Abstract
To examine the generality of cholinergic involvement in visual memory in primates, we trained macaque monkeys either on an object-in-place scene learning task or in delayed nonmatching-to-sample (DNMS). Each monkey received either selective cholinergic depletion of inferotemporal cortex (including the entorhinal cortex and perirhinal cortex) with injections of the immunotoxin ME20.4-saporin or saline injections as a control and was postoperatively retested. Cholinergic depletion of inferotemporal cortex was without effect on either task. Each monkey then received fornix transection because previous studies have shown that multiple disconnections of temporal cortex can produce synergistic impairments in memory. Fornix transection mildly impaired scene learning in monkeys that had received saline injections but severely impaired scene learning in monkeys that had received cholinergic lesions of inferotemporal cortex. This synergistic effect was not seen in monkeys performing DNMS. These findings confirm a synergistic interaction in a macaque monkey model of episodic memory between connections carried by the fornix and cholinergic input to the inferotemporal cortex. They support the notion that the mnemonic functions tapped by scene learning and DNMS have dissociable neural substrates. Finally, cholinergic depletion of inferotemporal cortex, in this study, appears insufficient to impair memory functions dependent on an intact inferotemporal cortex.
Keywords: acetylcholine, hippocampus, macaque, monkey, temporal lobe
Introduction
In primates, basal forebrain cholinergic projections innervate the entire cerebral cortex (Mesulam and Van Hoesen 1976). Cholinergic projections from the basal forebrain to cortical structures have been proposed to play an important role in some aspects of memory function (Aigner et al. 1991; Voytko et al. 1994; Tang et al. 1997; Baxter et al. 1999; Baxter and Chiba 1999; Easton et al. 2002; Janisiewicz et al. 2004; McGaughy et al. 2005). The most direct test of this hypothesis to date in primates is in an experiment in which selective lesions of the cholinergic afferents to part of the temporal cortex in macaque monkeys, the rhinal cortex, made with local cortical injections of cholinergic immunotoxin severely impaired delayed nonmatching-to-sample (DNMS), an object recognition memory task (Turchi et al. 2005).
Our aim in the present experiments was to test directly the generality of the effects of selective cholinergic depletion of inferotemporal cortex on learning and memory in monkeys. We used 2 tasks that have been studied extensively in primates, object-in-place scene learning, and DNMS. We have proposed that object-in-place scene learning represents a macaque monkey model of episodic memory because learning of new scene problems occurs very rapidly (mostly within a single trial) and because this rapid learning is the result of the discrimination problems occurring with the unique contexts of each individual scene (Gaffan 1994, 1998; Baxter et al. 2007). In the first experiment, we trained macaque monkeys preoperatively on object-in-place scene learning and then compared pre- and postoperative learning of new scene problems in monkeys that received either bilateral cholinergic depletion of inferotemporal cortex with ME20.4-saporin or saline injections into the same cortical region as a control procedure. In a second experiment, we used a similar experimental design to examine the effect of identical cholinergic depletions of inferotemporal cortex on performance of DNMS in monkeys trained preoperatively to perform DNMS with lists of 8 sample objects.
A second goal of the present study was to test for synergistic interactions between the effect of cholinergic depletion and the effect of bilateral fornix transection (Kim and Baxter 2001; Aggleton et al. 2005; Saunders and Aggleton 2007). Object-in-place scene learning was very severely impaired when fornix transection was combined with a disconnection of temporal cortex by sectioning the anterior temporal stem and amygdala (Gaffan et al. 2001). This impairment was synergistic because it was much more severe than that would be expected simply from an addition of the effect of fornix transection to the effect of section of the anterior temporal stem and amygdala. However, the nature of these synergistic impairments is unclear because those disconnections of temporal cortex were not selective for particular afferent or efferent pathways. Therefore, in the second stage of each experiment, having tested the effect of selectively destroying the cholinergic afferents to inferotemporal cortex, we performed a fornix transection in each monkey and reassessed scene learning and DNMS performance postoperatively.
Experiment 1: Object-In-Place Scene Learning
Materials and Methods
Subjects
All experiments were conducted in accordance with the UK Home Office regulations. In Experiment 1 (Object-in-place scene learning), 6 male macaque monkeys, 3 rhesus (Macaca mulatta), and 3 cynomolgus (Macaca fascicularis) of mean weight 7.3 kg at the start of training were used. Monkeys CON1, CON3, and ACh3 were rhesus, and monkeys CON2, ACh1, and ACh2 were cynomolgus. Two of the cynomolgus monkeys (control monkey CON2 and ACh1) had been control subjects in other visual learning experiments, and the remaining monkeys were naive. Monkeys were housed in social groups or singly in large enclosures with water provided ad libitum. Monkeys were preoperatively trained in object-in-place scene learning (see below). Each monkey was operated on twice, and each completed 10-day performance tests preoperatively and following each surgery. Each monkey's first neurosurgical procedure consisted of multiple injections of either sterile saline (controls) or ME20.4-saporin (cholinergic lesion) into the inferior temporal cortex bilaterally. Following recovery and behavioral testing, each monkey underwent a second procedure that was a bilateral transection of the fornix. Monkeys then completed the final performance test by which time the control monkeys had received saline injections in inferotemporal cortex combined with a fornix transection (identified throughout as “CON”), and the cholinergic lesioned monkeys had received saporin injections combined with a fornix transection (identified throughout as “ACh”).
Surgery
Neurosurgical procedures were performed in a dedicated operating theater under aseptic conditions. Steroids (methylprednisolone, 20 mg/kg) were given the night before surgery intramuscularly (i.m.), and 3 doses were given 4–6 h apart (intravenously [i.v.] or i.m.) on the day of surgery to protect against intraoperative edema and postoperative inflammation. The monkey was sedated on the morning of surgery with both ketamine (10 mg/kg) and xylazine (0.25–0.5 mg/kg) i.m. Once sedated, the monkey was given atropine (0.05 mg/kg) to reduce secretions, antibiotic (amoxicillin, 8.75 mg/kg) for prophylaxis of infection, opioid (buprenorphine 0.01 mg/kg, i.v., repeated twice at 4- to 6-h intervals on the day of surgery, i.v. or i.m.) and nonsteroidal anti-inflammatory (meloxicam, 0.2 mg/kg, i.v.) agents for analgesia, and an H2 receptor antagonist (ranitidine, 1 mg/kg, i.v.) to protect against gastric ulceration as a side effect of the combination of steroid and nonsteroidal anti-inflammatory treatment. The head was shaved and an intravenous cannula put in place for intraoperative delivery of fluids (warmed sterile saline drip, 5 mL/h/kg). The monkey was moved into the operating theater, intubated, placed on sevoflurane anesthesia (1–4%, to effect, in 100% oxygen), and then mechanically ventilated. A hot air blower (Bair Hugger) allowed maintenance of normal body temperature during surgery. Heart rate, oxygen saturation of hemoglobin, mean arterial blood pressure, end tidal CO2, body temperature, and respiration rate were monitored continuously throughout surgery.
In each surgery, the monkey was placed in a head holder and the head cleaned with alternating antimicrobial scrub and alcohol and draped to allow a midline or coronal incision. The skin and underlying galea were opened in layers. For the inferotemporal injections, the zygoma were removed to improve access to the temporal lobe, and the temporal muscles were retracted as necessary to expose the skull surface over the temporal lobe. A bone flap was turned over the site on both sides, and each craniotomy was extended ventrally with rongeurs as necessary. The dura was cut and reflected over the intended area of injections. When the injections were complete, the dura was sewn over the lesion site, the bone flap replaced and held with loose sutures, and the skin and galea were closed in layers. The monkey was removed from the head holder and anesthesia discontinued. The monkey was extubated when a swallowing reflex was observed, returned to the home cage, and monitored continuously until normal posture was regained (usually within 10 min). Nonsteroidal anti-inflammatory analgesic (meloxicam, 0.2 mg/kg, oral) and antibiotic (amoxicillin, 8.75 mg/kg, oral) treatment continued following surgery in consultation with veterinary staff, typically for 5 days. Operated monkeys that were socially housed rejoined their social groups as soon as practicable after surgery, usually within 3 days of the operation.
The injections were intended to infuse immunotoxin (or saline) into the entire inferotemporal cortex, including the surfaces of the inferior temporal gyrus and middle temporal gyrus (MTG) and the cortex in both banks of the anterior middle temporal sulcus (AMTS) and rhinal sulcus. Most injections were placed by exposing the lateral surface of the temporal lobe and gently retracting it from the base of the skull. Injections began ventral to the ventral lip of the superior temporal sulcus and were made in sequence proceeding from posterior to anterior, about 3 mm apart. One microliter of ME20.4-saporin (0.02 μg/μL; Advanced Targeting Systems, San Diego, CA) in cholinergic lesioned monkeys or 1 μL of sterile phosphate-buffered saline (PBS) in control monkeys was injected at each site, and the needle was held in place for several seconds before moving to the next site. Additional rows of injections were placed below the first, approximately 3 mm more ventrally each time. Once the entire extent of inferotemporal cortex that was accessible through this approach was injected, additional injections into the rostral entorhinal and perirhinal cortices were made through a subfrontal approach. Each monkey received between 56 and 64 injections in each hemisphere.
The fornix transections were made by aspiration with the aid of an operating microscope. A D-shaped bone flap was raised over the midline and one hemisphere. The dura mater was cut to expose the hemisphere up to the midline. Veins draining into the sagittal sinus were cauterized and cut. The hemisphere was retracted from the falx with a brain spoon. A glass aspirator was used to make a sagittal incision no more than 5 mm in length in the corpus callosum at the level of the interventricular foramen. The fornix was sectioned transversely by electrocautery and aspiration with a 20-gage metal aspirator, which was insulated to the tip. The dura mater was drawn back but not sewn, the bone flap replaced, and overlying tissue closed in layers.
Histology
At the completion of all behavioral testing, subjects were deeply anesthetized with intravenous barbiturate and transcardially perfused with physiological saline followed by 4% paraformaldehyde. The brains were extracted and cut on a freezing microtome in 50-μm sections in the coronal plane. A 1 in 10 series of sections was taken for cresyl violet staining using standard methods. In addition, a further 1 in 10 series of sections was taken for acetylcholinesterase (AChE) detection to assess the effectiveness of the immunotoxin. The protocol for AChE staining was as follows. Sections were collected directly into an incubation medium (680 mg sodium acetate, 100 mg copper sulfate, 120 mg glycine, 116 mg acetylthiocholine iodide, and 3 mg ethopropazine per 100 mL deionized water) and left overnight at 4 °C. Sections were rinsed 3 times in PBS and transferred to a sodium sulfide developing medium for 2 min (2 g sodium sulfide per 200 mL 0.1 M acetic acid). Sections were rinsed again 3 times in PBS before being mounted on gelatin-coated slides and air-dried over several days. Silver nitrate intensification proceeded with sections being dipped in aqueous silver nitrate for 7 s, rinsed in 0.1 M Tris buffer (pH 7.4), and left in 5% sodium thiosulfate for 2 min. Sections were then dehydrated in ascending alcohols and placed in Histoclear before being coverslipped.
Parvalbumin-, 5-hydroxytryptamine transporter (5HTT)–, and tyrosine hydroxylase (TH)–immunostained sections were, prior to processing, cryoprotected in 30% sucrose and phosphate buffer and stored at –80 °C. Sections were incubated in the appropriate primary antibody (parvalbumin:rabbit anti-parvalbumin, 1:15 000; Swant, Bellinzona, Switzerland; 5HTT:rabbit anti-5HTT, 1:12 000; Immunostar, Hudson, WI; TH:mouse anti-TH, 1:1000; Immunostar) in blocking serum for 2 days at 4 °C and developed with standard avidin–biotin complex methods using biotinylated secondary antibodies and diaminobenzidine with or without nickel intensification.
Cholinergic depletion of inferotemporal cortex was quantified by a fiber-counting method adapted from Mesulam (Geula and Mesulam 1989). Regions of interest were entorhinal cortex, perirhinal cortex, AMTS, and MTG and a control region of the superior temporal gyrus (STG) (see below). Either 3 (for entorhinal, perirhinal, and AMTS) or 5 (for MTG and STG) 10× images per region in each hemisphere were captured at 1280 × 1024 resolution. A counting grid was superimposed on binarized images using ImageJ software (National Institutes of Health download from http://rsbweb.nih.gov/ij/) and the number of crossings counted. To control for intrasubject differences in intensity of AChE staining (because it was not possible to process sections from all monkeys contemporaneously), mean crossings for each region counted were analyzed as a ratio to counts from the STG, which was not included in the lesion. The placement of regions analyzed were as follows: the inferior part of the STG, the superior part of the MTG, the medial bank of the AMTS, the lateral bank of the rhinal sulcus (perirhinal cortex), and the crown of the medial bank of the rhinal sulcus (entorhinal cortex). There was no significant effect of lesion on raw counts in the STG, F1,4 = 3.30, P = 0.14, validating the use of this area as a baseline in Experiment 1.
Object-In-Place Scene Learning: Stimulus Material and Task Procedure
Each trial consisted of an artificially constructed scene that occupied the whole area of the display screen. Foreground objects appeared within the scene and were 2 small randomly selected and colored typographic characters each placed in a constant location in the scene. The backgrounds were generated using an algorithm which drew a random number (between 2 and 7) of randomly located ellipses and ellipse segments of random color, size, and orientation on a randomly colored initial background and then drawing a single very large randomly selected typographic character in a random color somewhere in the scene. In each scene, 1 of the 2 foreground objects was the correct one for the monkey to touch and the other was incorrect. Figure 1 shows examples of 2 object-in-place scene problems.
Figure 1.
Examples of 2 object-in-place problems. The “foreground objects” are the small alphanumeric characters; “c” and “w” in the left panel and “T” and “6” in the right panel. Monkeys learnt which foreground object produced reward and learnt 20 new problems each session.
In each daily session, a list of new scenes was presented for at least 8 successive blocks within the session. The order of presentation of the scenes was the same in each of the 8 blocks. On each trial, the display remained on the screen until the monkey touched the screen. If the correct foreground object was touched, the object flashed on and off for a period of 2400 ms, whereas the background scene remained. The screen then blanked and simultaneously 1 reward pellet was dispensed into the food hopper. An intertrial interval (ITI) of 5 s then began, ending with the presentation of the next trial in the session. If the incorrect foreground object was touched, the screen blanked and an ITI of 20 s began. In blocks 2–8 in each session, choice of the incorrect object was followed by the next scheduled trial in the session. A correction trial was presented after any error in the first block of trials in each session, that is, the first run through the list of scenes. A correction trial consisted of the re-presentation of the same scene with only the correct foreground object in it. When this object was touched, the flashing and food followed as in the main trials of the task. Touching the screen during the ITI reset the interval. Touching any part of a displayed scene that was not one of the foreground objects in that scene resulted in screen blanking, an ITI of 20 s, and the presentation again of the same scene. These “inaccurate” touches were recorded separately from errors (i.e., choices of the incorrect object). For the final scene in the session, the food reward was a large food reward, and for this trial, a correction trial was presented if an error was made, thus ensuring that the large food reward would always be obtained. Within each day, the monkey's performance began at chance in the first block, when the monkey had no information as to which was the correct object to choose, and improved over the subsequent blocks. Thus, the proficiency of within-day learning was expressed by the average percent error in blocks 2–8.
Monkeys were trained to a stable level of within-day learning before the preoperative performance test. For this and all subsequent performance tests, monkeys CON1 and ACh3 completed lists of 20 scenes; monkeys CON2, CON3, and ACh2 completed lists of 10 scenes; and ACh1 completed lists of 25 scenes. Each monkey was then assigned to either the control group (to receive saline injections) or the experimental group (to receive saporin injections) equating for preoperative learning ability (in addition, training history was equated by ensuring that neither of the 2 sophisticated monkeys [see Subjects, above] were assigned to the same group). At each performance test, each monkey completed 13 sessions, the final 10 constituting the data set for that performance test. After each surgery, monkeys had between 12 and 15 days recovery before beginning each performance test.
Results
Figures 2 and 3 show examples of cresyl violet– and AChE-stained sections from monkey CON2 and monkey ACh2. In all monkeys who had received injections of immunotoxin, there was a marked loss of AChE-positive fibers throughout the intended area of depletion within inferior temporal cortex, and this could be seen clearly particularly at high magnifications (Fig. 2, sections D and L [control] and sections G and O [cholinergic]). The toxin was therefore effective in depleting acetylcholine from the cortex. In monkeys ACh1 and ACh3, slight sparing of cholinergic terminals could be seen in the fundus of the rhinal sulcus and in monkey ACh2 in the fundus of the AMTS (Fig. 3, left hemisphere, asterisk). In comparison to the control monkeys, the overall depletion was striking throughout the inferior temporal cortex. Sections stained for cresyl violet displayed no gross cell abnormalities, and this is illustrated in adjacent sections in Figure 2 at identical magnifications. The fornix transections were complete in all cases (Fig. 3, CON2 and ACh2). A loss of cholinergic fibers could also been seen in the entorhinal cortex of control monkeys as a result of the fornix transection.
Figure 2.
Examples of low- and high-magnification cresyl violet– and AChE-stained sections from monkey CON2, who received injections of saline into inferior temporal cortex and from monkey ACh2, who received injections of saporin. Photographs (A–D) and (I–L) show the perirhinal cortex and MTG, respectively, of control monkey CON2. Photographs (E–H) and (M–P) show the perirhinal cortex and MTG, respectively, of cholinergic lesioned monkey ACh2. In all monkeys, the toxin was effective, evidenced by the loss of AChE-postive fibers, and this is clearly seen at higher magnifications (sections G and O). Cresyl violet sections, adjacent to those processed for AChE, showed no gross abnormalities.
Figure 3.
Fornix transection and cholinergic depletion in monkey CON2, who received injections of saline into inferior temporal cortex, and monkey ACh2, who received injections of ME20.4-saporin. Complete transection of the fornix is shown in both cases. Coronal sections from monkey ACh2 show cholinergic depletion bilaterally within inferotemporal cortex, highlighted in the left hemisphere by arrows, compared with sections at similar anterior posterior levels in monkey CON2. sts, superior temporal sulcus; amts, anterior middle temporal sulcus; rs, rhinal sulcus; ots, occipitotemporal sulcus.
ME20.4-saporin injections produced dramatic decreases in AChE-positive fibers in all areas of inferotemporal cortex examined. Because some cholinergic afferents to the entorhinal cortex travel via the fornix and would have been removed by the fornix transection, we are unable to determine the relative contributions of fornix transection and ME20.4-saporin injections to loss of cholinergic fibers in this area. We first analyzed fiber-crossing counts in entorhinal cortex, perirhinal cortex, AMTS, and MTG (normalized to counts in STG) and found a significant main effect of lesion group, F1,4 = 80.28, P < 0.001, region, F3,12 = 15.65, P < 0.001, and an interaction of these factors, F3,12 = 10.84, P = 0.006. Thus, there was an overall difference in counts between the control and experimental groups, which varied across regions. There was a mean 46.9% loss of fibers in the entorhinal cortex, 64.4% loss in the perirhinal cortex, 81.3% loss in the AMTS, and 88.3% loss in the MTG. With the exception of the entorhinal cortex, each of these losses was statistically significant in follow-up 1-way analyses of variance (ANOVAs), F1,4 values > 54.5, P values < 0.002; entorhinal cortex on its own differed at trend level, F1,4 = 6.93, P = 0.058.
To demonstrate the selectivity of the cholinergic depletion, immunohistochemistry for parvalbumin (γ-aminobutyric acidergic [GABAergic] cortical interneurons), TH (dopamine and noradrenaline fibers), and serotonin transporter (serotonin fibers) within inferotemporal cortex of some cryoprotected sections from some of the cases in this experiment is shown in Figure 4. These high-power photomicrographs are taken from the MTG where the degree of cholinergic depletion was most extensive. There was no evidence for depletion of any of these neuromodulators, and the appearance and distribution of GABAergic interneurons is normal. This is consistent with other data from our laboratory analyzing neurochemical effects of ME20.4-saporin injections into other cortical regions, as well as a previous investigation in rhesus monkeys using this technique (Turchi et al. 2005).
Figure 4.
Neurochemical specificity of ME20.4-saporin injections. (a) Parvalbumin-stained section through the left temporal lobe of case CON2. The small box in the MTG shows the approximate location of the higher power images (b–g). This region was chosen for illustration because it is the area of maximal cholinergic depletion. (b, c) Parvalbumin immunohistochemistry in cases ACh2 (b) and CON2 (c) shows intact parvalbumin-immunoreactive neurons in the lesion case. (d, e) TH immunohistochemistry in cases ACh2 (d) and CON2 (e) shows intact TH-immunoreactive (presumably dopaminergic and noradrenergic) fibers in the lesion case. (f, g) Serotonin transporter (5HTT) immunohistochemistry in cases ACh2 (f) and CON2 (g) shows intact 5HTT-immunoreactive (presumably serotonergic) fibers in the lesion case. The scale bar in (b) is 0.1 mm and applies to panels (b–g).
Learning rates in object-in-place learning were measured at each stage as the mean number of errors made in trials 2–8 in the final 10 sessions of each period of testing. Table 1 shows the learning rates for these sessions expressed as percent error for the monkeys in Experiment 1. Cholinergic depletion of inferior temporal cortex was without effect on scene learning, but transection of the fornix caused a larger impairment in scene learning in monkeys with cholinergic lesions of inferotemporal cortex compared with monkeys whose cholinergic innervation of inferior temporal cortex was intact. Repeated measures ANOVA on the data using “operative stage” and “trial” as within-subjects factors and “experimental group” as the between-subjects factor revealed a significant main effect of operative stage (F2,8 = 54.6, P < 0.001) that interacted with experimental group (F2,8 = 8.22, P = 0.011), indicating that learning rates differed between the control and the lesioned group over the 3 performance tests. Comparison of “Preop” and “Postop1” measures revealed no main effect of operative stage (F1,4 = 1.10, P = 0.354) and no operative stage × experimental group interaction (F < 1), showing that injections of saline or injections of saporin into inferior temporal cortex was entirely without effect on learning rates on the task. A comparison of learning rates in Postop1 and “Postop2” performance tests revealed a significant effect of operative stage (F1,4 = 61.04, P = 0.001), trial (F7,28 = 129.16, P < 0.001), and an operative stage × trial interaction (F7,28 = 18.27, P < 0.001). Thus, learning rates differed between Postop1 and Postop2 performance tests across both experimental groups. Critically, a significant operative stage × experimental group interaction (F1,4 = 11.55, P = 0.027) showed that the impairments differed between groups. Transecting the fornix in monkeys who had previously received injections of saporin into the inferior temporal cortex caused a more severe impairment in learning rates than the same procedure in monkeys who had previously received injections of saline into inferior temporal cortex.
Table 1.
Percent error in trials 2–8 of object-in-place scene learning for both experimental groups and across the 3 operative stages
| Object-in-place scene learning |
||||
| Preop | Postop1 (injections) | Postop2 (fornix) | Decrement after fornix transection | |
| CON1 | 13.07 | 12.50 | 28.49 | 15.99 |
| CON2 | 6.86 | 7.71 | 12.57 | 4.86 |
| CON3 | 7.43 | 13.57 | 22.43 | 8.86 |
| Mean | 9.12 | 11.26 | 21.16 | 9.90 |
| ACh1 | 3.26 | 2.97 | 29.20 | 26.23 |
| ACh2 | 12.71 | 16.14 | 37.14 | 21.00 |
| ACh3 | 9.50 | 8.00 | 30.07 | 22.07 |
| Mean | 8.49 | 9.04 | 32.14 | 23.10 |
Note: Data in the column Preop were obtained in the preoperative performance test. Postop1 were obtained after the first surgery that was bilateral saline injections into inferior temporal cortex for “CON1–3” and bilateral saporin injections into inferior temporal cortex for “ACh1–3.” Postop2 were obtained after a second surgery that was fornix transection for all monkeys. “Decrement after fornix transection” was calculated by subtracting Postop1 from Postop2.
Figure 5 illustrates these effects by showing learning over trials by monkeys in the control group and by monkeys with cholinergic lesions of inferior temporal cortex in the object-in-place scene task. These graphs show that learning rates after either saline or saporin injections (Postop1) are comparable to preoperative learning rates. Figure 5 also shows that the magnitude of the impairment caused by fornix transection (Postop2) on learning rates in the object-in-place task depended on whether prior surgery was to inject saline or saporin into inferior temporal cortex. The effect of fornix transection was potentiated by prior depletion of acetylcholine from inferior temporal cortex.
Figure 5.
Learning curves in object-in-place scene learning at each stage of the experiment (Preop, Postop1, and Postop2). Each curve shows the mean percent error on each trial over the 10-session performance tests. The left panel shows the learning curves from control monkeys, who received saline injections followed by fornix transection (CON1–3 in Table 1), and the right panel shows the learning curves from cholinergic lesioned monkeys, who received saporin injections followed by fornix transection (ACh1–3 in Table 1).
Experiment 2: DNMS
Materials and Methods
Subjects
In Experiment 2 (DNMS), 6 male rhesus monkeys were used. Two monkeys, CON5 and ACh4, were behaviorally sophisticated, having served as control animals in other experiments, and the remainder were naive. Monkeys were housed in social groups or singly in large enclosures with water provided ad libitum. Monkeys were preoperatively trained in DNMS (see below). As in Experiment 1, each monkey was operated on twice and completed 10-day performance tests preoperatively and following each surgery. Each monkey's first neurosurgical procedure consisted of multiple handheld injections of either saline (controls) or ME20.4-saporin (cholinergic lesion) into the inferior temporal cortex bilaterally. Following recovery and behavioral testing, each monkey underwent a second procedure, which was a bilateral transection of the fornix. Monkeys then completed the final performance test by which time the control monkeys had received saline injections in inferotemporal cortex combined with a fornix transection (identified throughout as CON), and the cholinergic lesioned monkeys had received saporin injections combined with a fornix transection (identified throughout as Ach).
Surgery
This was the same as Experiment 1.
Histology
This was the same as Experiment 1.
There was no significant effect of lesion on raw counts in the STG, F1,4 = 1.12, P = 0.35, validating the use of this area as a baseline in Experiment 2.
DNMS: Stimulus Material and Task Procedure
The stimuli used for the DNMS task were taken from a pool of 5406 clipart images 128 × 128 pixels in size. Clipart “objects” contained a maximum of 20 colors and were presented on a white background. Objects were selected at random and without replacement until all the objects in the pool had been presented once. Objects were then selected from the pool using a different random seed so that the order of presentation of objects was different between runs through the pool and thus ensuring that any particular object had an equal chance of being presented as a sample or a choice stimulus.
In the initial period of training, monkeys learnt the basic nonmatching-to-sample principle. Each trial consisted of the presentation of the sample object in the center of the touch screen, and the monkey touched it to proceed. Touching the sample caused it to disappear 500 ms later (visual feedback). After a 2-s delay, 2 choice stimuli appeared on the left and right positions on the screen (vertically centered, 200 pixels from the edge of the screen and 400 pixels apart horizontally), one which was the sample object and one which was a novel object, and the monkey was rewarded with a food pellet for touching the novel choice stimulus. After a correct response, both objects remained on the screen for 500 ms. After an incorrect response, both objects disappeared immediately. The ITI began as soon as the choice stimuli disappeared from the screen. At this initial stage of training, the ITI was 8 s following a correct response and 16 s following an unrewarded response, and this interval was reset by touches to the screen during this time. For the final trial in a session, the food reward was a large food reward, constituting almost the total daily diet for the monkey. If the monkeys chose incorrectly on this trial, another trial was presented until the monkey chose correctly, thus ensuring that the large food reward would always be obtained. Monkeys were trained to a criterion of ≥90% correct choices in a session of 100 trials. The delay was then increased to 5 s, and monkeys were trained to a criterion of ≥90% correct choices on 3 consecutive sessions of 100 trials. Monkeys reached this criterion in 45 sessions on average.
Monkeys were then trained with lists of samples in the following manner. Several samples were presented one after another while monkeys touched each object in the list to proceed to the next. The delay between sample objects was 5 s. Having worked through the list of samples, monkeys worked through the list of choices. The presentation of lists of choice stimuli was in reverse order to the list of samples such that the hypothetical alphabetical list of samples A through H would be followed by choices H versus N1, G versus N2, F versus N3, E versus N4, D versus N5, C versus N6, B versus N7, and A versus N8, where N1–N8 are always novel objects (see Table 2). As throughout the experiment, the novel choice object was rewarded. The ITI after a choice trial in sessions with lists was 10 s regardless of the outcome of the trial, and this was not reset by touches to the screen during this time. As in the initial training period, the final trial in a session led to a large food reward. In sessions with lists, if the monkey made an incorrect choice on the last trial in a session, then the next and final trial was a forced choice correction trial. This consisted of the re-presentation of the correct object that remained until the monkey touched it to obtain the large food reward.
Monkeys began with lists of 2 objects, and the list length was increased at the experimenter's discretion until monkeys were completing sessions with lists of 8 objects. This was attained in 27 sessions on average. Thereafter, monkeys completed sessions of 120 trials except monkey ACh6 who completed sessions of 80 trials. Ten s of data were taken as a preoperative performance test. Each monkey was then assigned to either the control group (to receive saline injections) or the experimental group (to receive saporin injections) equating for preoperative learning ability. At each postoperative performance test, each monkey completed 11 sessions, the first postoperative session being half as long as a regular session and the final 10 sessions constituting the performance test. The dependent measure was the mean percent correct over these 10 sessions. After each surgery, monkeys had between 12 and 15 days recovery before beginning each performance test.
Results
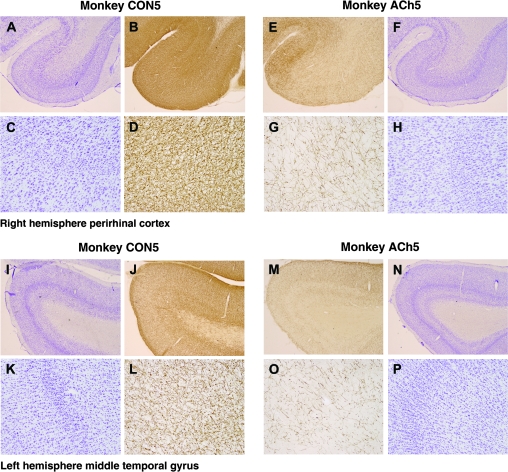
Figures 6 and 7 show examples of cresyl violet– and AChE-stained sections from monkey CON5 and monkey ACh5. Similar to Experiment 1, all monkeys who had received injections of immunotoxin showed a marked loss of AChE-positive fibers within the intended area of depletion within inferior temporal cortex. This could be seen clearly particularly at high magnifications (Fig. 6, sections D and L [control] for comparison with sections G and O [cholinergic]). The toxin was therefore effective in depleting acetylcholine from the cortex. Some minor sparing of cholinergic terminals could be seen in the fundus of the rhinal sulcus in monkey ACh5 (Fig. 7). As in Experiment 1, the overall depletion was striking throughout IT. Sections stained for cresyl violet displayed no gross cell abnormalities, and this is illustrated in adjacent sections in Figure 6 at identical magnifications. The fornix transections were complete in all cases (Fig. 7, CON5 and ACh5).
Figure 6.
Examples of low- and high-magnification cresyl violet– and AChE-stained sections from monkey CON5, who received injections of saline into inferior temporal cortex, and from monkey ACh5, who received injections of saporin. Photographs (A–D) and (I–L) show the perirhinal cortex and MTG, respectively, of control monkey CON5. Photographs (E–H) and (M–P) show the perirhinal cortex and MTG, respectively, of cholinergic lesioned monkey ACh5. In all monkeys, the toxin was effective evidenced by the loss of AChE-postive fibers, and this is clearly seen at higher magnifications (sections G and O). Cresyl violet sections, adjacent to those processed for AChE, showed no gross abnormalities.
Figure 7.
Fornix transection and cholinergic depletion in monkey CON5, who received injections of saline into inferior temporal cortex, and monkey ACh5, who received injections of ME20.4-saporin. Complete transection of the fornix is shown in both cases. Coronal sections from monkey ACh5 show cholinergic depletion bilaterally within inferotemporal cortex, highlighted in the left hemisphere by arrows, compared with sections at similar anterior posterior levels in monkey CON5. sts, superior temporal sulcus; amts, anterior middle temporal sulcus; rs, rhinal sulcus; ots, occipitotemporal sulcus.
As in Experiment 1, ME20.4-saporin injections produced dramatic decreases in AChE-positive fibers in all areas of inferotemporal cortex examined. We again analyzed fiber-crossing counts in entorhinal cortex, perirhinal cortex, AMTS, and MTG, normalized to counts in STG, and found a significant main effect of lesion group, F1,4 = 139.5, P < 0.001, region, F3,12 = 22.0, P < 0.001, and an interaction of these factors, F3,12 = 14.6, P < 0.001. Thus, there was an overall difference in counts between the control and experimental groups, which varied across regions. There was a mean 58.7% loss of fibers in the entorhinal cortex, 64.9% loss in the perirhinal cortex, 84.2% loss in the AMTS, and 87.8% loss in the MTG. Each of these losses was statistically significant in follow-up 1-way ANOVAs, F1,4 values > 37.4, P values < 0.004. To assess the comparability of the 2 experiments, the data were analyzed together in an overall ANOVA with the additional factor of experiment included. This analysis revealed expected effects of lesion, region, and their interaction as seen in the analyses from each experiment. Importantly, the experiment factor was not significant, F1,8 = 0.52, P = 0.49, nor did it interact with any of the other factors, F values < 1. Thus, the extent of the cholinergic lesions in both experimental groups was comparable. If anything, the cholinergic depletions in Experiment 2 are slightly greater than those in Experiment 1 (based on a numerical comparison).
Recognition memory was measured at each stage as the percent correct responses made at each of the 8 list positions in the 10 sessions, which constituted each period of testing. Table 3 shows the mean percent correct across all list positions for individual monkeys (CON4–6 and ACh4–6) at each stage of the experiment. Figure 8 shows these data for the control group and for the cholinergic lesioned monkeys. Repeated measures ANOVA using “stage” and “list position” as within-subject factors and “group” as the between-subjects factor revealed neither significant group differences nor significant group interactions with other factors (stage × group: F2,8 = 1.12, P = 0.351; list position × group: F7,28 = 1.67, P = 0.157; stage × list position × group: F14,56 < 1, P = 0.834). This indicates that the groups did not differ in their recognition memory at any stage of the experiment or at systematically any list positions. An overall effect of list position was found showing that monkeys’ performance was affected by where in the test list items occurred (F7,28 = 70.49, P < 0.001). However, as shown above, this effect was not different between groups of monkeys, and there was no significant 3-way interaction between list position, group, and stage. Apart from these nonsignificant findings, there was, however, an overall main effect of stage (F2,8 = 7.3, P = 0.016). Planned comparisons using the pooled error term showed that, across all monkeys, performance after saline or saporin injections was not different from preoperative performance (Preop vs. Postop1: t8 = 1.65, P > 0.05, 1 tailed) but that performance after fornix transection was significantly different from performance after injections (Postop1 vs. Postop2: t8 = 2.15, P < 0.05, 1 tailed). Fornix transection therefore produced a very mild impairment in DNMS in all monkeys, but this effect did not differ whether the monkeys had cholinergic depletion of inferotemporal cortex or not.
Table 3.
Percent correct at each list position in DNMS
| List position |
|||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean | |
| Preoperative | |||||||||
| CON4 | 97.3 | 96.0 | 92.7 | 92.7 | 86.0 | 85.3 | 82.0 | 87.3 | 89.9 |
| CON5 | 96.0 | 93.3 | 88.0 | 86.0 | 90.0 | 83.3 | 73.3 | 80.7 | 86.3 |
| CON6 | 96.0 | 95.3 | 89.3 | 90.7 | 86.0 | 91.3 | 78.0 | 84.7 | 88.9 |
| Mean | 96.4 | 94.9 | 90.0 | 89.8 | 87.3 | 86.7 | 77.8 | 84.2 | 88.4 |
| ACh4 | 100.0 | 96.0 | 94.0 | 94.7 | 89.3 | 91.3 | 86.7 | 88.0 | 92.5 |
| ACh5 | 95.3 | 93.3 | 90.7 | 92.0 | 84.7 | 87.3 | 84.0 | 82.7 | 88.8 |
| ACh6 | 97.0 | 96.0 | 96.0 | 92.0 | 86.0 | 78.0 | 80.0 | 84.0 | 88.6 |
| Mean | 97.4 | 95.1 | 93.6 | 92.9 | 86.7 | 85.6 | 83.6 | 84.9 | 90.0 |
| Postoperative 1 (injections) | |||||||||
| CON4 | 97.3 | 95.3 | 96.7 | 93.3 | 90.7 | 82.7 | 77.3 | 89.3 | 90.3 |
| CON5 | 89.3 | 86.0 | 87.3 | 87.3 | 83.3 | 80.7 | 73.3 | 72.7 | 82.5 |
| CON6 | 98.0 | 99.3 | 95.4 | 89.4 | 91.3 | 86.6 | 84.6 | 88.6 | 91.6 |
| Mean | 94.9 | 93.6 | 93.1 | 90.0 | 88.4 | 83.3 | 78.4 | 83.5 | 88.2 |
| ACh4 | 96.7 | 94.7 | 88.7 | 92.7 | 84.7 | 88.0 | 82.0 | 89.3 | 89.6 |
| ACh5 | 91.3 | 83.3 | 86.7 | 86.0 | 83.3 | 80.7 | 75.3 | 78.7 | 83.2 |
| ACh6 | 90.0 | 90.0 | 93.0 | 82.0 | 78.0 | 81.0 | 79.0 | 74.0 | 83.4 |
| Mean | 92.7 | 89.3 | 89.4 | 86.9 | 82.0 | 83.2 | 78.8 | 80.7 | 85.4 |
| Postoperative 2 (fornix) | |||||||||
| CON4 | 93.5 | 88.3 | 85.1 | 78.6 | 81.0 | 78.9 | 71.7 | 71.7 | 81.1 |
| CON5 | 95.3 | 90.7 | 89.3 | 81.3 | 81.3 | 81.3 | 75.3 | 70.7 | 83.2 |
| CON6 | 93.4 | 96.7 | 91.4 | 85.4 | 87.9 | 84.6 | 73.8 | 75.2 | 86.0 |
| Mean | 94.1 | 91.9 | 88.6 | 81.8 | 83.4 | 81.6 | 73.6 | 72.5 | 83.4 |
| ACh4 | 94.7 | 95.4 | 94.0 | 90.1 | 88.6 | 83.2 | 79.9 | 77.9 | 88.0 |
| ACh5 | 97.3 | 95.3 | 91.3 | 77.3 | 83.3 | 83.3 | 74.7 | 79.3 | 85.3 |
| ACh6 | 93.0 | 91.0 | 77.0 | 76.0 | 70.0 | 70.0 | 78.0 | 71.0 | 78.3 |
| Mean | 95.0 | 93.9 | 87.5 | 81.1 | 80.6 | 78.9 | 77.5 | 76.1 | 83.8 |
Note: Preoperative data were obtained in the preoperative performance test having acquired the nonmatching rule. “Postoperative 1” data were obtained after the first surgery that was bilateral saline injections for “CON4–6” and bilateral saporin injections for “ACh4–6.” “Postoperative 2” data were obtained after a second surgery that was fornix transection in all monkeys.
Figure 8.
Percent correct scores on DNMS by each group of monkeys after each surgical stage: Preoperative, Postop1, and Postop2. Each curve shows the mean percent correct at each list position. The left panel shows the learning curves from control monkeys (CON4–6 in Table 3) who received injections of saline followed by fornix transection. The right panel shows learning curves from cholinergic lesioned monkeys (ACh4–6 in Table 3) who received injections of saporin followed by fornix transection.
Table 2.
Structure of sample list and test list in DNMS
| List position |
||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Sample list | A | B | C | D | E | F | G | H |
| Test list | H versus N1 | G versus N2 | F versus N3 | E versus N4 | D versus N5 | C versus N6 | B versus N7 | A versus N8 |
Note: Eight samples were presented one after another, and monkeys touched each object in the sample list to proceed to the next. The delay between sample objects was 5 s. Having worked through the list of samples, monkeys worked through the test list. The choice stimuli in the test list was presented in reverse order to the list of samples such that the hypothetical alphabetical list of samples A through H, shown above, would be followed by choices H versus N1, G versus N2, F versus N3, E versus N4, D versus N5, C versus N6, B versus N7, and A versus N8, where N1–N8 are always novel objects. The delay between choice trials was always 10 s.
General Discussion
The present experiments produced 3 new findings. First, monkeys with selective cholinergic lesions of inferotemporal cortex were not impaired in scene learning or in DNMS. These findings contrast with the positive effect of cholinergic depletion of rhinal cortex on DNMS reported by Turchi et al. (2005). Additionally, these findings argue against the simple hypothesis put forward by Easton et al. (2002) that cholinergic afferents to temporal cortex are necessary for visual learning. However, cholinergic depletion of inferotemporal cortex was not behaviorally ineffective because the deleterious effect of fornix transection on scene learning was greatly potentiated by prior depletion of acetylcholine from inferior temporal cortex. This second finding shows that previous potentiated effects of fornix transection after extensive white matter disconnections of temporal cortex (Gaffan et al. 2001) are almost entirely due to the prior deafferentation of cholinergic input of temporal cortex. Third, fornix transection produced a mild impairment in DNMS in both control monkeys and in monkeys who had received cholinergic lesions of inferotemporal cortex, but the magnitude of the impairment was not increased in monkeys with cholinergic depletion of inferior temporal cortex. This finding shows that the impairments previously shown in scene learning and recognition memory in monkeys with extensive white matter disconnections of temporal cortex are dissociable.
Our results from DNMS contrast with the finding by Turchi et al. (2005) that cholinergic depletion of rhinal cortex in macaques severely impaired object recognition memory. One possible explanation of the discrepancy between these 2 findings is that our cholinergic depletions were less complete than those of Turchi et al. (2005). We are unable to definitively discount this explanation, not least because the histological analysis of the cholinergic depletions in the monkeys behaviorally tested by Turchi et al. (2005) has not yet been published. However, our cholinergic depletions were extensive, as our histological analysis showed; the lesions covered a very large area of the inferior temporal cortex and had a marked behavioral effect on scene learning in combination with fornix transection. The cholinergic depletions that had a severe effect on scene learning were of the same magnitude as those that were without effect on DNMS. It is notable that the object-in-place scene memory task is either equally sensitive to or more sensitive than DNMS to other surgical manipulations, including perirhinal cortex ablation (Meunier et al. 1993; Easton and Gaffan 2000), frontal–temporal disconnection by uncinate fascicle section (Gaffan and Eacott 1995; Browning and Gaffan 2008), and fornix transection alone (Gaffan 1994; Charles et al. 2004). Similarly, although it may be argued that preoperative training on DNMS with lists of objects may inoculate the monkeys against any lesion effects, DNMS presented in this way is devastated by ablations of rhinal cortex (Eacott et al. 1994) or of perirhinal cortex alone (P.G.F. Browning, D. Gaffan, P.L. Croxson, M.G. Baxter, unpublished observation). The memory demands of our DNMS task were comparable to those that were associated with impairment in Turchi et al. (2005) as well (monkeys in that study were substantially impaired on lists of 3 and 5 objects, making ca., 25% errors vs. less than 10% errors in controls on these conditions, estimated from their Figure 2). On the basis of these considerations, we suggest that our negative result from cholinergic depletion alone, in contrast to the strongly positive result seen by Turchi et al. (2005), is unlikely to be simply explained by the different tasks employed or by possible incompleteness of our cholinergic depletions.
Although preoperative training does not appear to, in general, protect against postoperative impairment induced by lesions, it may do so in the case of selective cholinergic depletions to the extent that the cholinergic innervation of temporal cortex may be required to adjust to new demands on information processing encountered only postoperatively. Because the monkeys tested by Turchi et al. (2005) only encountered extended delays and list lengths postoperatively, this could explain the impairment observed in their study and the intact performance in monkeys in our study. This may be related to a role for cortical cholinergic input in plasticity of sensory representations as a consequence of learning (Kilgard and Merzenich 1998; Ikonen et al. 2002; Conner et al. 2003). However, this cannot be the only factor at work in determining impaired or intact memory function following selective cholinergic lesions because preoperative training in scene learning did not protect against the synergistic effect of cholinergic inferotemporal lesions and fornix transection. But it may account for, at least partially, the different results of cholinergic temporal cortical lesions on DNMS between the 2 studies.
The combination of temporal cholinergic depletion with fornix transection produced a severe impairment in scene learning. This aspect of our present results is in agreement with Easton et al. (2002), who found a severe impairment of scene learning when inferior temporal cortex was disconnected, by crossed unilateral lesions, from both the fornix and the cholinergic basal forebrain. Easton et al. (2002) made unilateral lesions of the cholinergic basal forebrain in the same hemisphere as a unilateral fornix transection by injecting cholinergic immunotoxin directly into the basal forebrain. The method we have adopted in the present experiment, following Turchi et al. (2005), involving cortical injections of immunotoxin in preference to injections of immunotoxin directly into the cholinergic basal forebrain, is clearly superior to the method employed by Easton et al. (2002). Our experiment adds to that of Easton et al. (2002) by including an assessment of the effect of the cholinergic lesion alone, without fornix transection. The present experiment and the experiment by Easton et al. (2002) nevertheless concur in showing that a severe impairment in scene learning is produced by the combined disconnection of inferior temporal cortex, both from the fornix and from the cholinergic basal forebrain, even though the neurons of the inferior and medial temporal cortex are themselves intact.
The severe impairment we saw following the combination of cholinergic depletion with fornix transection was similar to the synergistic effect of fornix transection given after section of anterior temporal stem and amygdala in scene learning (Gaffan et al. 2001), except that the section of anterior temporal stem and amygdala without fornix transection did produce some impairment, whereas cholinergic depletion without fornix transection produced no detectable impairment at all in the present task (Fig. 5). Synergistic effects such as those reported here and those seen by Gaffan et al. (2001) could arise because adding fornix transection to another temporal cortical disconnection results in a critical threshold or extent of cholinergic depletion being exceeded throughout the medial and inferior temporal cortices. The present results depend upon a sequential lesion experimental design that makes it impossible to know the extent of cholinergic depletion after saporin injections alone, prior to fornix transection. Therefore, it is possible that the synergistic effect arises because together cholinergic depletion of inferotemporal cortex combined with fornix transection produces a more complete cholinergic depletion throughout inferotemporal cortex and rhinal cortex. Cholinergic depletion was observed in the entorhinal cortex of the monkeys with fornix transection alone, although it is unlikely that the entorhinal cortex is the key component in producing severe learning impairments because selective entorhinal ablation produces only mild impairment in scene learning (Charles et al. 2004). The above interpretation, however, cannot apply generally to visual learning because combined fornix and temporal cortical cholinergic depletion in the present experiment was without effect on DNMS, but we note that we cannot rule out a “mass action” explanation with respect to scene learning. Our findings support the observation that DNMS and scene learning have, to some extent, dissociable neural substrates.
Another interpretation of these observations is that cholinergic innervation of inferior temporal cortex is able, to some extent, to compensate for the loss of neuromodulatory influences carried by the fornix. On this view, temporal cortical acetylcholine is not necessary for scene memory when other structures are intact but becomes more critical once connections through the fornix are lost. Experiments in rats have provided some support for this viewpoint, in that lesions of basal forebrain cholinergic neurons are without effect on spatial memory on their own but can exacerbate the deleterious effects of intraseptal drug treatments that impair memory (Pang and Nocera 1999). This is also consistent with the observation that procholinergic drug treatments can rescue memory impairments caused by nonselective pharmacological inactivation of the basal forebrain (Degroot and Parent 2000) and with the view that these projections are involved in, but not necessary for, memory function (Parent and Baxter 2004).
A weakness of the current experiment is that we do not have data from cholinergic depletion of inferotemporal cortex and fornix transection performed in the opposite order: all the monkeys in the current study received fornix lesions after the lesions of cholinergic input to inferotemporal cortex. This was done mainly because the effects of selective cholinergic lesions of inferotemporal cortex were of primary interest in the current study, and performing these lesions after fornix transection would not provide any information on the effect of these depletions alone. Because the synergistic effect we saw was only present in scene learning and not in DNMS, we can be confident that it is not simply the result of, for example, 2 brain lesions that occur one after the other. Nevertheless, we must limit our conclusions about synergistic interactions to the case in which fornix damage occurs after cholinergic lesions of inferotemporal cortex. A mass action explanation, as outlined above, would be supported if this interaction remained significant when the lesions are given in the opposite order, that is, fornix transection followed by cholinergic depletion of temporal cortex. However, if this interaction was not present when the lesions were given in the opposite order, it would imply that there is something about the presence of acetylcholine in the inferotemporal cortex that mitigates the behavioral effect of fornix transection and that does not occur when the cholinergic projections to inferotemporal cortex have been removed prior to fornix transection. We are currently investigating these issues but at present we cannot rule out either explanation.
The results presently available from specific cholinergic lesions in macaque monkeys, those of Easton et al. (2002), Turchi et al. (2005), and the present experiment, clearly indicate that acetylcholine has an important role in memory in macaques. However, the involvement of acetylcholine in memory in primates may take a somewhat unexpected form: rather than playing a general role in supporting cortical memory encoding, acetylcholine may support adaptation to new demands on information processing as well as compensate for effects of lesions elsewhere in neural circuits for memory. It will be important for future experiments to investigate not only the different sensitivities of different memory tasks to cholinergic depletion alone but also the interactions of cholinergic depletion with other kinds of disconnection in producing memory impairment.
Funding
Medical Research Council (UK; Grant G9119577) to P.G.F.B. and D.G.; Wellcome Trust (Grant 071291/Z/03/Z) to M.G.B. and P.L.C.
Acknowledgments
We thank Carinne Piekema and Charles R. E Wilson for their help in training the monkeys. We also thank Zach Sulkowski for help with immunohistochemistry. Conflict of Interest: None declared.
References
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Aigner TG, Mitchell SJ, Aggleton JP, DeLong MR, Struble RG, Price DL, Wenk GL, Pettigrew KD, Mishkin M. Transient impairment of recognition memory following ibotenic-acid lesions of the basal forebrain in macaques. Exp Brain Res. 1991;86:18–26. doi: 10.1007/BF00231036. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Holland PC, Gallagher M. Impairments in conditioned stimulus processing and conditioned responding after combined selective removal of hippocampal and neocortical cholinergic input. Behav Neurosci. 1999;113:486–495. doi: 10.1037//0735-7044.113.3.486. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Orbital prefrontal cortex is required for object-in-place scene memory but not performance of a strategy implementation task. J Neurosci. 2007;27:11327–11333. doi: 10.1523/JNEUROSCI.3369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning PG, Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav Neurosci. 2008;122:477–482. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci. 2004;24:2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38:819–829. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Degroot A, Parent MB. Increasing acetylcholine levels in the hippocampus or entorhinal cortex reverses the impairing effects of septal GABA receptor activation on spontaneous alternation. Learn Mem. 2000;7:293–302. doi: 10.1101/lm.32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Easton A, Gaffan D. Comparison of perirhinal cortex ablation and crossed unilateral lesions of the medial forebrain bundle from the inferior temporal cortex in the rhesus monkey: effects on learning and retrieval. Behav Neurosci. 2000;114:1041–1057. doi: 10.1037//0735-7044.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Easton A, Ridley RM, Baker HF, Gaffan D. Unilateral lesions of the cholinergic basal forebrain and fornix in one hemisphere and inferior temporal cortex in the opposite hemisphere produce severe learning impairments in rhesus monkeys. Cereb Cortex. 2002;12:729–736. doi: 10.1093/cercor/12.7.729. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Idiothetic input into object-place configuration as the contribution to memory of the monkey and human hippocampus: a review. Exp Brain Res. 1998;123:201–209. doi: 10.1007/s002210050562. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Eacott MJ. Uncinate fascicle section leaves delayed matching-to-sample intact, with both large and small stimulus sets. Exp Brain Res. 1995;105:175–180. doi: 10.1007/BF00242192. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia. 2001;39:51–70. doi: 10.1016/s0028-3932(00)00097-x. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Cortical cholinergic fibers in aging and Alzheimer's disease: A morphometric study. Neuroscience. 1989;33:469–481. doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Janisiewicz AM, Jackson O, 3rd, Firoz EF, Baxter MG. Environment-spatial conditional learning in rats with selective lesions of medial septal cholinergic neurons. Hippocampus. 2004;14:265–273. doi: 10.1002/hipo.10175. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Baxter MG. Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends Neurosci. 2001;24:324–330. doi: 10.1016/s0166-2236(00)01818-x. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J Neurosci. 2005;25:10273–10281. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW. Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res. 1976;109:152–157. doi: 10.1016/0006-8993(76)90385-1. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KC, Nocera R. Interactions between 192-IgG saporin and intraseptal cholinergic and GABAergic drugs: role of cholinergic medial septal neurons in spatial working memory. Behav Neurosci. 1999;113:265–275. doi: 10.1037//0735-7044.113.2.265. [DOI] [PubMed] [Google Scholar]
- Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17:396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- Tang Y, Mishkin M, Aigner TG. Effects of muscarinic blockade in perirhinal cortex during visual recognition. Proc Natl Acad Sci USA. 1997;94:12667–12669. doi: 10.1073/pnas.94.23.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J, Saunders RC, Mishkin M. Effects of cholinergic deafferentation of the rhinal cortex on visual recognition memory in monkeys. Proc Natl Acad Sci USA. 2005;102:2158–2161. doi: 10.1073/pnas.0409708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]