin shell and linear oscillations, the shell parameters in each of the different models are related to each other; (3) the shell parameters increase with bubble size, in agreement with previously published data; and (4) light scattering is a very useful technique for characterizing UCA microbubbles.
SHELLED UCA MODELS
Modeling the response of UCAs to pulsed US is non-trivial due to the addition of a coated shell. In the present work, three models (i.e., the Hoff, Sarkar, and Marmottant models) were compared to determine if a “best” model can be selected for microbubbles encapsulated with lipid monolayer membranes. While not inclusive, the models are representative. All these models are modified from a RP-like equation, which describes the response of a spherical free gas bubble to a time-varying acoustic pressure field in an incompressible liquid. A general RP-like (unshelled) equation goes like
| (1) |
where R0 is the ambient bubble radius, ρL is the density of a Newtonian liquid, P0 is the ambient pressure, Pv is the vapor pressure, σ is the surface tension, γ is the polytropic exponent of the gas, δ is the damping coefficient, Pa is the amplitude of the incident acoustic pressure, ω is the angular frequency of driving signal, and Pg is the gas pressure inside the bubble (Pg=P0−Pv+2σ∕R0).
The assumptions for this equation are as follows: (1) The motion of the bubble is symmetric; (2) the wavelength of US is much larger than the bubble radius; (3) no rectified diffusion occurs; and (4) the bubble contains gas or vapor, which is compressed and behaves like an ideal gas with a constant polytropic exponent.
Hoff model
A classical three-region RP-like equation [Eq. 2] describing the dynamics of shelled gas microbubbles was derived by Church.10 In this model a continuous layer of incompressible, solid elastic shell with damping separates the gas microbubble from the bulk Newtonian liquid. The elastic surface layer stabilizes the microbubble against dissolution by supporting a strain that counters the Laplace pressure. Viscous damping is considered in this model.
| (2) |
where R1 and R2 are the inner and outer radii of the elastic shell, R01 are R02 are corresponding ambient radii, ρs is the shell density, σ1 is the surface tension of the gas-shell interface, σ2 is the surface tension of the shell-liquid interface, PG,eq=P0 for the surface layer permeable to gas, ηs and ηL are shell and liquid shear viscosities, respectively, and Gs is the shell shear modulus
and
| (3) |
Hoff11 simplified Church’s equation for the case of a bubble whose shell thickness is thin, ds(t)⪡R2,
| (4) |
Throughout this paper, Eq. 4 is referred to as the “Hoff” model. For microbubbles coated with a lipid monolayer shell, we will set ds=4 nm according to Hoff’s report.11
Sarkar model
Chatterjee and Sarkar12 developed a new model for encapsulated contrast agent microbubbles
| (5) |
They assumed the encapsulation of a contrast agent to be an interface of infinitesimal thickness with complex interface rheological properties, where the interfacial tension (σi) and dilatational viscosity (κs) are unknown interface and shell parameters. The other symbols used are the same as above. For simplicity, we refer to Eq. 5 as the “Sarkar” model.
Marmottant model
Most shelled UCA models assume constant surface tension coefficients and small deformations of the microbubble surface. However, for phospholipid monolayer coatings, the surface area available per phospholipid molecule apparently varies as the microbubble oscillates. Thus, Marmottant et al.13 derived an improved model specifically for microbubbles with lipid monolayer coatings. The model considers the microbubble shell as a two-dimensional viscoelastic medium and suggests that the shell elasticity can be modeled with a radius-dependent surface tension that takes into account shell buckling and rupture. For our purposes, we use the linearized version (no rupture or buckling), from Van der Meer et al.23 There are two parameters to model the shell properties: the shell elastic compression modulus χ and a shell dilatational viscosity κs.
| (6) |
Again, for simplicity, Eq. 6 is referred to throughout this paper as the linearized “Marmottant” model. The model has been applied very successfully to SonoVue® and BR14, from Bracco Diagnostics.
COMPARISON OF THREE SHELLED BUBBLE DYNAMIC MODELS
In Sec. 5, it will be necessary to use a specific bubble dynamics model to fit the measured dynamical response of SonoVue® microbubbles from our light scattering system. We do not know a priori which model is most appropriate. The three models described above (i.e., the Hoff, Sarkar, and Marmottant models) provide a means for comparison. It will be useful to first compare these models by fitting some experimental data obtained from literature published by Van der Meer et al.23 As described in their paper, high-speed optical imaging was used to record the R(t) curves for individual lipid-shelled UCA microbubbles (BR14, Bracco Diagnostics) up to 25×106 frames∕s. Optical imaging allows direct measurement of the bubble radius, which can be an advantage over other indirect experimental methods such as acoustic backscattering and light scattering, for which a secondary means of calibrating the size is required. However, even optical imaging suffers from inaccuracies in identifying a bubble’s indistinct edge. Still, it is the gold standard for measuring sizes and, thus, provides a good framework for our purposes of comparing bubble dynamics models.
For the comparison, we chose a specific R(t) curve from Fig. 4 in Van der Meer et al.’s paper,23 with an ambient radius of 1.7 μm. To simulate a forcing function, we assume (as they did) an 8-cycle acoustic pulse [Fig. 1a], tapered with a Gaussian envelope. The amplitude of the driving signal was 40 kPa with the frequency of 2.5 MHz, again as was done in Ref. 23. Since R0 was measured to be 1.7 μm, the two-parameter fitting sets used here were (χ,κs), (σi,κs), and (ηs,Gs) for the linearized Marmottant, Sarkar, and Hoff models, respectively. Minimum standard deviation (STD) evaluation is applied to determine the best fitting parameters. The STD value is defined as
| (7) |
where tstart and tend are the starting and ending points of the fitting region, Rexp,i and Rcal,i are the ith measured and simulated microbubble radius along the time span, respectively, and N is the total number of the fitting points. The following are some physical constants used for the simulation: P0=1.013×105 Pa, Pv=2.33 kPa, ρg=1.161 kg∕m3, σw=0.072 N∕m, γ=1.07, ρL=103 kg∕m3, ρs=1100 kg∕m3, c=1500 m∕s, and ηL=2×10−3 Pa s (effective liquid viscosity accounts for thermal damping23). The best fit results are shown in Fig. 1b for all three models. Table 1 lists the best fit shell parameters and the minimum STD values given by these three models.
Figure 1.
(a) A Gaussian-tapered, 8-cycle, 2.5-MHz, 40-kPa forcing function is used to generate simulated acoustic microbubble responses. (b) The best-fit simulated responses for the three models are compared to each other and to the data (R0=1.7 μm) available in Ref. 23. Specific values for the best fit shell parameters are given in Table 1. Only slight differences between the models can be observed.
Table 1.
Best fit shell parameters and minimum STD for the data from Ref. 23.
| UCA model | Shell elasticity | Shell viscosity | Minimum STD |
|---|---|---|---|
| Marmottant | Shell elasticity χ=0.25 N∕m | Dilatational viscosity κs=4×10−9 kg∕s | 0.054 |
| Sarkar | Interfacial tension σi=0.32 N∕m | Dilatational viscosity κs=4×10−9 kg∕s | 0.054 |
| Hoff | Shear modulus Gs=23 MPa | Shear viscosity ηs=0.5 Pa s | 0.059 |
The results shown in Fig. 1b suggest that all three models perform equally well in describing the experimental data in the central region, and all models show deviations from the experimental data at the beginning and end stages. The minimum STD values are similar for all three sets of shell parameters. The relative equality between the models suggests that any of these models can be used to fit our light scattering data.
MEASUREMENTS OF SONOVUE® MICROBUBBLE DYNAMICS USING LIGHT SCATTERING
Having determined that any of these three models can be used successfully to characterize a thin lipid-shelled microbubble from direct optical imaging data, we attempted to determine if an identical fitting analysis can be applied to light scattering data, an indirect measure of a bubble’s oscillation that must be converted to an R(t) curve using Mie scattering theory. A modification of Mie scattering theory, applicable to a coated sphere, was first developed by Aden and Kerker,25 and later applied to coated bubbles by Marston et al.26, 27 Our implementation of the model for coated SonoVue® microbubbles is based on the equations in the book by Bohren and Huffman.28
Experimental setup
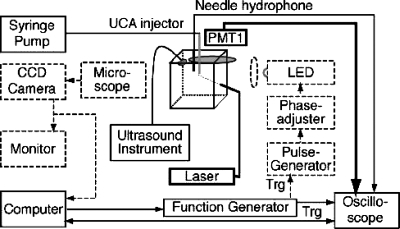
Figure 2 illustrates the experimental setup. Details of the design and methods have been described elsewhere.29 In brief, highly diluted SonoVue® suspensions were injected into the region of interest (ROI) (a small volume within a water tank where the US and laser beams intersect with SonoVue® microbubbles) using a syringe pump (74900 series, Cole-Parmer Instrument Co., Vernon Hills, IL) at a rate of 10 ml∕h with a 0.5 mm inner-diameter tube. Acoustic interrogation pulses were sent from a probe of a diagnostic US instrument (Ultramark 4 Plus, ATL-Philips, Andover, MA) operated in M-mode at 1-kHz pulse-repetition-frequency and monitored using a calibrated needle hydrophone (NTR Systems Inc., Seattle, WA). Although the Ultramark 4 Plus is an older instrument, its output does more closely resemble a clinical pulse than does a research transducer.
Figure 2.
Diagram of the light scattering system that was used to measure SonoVue® microbubble responses to pulsed US from a diagnostic US system. Dark solid lines refer to laser probe, whereas dashed lines refer to light scattering calibration components.
A HeNe laser (Melles Griot, Carlsbad, CA) was used as the light scattering probe. The waist of laser beam was focused to less than 100 μm at the ROI. The scattered light signals from the microbubbles in the ROI were collected and focused onto a photo-multiplier tube (PMT) detector (Hamamatsu, model 2027). The output signals from the PMT and the hydrophone were recorded using a high-speed digital oscilloscope (LeCroy, Chestnut Ridge, NY) in sequence mode, and then transferred to a computer and post-processed using MATLAB (Mathworks Inc., Natick, MA).
Although optical imaging has the advantage of direct measurements of microbubble radius, an ultrahigh-speed camera is very expensive and not commonly found in laboratories. Previously Guan and Matula29 showed that UCA dynamics could be measured with light scattering, and that these signals could be interpreted as volume mode oscillations of shelled microbubbles using bubble dynamics equations such as those described above. The question remains, and we will try to answer, whether or not one can use these models to accurately (or consistently) interpret the light-scattered signal to estimate shell parameters.
Shell properties estimation of a sample microbubble
Individual SonoVue® microbubbles were exposed to diagnostic US pulses with pressure amplitude 150 kPa and frequency 2.5 MHz [Fig. 3a]. The microbubble response [i.e., R(t) curve] was measured with light scattering. Backlit optical imaging was used to calibrate the light scattering amplitude,29 and for these data, R0≈1.78 μm. A relatively low signal∕noise may affect the overall calibration constant, which will be discussed further in Sec. 6. As before, the parameter sets used to fit the data were (χ,κs), (σi,κs), and (Gs,ηs) for the linearized Marmottant, Sarkar, and Hoff models, respectively. The best fit R(t) curves for these models are shown in Fig. 3b. Again, we assume a constant shell thickness of 4 nm, according to the report by Hoff.11 The corresponding best-fit shell parameters and minimum STD values are listed in Table 2.
Figure 3.
(a) A 150 kPa, 2.5-MHz M-mode pulse from an Ultramark 4 Plus diagnostic US system is used to excite SonoVue® oscillations. (b) The best-fit simulated responses for the three models are compared to each other and to the light scattering data (R0=1.78 μm). Specific values for the best fit shell parameters are given in Table 2.
Table 2.
Best fit shell parameters and minimum STDs for SonoVue® microbubbles (calibrated R0=1.78 μm) measured with light scattering.
| UCA model | Shell elasticity | Shell viscosity | Minimum STD |
|---|---|---|---|
| Marmottant | Shell elasticity χ=0.3 N∕m | Dilatational viscosity κs=3.2×10−9 kg∕s | 0.132 |
| Sarkar | Interfacial tension σi=0.4 N∕m | Dilatational viscosity κs=4×10−9 kg∕s | 0.134 |
| Hoff | Shear modulus Gs=20 MPa | Shear viscosity ηs=0.6 Pa s | 0.136 |
Figure 3b and the minimum STD values listed in Table 2 show that SonoVue® microbubble R(t) curves can be fitted reasonably well by each of the three models. Overall, the best fit shell parameters from the light scattering data are quite close to those values obtained for BR14 microbubbles reported in Van der Meer et al.’s work.23
Comparing Fig. 1b with Fig. 3b, and the minimum STDs in Tables 1, 2, it is clear that better fits were obtained with Van der Meer’s data. One possible reason for this disparity may lie in the microbubble oscillation amplitude. In Van der Meer’s experiment, the maximum driving amplitude is 40 kPa. The present experiment used a relatively higher driving amplitude (150 kPa), and nonlinear effects might negate some of the assumptions (such as constant shell thickness).
SONOVUE® SHELL PARAMETERS VERSUS AMBIENT MICROBUBBLE SIZE
Having shown that light scattering can be used with any of the three models to estimate the shell parameters for a microbubble, we now describe light scattering experiments performed on a series of SonoVue® microbubbles, whose ambient radii were determined based on the calibration constant for the received relative scattered light intensity.29 In this experiment, the bubbles were injected into the ROI via a syringe pump, while continuously taking data. Although we could have chosen to use any of the three models for our fitting algorithms, we used the linearized version of Marmottant’s model for consistency and for later comparison (Sec. 6 describes the relationship between the shell parameters for the various models so that one can convert between the various parameter sets). As before, the Ultramark 4Plus was used to excite microbubble oscillations at 150 kPa and 2.5 MHz. A total of 50 Sonovue® microbubbles were examined, although in principal, because the acquisition speed is rapid, the sample number can be increased significantly. The results, shown in Fig. 4, illustrate that both the shell elastic compression modulus χ and shell dilatational viscosity κs increase with increasing bubble ambient radius (R0).
Figure 4.
SonoVue® shell parameters estimated from fitting the experimental light scattering data with the linearized Marmottant model. (a) The relationship between shell elasticity and R0. (b) The relationship between shell dilatational viscosity and R0. Standard deviation error bars are used when multiple bubbles are “fitted” to the same radius.
In Fig. 4b, we add the data from Van der Meer et al.23 to our own data. The comparison is quite good. The two independent methods for measuring UCA characteristics suggest that the variability in dilatational viscosity is real. These results agree with previous experimental observations on other lipid-shelled microbubbles.17, 23, 30 Van der Meer et al.23 suggested that the physical significance of the viscosity variation involves rheological thinning processes.
DISCUSSION
Shell parameters
In our comparative study, a different set of shell parameters exists for each model, (χ,κs), (σi,κs), and (ηs,Gs), for the linearized Marmottant, Sarkar, and Hoff models, respectively. However, it is natural to ask whether or not these parameters are related. Comparing the shell parameter terms in Sarkar’s model [Eq. 5] with those in Hoff’s model [Eq. 4], we have
| (8) |
| (9) |
In order to make progress we note that under small-amplitude oscillation, the shell volume can be assumed constant, in which case , where ds0 is the initial shell thickness. Using this in Eq. 8 gives
| (10) |
The fits in Table 1 show good agreement with this analysis.
Now, looking at Eq. 9, and allowing γ=1.07≈1 in the left hand side, we have
| (11) |
The right hand side of Eq. 9 is analyzed as follows: The variable χ is used in Marmottant’s model to describe the shell elasticity. This elastic compression modulus is related to the shear modulus under thin-shell elasticity theory by31
| (12) |
Substituting this into the right hand side of Eq. 9 gives
| (13) |
Substituting Eqs. 11, 12 into Eq. 9 gives
| (14) |
which, upon using the small-amplitude assumption, suggests that σi should be of the same magnitude as χ. Table 1 suggests a good agreement between the values of Gs in Hoff’s model and χ in Marmottant’s model (assuming a shell thickness of 4 nm) when using thin plate elasticity theory. The best-fit value of χ is also on the same order of magnitude as previously reported for SonoVue® microbubbles.32
The best fit shell dilatational viscosities (κs) for both Van de Meer’s and Sarkar’s model are 4 nm Pa s (viz., 4×10−9 kg∕s), which also agrees with the results of Van de Meer et al.23 Finally, we note that when applying Eq. 10, there is a reasonable agreement between the best-fit ηs in Hoff’s model (0.5 Pa s) to the κs values found in Marmottant’s and Sarkar’s models (4×10−9 kg∕s).
The above analysis suggests that although different shell parameters are applied in each model, they appear to be related to each other under the assumption of small-amplitude oscillation and constant (yet thin) shell thickness. The results are consistent with optical imaging data (Table 1), as well as our light scattering data (Table 2). We have not, however, performed an analysis on the shell thickness parameter, which is assumed constant at 4 nm. We hypothesize that other types of bubbles would show a similar agreement between models. This analysis is restricted to small-amplitude behavior, and thus “degeneracy” in the models might be expected. Nonlinearities introduced with stronger forcing conditions may lead to a “splitting” in the degeneracy in which the various models might show differences. Indeed, Doinikov et al.33 suggested that the increase in shell shear modus and viscosity of SonoVue® bubbles with radius might result from material rheological properties of both shear-thinning and strain-softening processes. Due to complicated rheological changes in the lipid shell, SonoVue® microbubbles may generate stronger nonlinear responses when insonified by stronger US pulses, making these bubbles an ideal choice to explore nonlinear dynamical shell effects. One model ideally suited for such exploration is the full Marmottant model, which includes rupture and buckling effects—an elastic parameter that depends on the bubble radius.
Light scattering
Our implementation of light scattering is straightforward, and shows great promise for characterizing shelled microbubbles. The additional imaging system used for calibration (see dashed lines in Fig. 2) is somewhat inconvenient. For comparative purposes, we re-analyzed the light scattering data by including a third fitting parameter, R0, into the routine instead of assuming the measured calibration constant. For that case, the best-fit value for R0 is 1.72 μm, and the corresponding best-fit shell parameters and minimum STD are listed in Table 3. The R(t) curve using this slightly lower value for R0 is shown in Fig. 5. Comparing to the R(t) curve using the calibrated value R0=1.78 μm [Fig. 3b], we note that the addition of R0 as a fitting parameter does not appreciably change the fits to the data. The corresponding shell parameters are slightly lower (see Table 3), but the trend shown in Fig. 4 does not change.
Table 3.
Best fit shell parameters and minimum STDs for SonoVue® microbubbles (best-fitted R0=1.72 μm) measured with light scattering.
| UCA model | Shell elasticity | Shell viscosity | Minimum STD |
|---|---|---|---|
| Marmottant | Shell elasticity χ=0.22 N∕m | Dilatational viscosity κs=2.5×10−9 kg∕s | 0.131 |
| Sarkar | Interfacial tension σi=0.3 N∕m | Dilatational viscosity κs=3.5×10−9 kg∕s | 0.132 |
| Hoff | Shear modulus Gs=19 MPa | Shear viscosity ηs=0.4 Pa s | 0.135 |
Figure 5.
The data in Fig. 3b are re-analyzed by adding a third fitting parameter, R0. The best fit R0=1.72 μm. The best fit shell parameters are listed in Table 3.
There is relatively close agreement between our calibrated and fitted data. Indeed, professional multi-beam light scattering systems are already used to size microbubbles.34, 35 That a simple light scattering system can estimate microbubble size and shell parameters suggests that it should be possible to implement a light scattering system for microbubble characterization in the laboratory or industrially, for both sizing and shell characterization. In particular, with the addition of an US driver, an off-the-shelf particle sizer could be modified to characterize both size and shell properties. Such an effort is already being undertaken in our laboratory, with promising results.
CONCLUSION
Three shelled UCA bubble dynamic models (i.e., the Hoff, Sarkar, and linearized Marmottant models) were compared by fitting to experimental data obtained from previously published literature. The comparison results show that it is difficult to rank these models for lipid monolayer shelled bubbles undergoing small-amplitude oscillations. Although different shell parameters are used in each of the models, they appear to be related to each other under the assumptions of small-amplitude oscillation and constant shell thickness. SonoVue® bubble shell parameters were quantified by fitting the measured R(t) curves generated with light scattering to a linearized version of the Marmottant model. The results show that the shell elastic modulus χ and shell dilatational viscosity κs increase with increasing ambient radius. Finally, as an industrial method for characterizing microbubbles, light scattering appears to have an advantage of low cost, high speed of data acquisition and analysis, and high throughput.
ACKNOWLEDGMENTS
The authors wish to thank Peter Frinking and Bracco Imaging for supplying the SonoVue® microbubbles. We also wish to thank Michel Versluis for providing the MATLAB program for simulating the 8-cycle Gaussian windowed acoustic pulse used in Ref. 23 and shown in Fig. 2 of this paper. This work was funded in part by NIH Grant No. 5R01EB000350 (NIBIB), the National Natural Science Foundation of China (Grant Nos. 10434070 and 10704037), the Young Scholar Technological Innovation Projects of Jiangsu Province (China) Grant No. BK2007569, and the Research Fund for the Doctoral Program (for new scholar) of Higher Education of China (Grant No. 20070284070).
References
- Goldberg B. B., Raichlen J. S., and Forsberg F., Ultrasound Contrast Agents—Basic Principles and Clinical Applications, 2nd ed. (Martin Dunitz, London, 2001). [Google Scholar]
- Dijkmans P. A., Juffermans L. J. M., Musters R. J. P., van Wamel A., ten Cate F. J., van Gilst W., Visser C. A., de Jong N, and Kamp O., “Microbubbles and ultrasound: from diagnosis to therapy,” Eur. J. Echocardiogr. 5, 245–256 (2004). 10.1016/j.euje.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Regine G., Atzori M., Miele V., Buffa V. V., Galluzzo M., Luzietti M., and Adami L., “Second-generation sonographic contrast agents in the evaluation of renal trauma,” Radiol. Med. (Torino) 112, 581–587 (2007). 10.1007/s11547-007-0164-2 [DOI] [PubMed] [Google Scholar]
- Kabalnov A., Klein D., Pelura T., Schutt E., and Weers J., “Dissoluton of multicomponent microbubbles in the bloodstream: 1. Theory,” Ultrasound Med. Biol. 24, 739–749 (1998). 10.1016/S0301-5629(98)00034-9 [DOI] [PubMed] [Google Scholar]
- Tinkov S., Bekeredjian R., Winter G., and Coester C., “Microbubbles as ultrasound triggered drug carriers,” J. Pharm. Sci. 98, 1935–1961 (2009). 10.1002/jps.21571 [DOI] [PubMed] [Google Scholar]
- Kennedy J. E., “High-intensity focused ultrasound in the treatment of solid tumours,” Nat. Rev. Cancer 5, 321–327 (2005). 10.1038/nrc1591 [DOI] [PubMed] [Google Scholar]
- May D. J., Allen J. S., and Ferrara K. W., “Dynamics and fragmentation of thick-shelled microbubbles,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 49, 1400–1410 (2002). 10.1109/TUFFC.2002.1041081 [DOI] [PubMed] [Google Scholar]
- de Jong N., Cornet R., and Lancée C. T. “Higher harmonics of vibrating gas-filled microspheres. Part two: Measurements,” Ultrasonics 32, 455–459 (1994). 10.1016/0041-624X(94)90065-5 [DOI] [Google Scholar]
- de Jong N., Cornet R., and Lancee C. T., “Higher harmonics of vibrating gas-filled microspheres. Part one: Simulation,” Ultrasonics 32, 447–453 (1994). 10.1016/0041-624X(94)90064-7 [DOI] [Google Scholar]
- Church C. C., “The effects of an elastic solid surface layer on the radial pulsations of gas bubbles,” J. Acoust. Soc. Am. 97, 1510–1521 (1995). 10.1121/1.412091 [DOI] [Google Scholar]
- Hoff L., Acoustic Characterization of Contrast Agents for Medical Ultrasound Imaging (Kluwer, Dordrecht, 2001). [Google Scholar]
- Chatterjee D. and Sarkar K., “A Newtonian rheological model for the interface of microbubble contrast agents,” Ultrasound Med. Biol. 29, 1749–1757 (2003). 10.1016/S0301-5629(03)01051-2 [DOI] [PubMed] [Google Scholar]
- Marmottant P., van der Meer S., Emmer M., Versluis M., de Jong N., Hilgenfeldt S., and Lohse D. A., “A model for large amplitude oscillations of coated bubbles accounting for buckling and rupture,” J. Acoust. Soc. Am. 118, 3499–3505 (2005). 10.1121/1.2109427 [DOI] [Google Scholar]
- Rayleigh L., “On the pressure developed in a liquid during the collapse of a spherical cavity,” Philos. Mag. 34, 94–98 (1917). [Google Scholar]
- Plesset M. S., “The dynamics of cavitation bubbles,” ASME J. Appl. Mech. 16, 277–282 (1949). [Google Scholar]
- de Jong N. and Hoff L., “Ultrasound scattering properties of Albunex microspheres,” Ultrasonics 31, 175–181 (1993). 10.1016/0041-624X(93)90004-J [DOI] [PubMed] [Google Scholar]
- Morgan K. E., Allen J. S., Dayton P. A., Chomas J. E., Klibaov A. L., and Ferrara K. W., “Experimental and theoretical evaluation of microbubble behavior: Effect of transmitted phase and bubble size,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 47, 1494–1509 (2000). 10.1109/58.883539 [DOI] [PubMed] [Google Scholar]
- Basude R. and Wheatley M. A., “Generation of ultraharmonics in surfactant based ultrasound contrast agents: Use and advantages,” Ultrasonics 39, 437–444 (2001). 10.1016/S0041-624X(01)00080-4 [DOI] [PubMed] [Google Scholar]
- Chang P. H., Shun K. K., and Levene H. B., “Second harmonic imaging and harmonic Doppler measurements with Albunex,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 42, 1020–1027 (1995). 10.1109/58.476546 [DOI] [Google Scholar]
- Shi W. T., Forsberg F., Hall A. L., Chia R. Y., Liu J. B., Miller S., Thomenius K. E., Wheatley M. A., and Goldberg B. B., “Subharmonic imaging with microbubble contrast agents: Initial results,” Ultrason. Imaging 21, 79–94 (1999). [DOI] [PubMed] [Google Scholar]
- Dayton P. A., Morgan K. E., Klibanov A. L., Brandenburger G., and Ferrara K. W., “Optical and acoustical observation of the effects of ultrasound on contrast agents,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 46, 220–232 (1999). 10.1109/58.741536 [DOI] [PubMed] [Google Scholar]
- de Jong N., Frinking P. J. A., Bouakaz A., Goorden M., Schourmans T., Jingping X., and Mastik F., “Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera,” Ultrasound Med. Biol. 26, 487–492 (2000). 10.1016/S0301-5629(99)00159-3 [DOI] [PubMed] [Google Scholar]
- Van der Meer S. M., Dollet B., Voormolen M. M., Chin C. T., Bouakaz A., de Jong N., Versluis M., and Lohse D., “Microbubble spectroscopy of ultrasound contrast agents,” J. Acoust. Soc. Am. 121, 648–656 (2007). 10.1121/1.2390673 [DOI] [PubMed] [Google Scholar]
- Sboros V., Ramnarine K. V., Moran C. M., Pye S. D., and McDicken W. N., “Understanding the limitations of ultrasonic backscattering measurements from microbubble populations,” Phys. Med. Biol. 47, 4287–4299 (2002). 10.1088/0031-9155/47/23/313 [DOI] [PubMed] [Google Scholar]
- Aden A. L. and Kerker M., “Scattering of electromagnetic waves from two concentric spheres,” J. Appl. Phys. 22, 1242–1246 (1951). 10.1063/1.1699834 [DOI] [Google Scholar]
- Marston P. L., Billette S. C., and Dean C. E., “Scattering of light by a coated bubble in water near the critical and Brewster scattering angles,” Ocean Optics IX, edited by Blizard M. A., Proc. SPIE 925, 308–316 (1988). [Google Scholar]
- Marston P. L., “Colors observed when sunlight is scattered by bubble clouds in sea water,” Appl. Opt. 30, 3479–3484 (1991). 10.1364/AO.30.003479 [DOI] [PubMed] [Google Scholar]
- Bohren C. F. and Huffman D. R., Absorption and Scattering of Light by Small Particles (Wiley, New York, 1998). [Google Scholar]
- Guan J. and Matula T. J., “Using light-scattering to measure the response of individual ultrasound contrast microbubbles subjected to pulsed ultrasound in vitro,” J. Acoust. Soc. Am. 116, 2832–2842 (2004) 10.1121/1.1795334 [DOI] [PubMed] [Google Scholar]; Matula T. J. and Guan J., “Using light-scattering techniques to better understand the interaction of pulsed ultrasound with contrast microbubbles,” Proc. SPIE 5750, 117–126 (2005). 10.1117/12.595847 [DOI] [Google Scholar]
- Doinikov A. A. and Dayton P. A., “Nonlinear dynamics of lipid-shelled ultrasound microbubble contrast agents,” Computational Methods in Multiphase Flow IV, edited by Mammoli A. A. and Brebbia C. A. (WIT, Southampton, United Kingdom, 2007), pp. 261–270. [Google Scholar]
- Boal D., Mechanics of the Cell (Cambridge University Press, Cambridge, 2002). [Google Scholar]
- Gorce M., Arditi M., and Schneider M., “Influence of bubble size distribution on the echogenicity of ultrasound contrast agents. A study of SonoVue,” Invest. Radiol. 35, 661–671 (2000). 10.1097/00004424-200011000-00003 [DOI] [PubMed] [Google Scholar]
- Doinikov A. A., Haac J. F., and Dayton P. A., “Modeling of nonlinear viscous stress in encapsulating shells of lipid-coated contrast agent microbubbles,” Ultrasonics 49, 269–275 (2009). 10.1016/j.ultras.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeffinger B. E. and Wheatley M. A., “Development and characterization of a nano-scale contrast agent,” Ultrasonics 42, 343–347 (2004). 10.1016/j.ultras.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Bloch S. H., Short R. E., Ferrara K. W., and Wisner E. R., “The effect of size on the acoustic response of polymer-shelled contrast agents,” Ultrasound Med. Biol. 31, 439–444 (2005). 10.1016/j.ultrasmedbio.2004.12.016 [DOI] [PubMed] [Google Scholar]