Abstract
Cognitive control is an inherently multivariate phenomenon, and its neural basis is currently unclear. Here we examined using functional magnetic resonance imaging how participants retrieve prelearnt information from memory, use this information to guide responses for an impending decision, and adjust their responses based on outcome feedback. We developed a behavioral task designed to manipulate memory outcome–retrieval load, outcome-anticipation interval, and outcome-feedback processes. This allowed us to understand the neural basis of these cognitive processes in isolation and how they interact. Extending previous work, we found a retrieval-load by outcome-feedback interaction in the left globus pallidus; an outcome-feedback by anticipation-interval interaction in the inferior prefrontal cortex; a retrieval-load by anticipation-interval interaction in the midcingulate gyrus and a load by interval by outcome interaction in the right frontal pole. These results further advance our knowledge of how fundamental cognitive processes interact physiologically to give rise to higher-level behavioral control.
Keywords: feedback, magnetic resonance imaging, memory, motivation, reaction time
Introduction
The term cognitive control has been broadly defined as the ability to coordinate thoughts and actions in order to attain internal goals (Koechlin et al. 2003). This general definition can be broken down into a number of different subprocesses, though at present there is no agreement on what these subprocesses should be (Driver et al. 2007).
In typical cognitive control tasks, the experimenter is interested in the effects of different task factors on the dependent variables of performance accuracy and reaction time (RT). Usually the participant is cued with a preparatory signal to get ready to make a particular response. Following a fixed period of time (known as the foreperiod or preparatory interval) the onset of a warning signal cues the participant to initiate the selected response (the RT response). In the present study we aimed to extend this basic approach by examining the neural basis of performance of a novel cognitive control task that involves pretraining participants to retrieve from memory information (knowledge), when given certain cues that are then used to determine what response to make when given a warning signal. In general this paradigm allows examination of the neural basis of how participants are able to retrieve prelearnt information regarding what outcome to expect from memory, use this information to guide when in time an impending decision needs to be made, and adjust their responses on the basis of feedback that provides outcome information. This work extends ideas that the ability to predict the future rests on the same neural systems as that to retrieve past knowledge (Schacter et al. 2007). It furthermore provides the neural mechanisms for the test-operate-test-exit (TOTE) mechanism first proposed by (Miller et al. 1960).
Therefore, we are interested in the role of 3 cognitive control variables, namely memory retrieval load, interval preparation duration, and response to outcome feedback information. This work is important, as cognitive control is an inherently multivariate phenomenon with multiple neural systems interacting and not independent of one another. Understanding the nature of the neural systems associated with, and mediating the interaction between, different cognitive control processes will likely lead to a better understanding of the neural mechanisms associated with cognitive dysfunction observed in many neurological and psychiatric disorders.
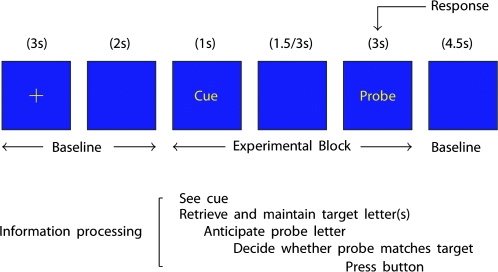
The task we developed to test 3 processes involved participants, prior to scanning learning cue–outcome relationships between letters (see Fig. 1). Following a particular cue letter, participants then had to retrieve either: 1, 3, or 5 letters from memory (outcome-retrieval factor). Participants then had either a 1.5- or 3-s preparatory interval to retrieve and maintain these target letters, used to anticipate what outcome was expected (anticipation-interval factor). Participants were then shown a probe letter and, given this feedback, were required to make a decision on whether the probe letter matched one of the target letters held in memory (outcome-feedback factor). Thus, using this factorial design we were able to examine the main effects and interactions of retrieval-load, anticipation-interval and outcome-feedback processes.
Figure 1.
Task procedure for a single trial. Participants were pretrained to associate cue letters with subsequently presented target letters. The number of target letters to be retrieved was either 1, 3, or 5 letters. In addition the interval between cue and probe was either 1.5 or 3 s.
As further background, this paradigm is a modification of other classic cognitive paradigms, such as the Sternberg working memory paradigms (Sternberg 1966), delayed match to sample (DMTS) paradigms (Elliott and Dolan 1999; Habeck et al. 2005), Stroop tasks (MacLeod 1991) and simple and choice RT tasks (Bertelson and Boons 1960; Niemi and Naatanen 1981). Both Sternberg and DMTS tasks have an encoding, maintenance and retrieval phase with the maintenance interval varied on DMTS tasks. The present study differs from these tasks as we eliminated the encoding phase. The task is similar to Stroop type tasks as participants are required to suppress an automatic, but inappropriate response on trials when probe letter feedback differs from that expected. Finally the task shares components to classic “variable foreperiod RT tasks” due to the variable anticipation interval employed. However, the novel contribution of the present work is that it allows multiple cognitive processes to be investigated concurrently in the same sample, allowing the testing of interaction effects not normally examined (though see [Fan et al. 2002] for a related approach).
This wealth of prior literature, both from behavioral and neuroimaging domains enables clear hypotheses to be drawn for each main effect. In line with previous studies investigating memory retrieval, we hypothesized an increase in RT with increasing memory load (Sternberg, 1966) and widespread increases in cortical and subcortical activity with increasing memory load (Braver et al. 1997; Rypma et al. 1999; Owen et al. 2005; Mansouri et al. 2007).
Regarding the interval hypothesis, previous work examining the foreperiod effect has shown that RT decreases as a function of the foreperiod length (Niemi and Naatanen 1981), however this only occurs under variable foreperiods. It is thought that this is due to participants building up an expectancy of when the respond/probe cue will occur. This is thought to occur through a trace conditioning process (Los et al. 2001; Steinborn et al. 2008). By varying the foreperiod we vary the conditional probability of the probe (respond stimulus), thus soon after the initial presentation of the cue there is high “time” uncertainty about the timing of the probe and this uncertainty decreases as a function of time. Previous work has implicated the basal ganglia in working with cortical regions to generate an internal timing mechanism (Buhusi and Meck 2005). Important experimental work has shown that dopamine manipulations can disrupt this interval timing mechanism (MacDonald and Meck 2004). Using lesions and virtual lesions with transcranial magnetic stimulation (TMS), previous studies have found a region of the right prefrontal cortex to underlie the foreperiod effect (Stuss et al. 2005; Vallesi, Mussoni, et al. 2007; Vallesi, Shallice, et al. 2007, 2009).
For the third experimental factor of processing outcomes we predict an adverse effect of RT following negative (expectation mismatch) feedback, as participants need to adjust their response mapping. Previous work has shown that the anterior cingulate/medial prefrontal cortex reduces in activity following nonmatch versus match feedback (McIntosh et al. 1996; Kronhaus and Willshaw 2006; Summerfield and Koechlin 2008). Other work examine RT responses following negative feedback has found the lateral prefrontal cortex increases in activity as participants need to adjust their response following negative feedback (Kerns et al. 2004; Mansouri et al. 2007).
The neural basis of how the 3 factors interact is currently unknown, however. The aim of this study was therefore to evaluate the neural basis of performance of a novel cognitive control task that could be employed in subsequent studies of clinical populations. This paradigm allows examination of the neural basis of each of the distinct psychological processes involved in the task, in addition to examination of the neural basis of the interaction between these different processes.
Materials and Methods
Participants
Informed consent was obtained from all participants. The Institutional Review Board at the University of Pittsburgh approved the study protocol. 20 right-handed participants were included in the study (10 females, mean age = 25 ± 5.2 years old). Exclusion criteria were: current or past history of psychiatric illness as determined by the structured clinical interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 1997); no history of psychiatric history in first degree relatives; current of past history of drug or alcohol abuse as determined by the SCID; pregnancy in female subjects as determined by urine sample for pregnancy test; North American Adult Reading Test (Uttl 2002) score >85; the presence of metallic foreign objects in the body, such as aneurysm clips or pacemakers or a questionable history of metallic fragments. Participants were paid $40 compensation for their time.
Procedure
An initial screening session took place 2–3 days prior to the scanning session.
Following the initial screening interview, participants completed a 10-min training session to prepare them for the fMRI scan. During training participants completed short runs until they were completely familiar with task to a criterion of 100% accuracy.
The task involved associating cue letters with a subsequently presented probe letters (Fig. 1). All stimuli were presented in yellow on a blue background. On every trial participants viewed a fixation cross for 3 s and then a blank screen (2 s). Participants then viewed a cue letter (1 s) followed by an interval of either 1.5 or 3 s. Participants then viewed a probe letter (3 s) where they were make a response and then a blank screen (4.5 s). Participants were trained to associate cue letters with subsequently presented target letters. These associations were A = K, B = GLV, C = JMQTW. Thus during the presentation of the cue letter, participants had to retrieve from memory the associated target letters and maintain them until they saw probe letter. Following the presentation of the probe letter participants had to respond and say whether the probe letter matched one of the target letters held in memory. In addition, X trials were used as an additional control condition, whereby participants were presented with an X both as a cue and always as a probe letter. During this condition, participants knew with 100% certainty what response to make following the cue. For 5 min, participants completed the task with a prompt sheet displayed next to the computer screen. This was to ease the initial cognitive demand placed on participants and to get them used to pressing the buttons following the probe letter. For the last 5 min, participants completed the task without the prompt sheet, to help them understand what performing the task will be like under test conditions. Following the initial training, participants were given the prompt sheet and asked to practice, in the days between training and testing, the letter associations up to a level where they are able to retrieve automatically the target letters following the cue. All participants were able to do this with ease when assessed prior to the scan. The task consisted on 3 experimental factors examining the effects of memory load (1, 3, and 5 letters), cue-probe letter interval (1.5 vs. 3 s) and outcome (match vs. no-match). There were 7 trial types per condition. This consisted of [7 × delay (2) × load (3) × outcome (2)] 84 trials. In addition, there were 7 × trials per delay, serving as an additional control condition, giving a total of 112 experimental trials. The length of each trial was on average 15.75 s. This gave a total length of experiment of 1764 s (24 min 24 s).
Data Analysis
Behavioral Data Analysis
The behavioral data were analyzed in a 2 × 3 × 2 factor repeated-measures design with either accuracy or RT as the dependent variables. Harmonic means of RTs were calculated within subjects for each condition as harmonic means are a better measure of central tendency (Ratcliff 1993).
fMRI Acquisition and Analysis
Neuroimaging data was collected using a 3.0 Tesla Siemens Allegra MRI scanner at the MRI Research Centre, Presbyterian Hospital, Pittsburgh, PA. For all scans, the subject was positioned comfortably in a supine orientation with their head located in a head RF coil that is electrically isolated from the subject. The head was stabilized with foam pads to minimize head motion. Participants wore sound-insulating earplugs to reduce discomfort associated with scanner noise. Participants viewed a screen, via an angled adjustable mirror, on which all visual stimuli were presented using a back-projection system attached to a PC. Structural data were acquired prior to blood-oxygen-level dependent (BOLD) functional Magnetic Resonance Imaging (fMRI) scanning. BOLD functional images were acquired with a gradient echo echo-planar imaging sequence, and covered 26 coronal slices (3 mm thick, 0 mm gap, time repetition/time echo = 1500/25 ms, field of view = 24 cm, matrix = 64 × 64). Slices were tilted to cover the frontal lobe, temporal lobes and cerebellum; however we were unable to completely acquire data in the parietal and occipital lobes. One thousand one hundred and seventy-six volumes were acquired in one run. Performance measures were collected via a MR compatible button glove placed under participant's right hand. Participants were required to press either the buttons under their index/pointer finger and middle finger.
During each volume, Siemens “MoCo” software (Siemens, www.medical.siemens.com) corrected for intrascan movement retrospectively, via standard 6 body affine transformations; and the prospective acquisition correction algorithm (Thesen et al. 2000) reduced motion-induced effects on magnetization history by adjusting slice position and orientation prospectively. Data was preprocessed and analyzed using statistical parametric mapping software (SPM5; Welcome Department of Imaging Neuroscience, London, UK). The single-subject data was first corrected for differences in acquisition times between slices; realigned using the first scan as a reference and unwarped to correct for static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. They were then normalized resampled to 3 × 3 × 3 mm3 voxels, and spatially smoothed to facilitate group level statistics with a Gaussian kernel of 8-mm full-width at half-maximum. Following preprocessing, a first-level fixed effects model was constructed with trials modeled as either 5.5- or 7-s epochs with design matrix regressors for each trial type. We used this epoch design rather than an finite impulse response type event-related design due to the superior efficiency and power afforded when using the canonical hemodynamic response modeled using SPM5 (Henson, 2007). Additionally error trials and movement parameters were entered into the model as nuisance variables. This generated 12 experimental contrasts plus a contrast for the X trials (average of the 2 delays), plus nuisance regressors. A second-level random effects group analysis was conducted on the 12 t-contrasts generated in the previous single-subject analyses in a 2 × 3 × 2 repeated-measures ANOVA. We report significant activations at a threshold of P < 0.001 uncorrected and with a minimum cluster size >5 voxels. To ease readers, we focus in this main article on the significant 2- and 3-factor interactions, and we report significant main effects in supplementary material. Regions of significant activation were extracted using the MARSBAR software (Brett et al. 2002). Correlations of extracted significant brain responses with behavioral variables were conducted using SPSS software v.16 (SPSS, www.spss.com).
Results
Behavioral Results
Accuracy
On average, participants performed the task to a 97% level of accuracy. There was a main effect of anticipation-interval on accuracy (F1,19 = 6.92, P < 0.05, ηp2 = 0.27). This effect was driven by participants demonstrating lower accuracy for 1.5-s interval trials (96.1% correct) compared with 3-s trials (97.4% correct). No other significant main effects or interactions (see Supplemental Results).
Response Times
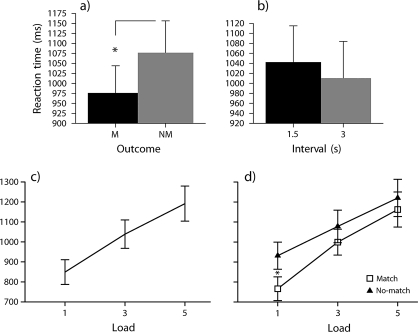
There was a significant main effect of outcome-feedback on participants’ RT, F1,19 = 11.98, P < 0.01, ηp2 = 0.39. This was due to participants’ RT being longer for nonmatch trials than match trials (Fig. 2a). There was a significant main effect of retrieval-load on participants’ RT, F1.6,29.5 = 79.92, P < 0.01, ηp2 = 0.81. This was due to participants’ RT increasing linearly with increasing load (Fig. 2c).
Figure 2.
(a) Main effect of outcome on RT (mean SEM across subjects averaged over correct responses; M = match outcome, NM = no-match outcome). (b) Main effect of anticipation interval on RT (mean SEM across subjects averaged over correct responses). (c) Main effect of load on RT (mean SEM across subjects averaged over correct responses). (d) Outcome-feedback by retrieval-load interaction, this was driven by participants being slower on no-match trials at load 1 (mean ± SEM across subjects averaged over correct responses).
The main effect of anticipation-interval was on the threshold of statistical significance, F1,19 = 4.37, P = 0.05, ηp2 = 0.19. With increasing anticipation-interval, participants’ RT decreased (Fig. 2b).
There was a significant interaction of outcome-feedback and retrieval-load, F2,38 = 3.917, P < 0.05, ηp2 = 0.17 (Fig. 2d). The outcome-feedback by retrieval-load interaction effect was analyzed using a simple main effects analysis (see Supplemental Results). This demonstrated that negative outcome-feedback results in a longer RT when one has in mind one potential outcome compared with 3 or 5 outcomes.
Neuroimaging Results
Main Effect of Outcome-Feedback
A number of regions demonstrated a significant main effect of outcome-feedback (Table S1). The 2 largest regions were in the left middle frontal gyrus (approximately BA46 [BA, Brodmann area]) and the anterior cingulate cortex (approximately BA24). Extraction of the activations in these 2 regions revealed significantly increased activation to no-match trials compared with match trials in the left dorsolateral prefrontal cortex (DLPFC) (Fig. S1) whereas in the ACC activation was decreased significantly more than on match trials (Figs S2/S3). Further correlations demonstrated that behavioral switch cost (no-match–match RT) significantly correlated with the difference in neural activation (no-match–match) in the left DLPFC (r = 0.592, P < 0.01) and ACC cluster (r = 0.474, P < 0.05).
Main Effect of Retrieval Load
A number of regions demonstrated a significant main effect of retrieval-load (Table S2). The largest cluster was located in the left DLPFC (Fig. S4). This region showed a linear increase in activation with increasing memory load. Other regions that also showed an increase in activation with increasing memory load included the cerebellum (Fig. S6), left inferior temporal gyrus (Fig. S5), dorsal anterior cingulate (dACC) gyrus and inferior frontal gyrus. There was also a significant positive correlation between RT and activation in the left DLPFC (r = .556, P < 0.05).
Main Effect of Anticipation Interval
Table S3 shows regions that demonstrated a main effect of anticipation-interval. The largest cluster was in the superior medial frontal gyrus (Figs S7/S8). This cluster showed a significant decrease in activation at the 3-s delay compared with 1.5-s delay. Additionally significant activation was found in the midbrain. Although functional localization of midbrain nuclei is difficult, the region appears to be the ventral tegmental area/substantia nigra. Activation in this region was significantly increased in this region at the 3 s compared with the 1.5-s interval (Figs S9/S10). There were no significant associations between RT and activation in these 2 regions.
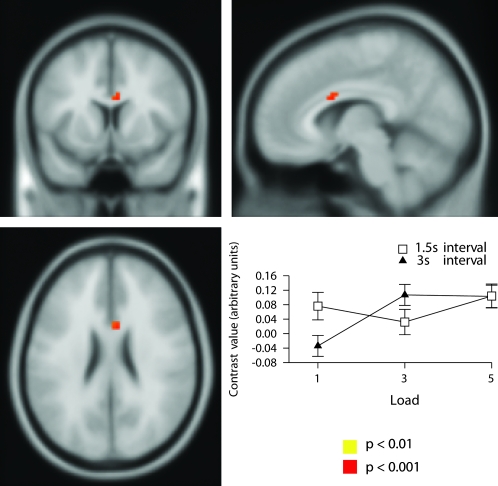
Outcome-Feedback by Retrieval-Load Interaction
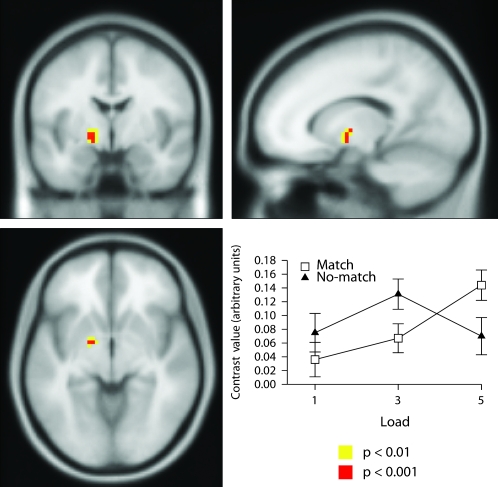
Only one region demonstrated a significant outcome-feedback by retrieval-load interaction and this was located in the left medial globus pallidus (Figs 3, S11, and Table 1). The outcome by load interaction effect was analyzed using a simple main effects analysis (see Supplemental Results). This demonstrated that activation increased with increasing load in the match condition but not in the no-match condition.
Figure 3.
Neural correlates of outcome-feedback by retrieval-load interaction in left globus pallidus; shown at P < 0.001 uncorrected (Z = 3.52, Montreal Neurological Institute (MNI) coordinates: −15, −3, −6). This interaction was due to activation increasing as a function of load in the match condition but did not in the no-match condition.
Table 1.
Brain regions demonstrating significant interaction effects (P < 0.001 uncorrected)
| Interaction | Region | Cluster size | Z score | x | y | z |
| Outcome by load | L GPi | 8 | 3.52 | −15 | −3 | −6 |
| Outcome by interval | L Inf. PFC | 62 | 3.98 | −33 | 36 | 12 |
| 3.68 | −33 | 39 | 0 | |||
| 3.48 | −30 | 24 | −3 | |||
| R Inf. PFC | 30 | 3.87 | 30 | 21 | −15 | |
| 19 | 3.67 | 36 | 39 | −9 | ||
| Load by interval | R ACC | 6 | 3.46 | 6 | 9 | 24 |
| Outcome by load | R Frontal pole | 15 | 3.75 | 18 | 57 | 9 |
| by interval | R Inf. parietal lobe | 6 | 3.45 | 36 | −24 | 30 |
Because there was a significant outcome-feedback by retrieval-load interaction in behavior, and significant main effects of outcome-feedback and retrieval-load in the left DLPFC, we expected that this region to also show a significant outcome-feedback by retrieval-load interaction. We therefore conducted a conjunction analysis (Price and Friston 1997; Nichols et al. 2005) in order to formally test that left DLPFC cluster activated in these 2 factors was the same region. This analysis demonstrated that the clusters identified in the outcome-feedback and retrieval-load main effects were the same region. In order to understand how 2 independent processes could be instantiated in one region, we derived the time-course of the activity in this region (Fig. S14). This analysis revealed outcome influenced load 1 at scans 6, 7, and 8; influenced load 3 at scans 6, 7, 8, and 9. Outcome-feedback did not influence retrieval-load 5 at any scan. This further analysis revealed that the outcome-feedback by retrieval-load interaction was present, but only when considered dynamically, thus clarifying the behavioral interaction.
Outcome-Feedback by Anticipation-Interval Interaction
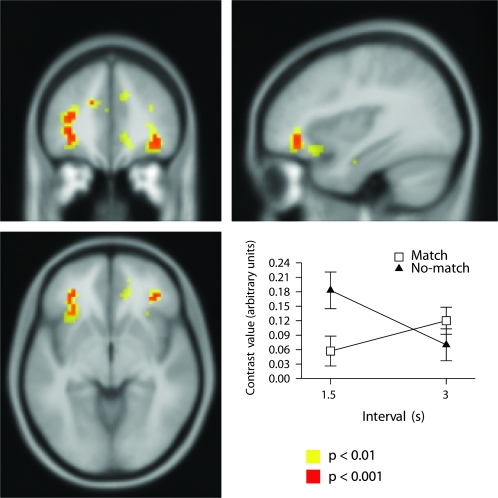
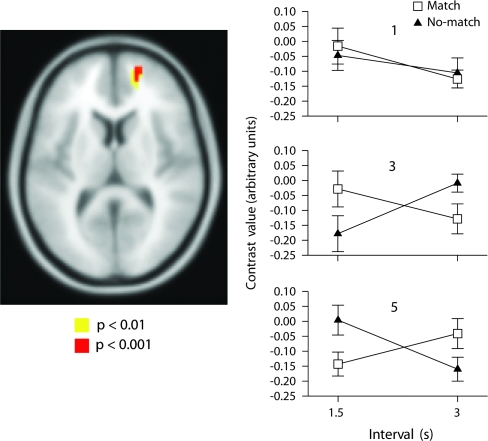
There was a significant outcome-feedback by anticipation-interval interaction bilaterally in inferior frontal gyrus (Figs 4, S12, and Table 1). The outcome-feedback by anticipation-interval interaction effect was analyzed using a simple main effects analysis (see Supplemental Results). This revealed Outcome-feedback influenced anticipation-interval in the 1.5-s interval but not the 3-s interval.
Figure 4.
Neural correlates of outcome-feedback by anticipation-interval interaction: Top left, bottom left, and bottom right: significant activation bilaterally in inferior prefrontal cortex. Top right: Plot of interaction in left inferior frontal cortex shown at P < 0.001 uncorrected (Z = 3.98, MNI coordinates: −33, 36, 12).
Retrieval-Load by Anticipation-Interval Interaction
There was one region that showed a significant retrieval-load by anticipation-interval interaction located in the midcingulate gyrus (Figs 5, S13 and Table 1). The retrieval-load by anticipation-interval interaction effect was analyzed using a simple main effects analysis (see Supplemental Results). This demonstrated that interval influenced load in the 1 load condition but not the 3 or 5 load conditions shown in Figure 5 and demonstrates that activation was increased at the 1 item load in the midcingulate gyrus more at the 1.5 s compared with 3-s interval.
Figure 5.
Neural correlates of retrieval-load by anticipation-interval interaction in midcingulate gyrus; shown at P < 0.001 uncorrected (Z = 3.46, MNI coordinates: 6, 9, 24).
Outcome-Feedback by Retrieval-Load by Anticipation-Interval Interaction
Only one region, located in the superior frontal gyrus demonstrated a significant outcome-feedback by retrieval-load by anticipation-interval interaction (Fig. 6 and Table 1). The retrieval-load by anticipation-interval interaction effect was analyzed using a simple main effects analysis (see Supplementary Results). These results demonstrated that activation was significantly higher on no-match trials after a 3 s compared with a 1.5-s interval when one retrieves 3 letters; whereas activation was significantly reduced after a 3 s compared with a 1.5-s interval when one retrieves 5 letters from memory.
Figure 6.
Neural correlates of outcome-feedback by retrieval-load by anticipation-interval interaction in frontal pole; shown at P < 0.001 uncorrected (Z = 3.75, MNI coordinates: 18, 57, 9).
Comparison of X Trials versus Fixation
As a final comparison we report activation on X-trials (vs. fixation) and on fixation (vs. X-trials). The largest activations found on X trials were in the putamen and cerebellum (Table S5 and Fig. S15). In contrast during fixation, significant activity was found in anterior and posterior cingulate cortices (Table S6 and Fig. S15).
Discussion
The aim of this study was to investigate how cognitive control, an inherently multivariate phenomenon, can be decomposed into a number of interacting subprocesses. We found evidence for these subprocesses occurring in a number of different brain regions, including the left globus pallidus, inferior prefrontal cortex, midcingulate gyrus and the frontal pole. These results help further advance our knowledge of the multivariate nature of human cognitive control processes and how fundamental cognitive processes interact neurophysiologically to give rise to higher-level behavioral control. In the following sections we discuss these results with a particular emphasis on the interaction findings.
Main Effect of Outcome-Feedback
We found a significant main effect of outcome-feedback on behavioral performance, due to RTs being longer on no-match trials compared with match trials (Fig. 2). It should be noted that the feedback is internal feedback, rather than external feedback commonly used in learning tasks. Thus participants know what the target is because they have been pretrained to form a mental model that allows one to predict target letters following different cues. This “switch cost” (Monsell 2003) is very likely due to participants adjusting their response mapping, when the outcome did not match their expectation. The fMRI data underlying this main effect, revealed 2 clusters located in the left DLPFC and bilateral pregenual ACC (although including the subgenual ACC at a lower threshold). The activity in the left DLPFC was increased on no-match trials compared with match trials (Fig. S1). In contrast the ACC activation demonstrated an opposite pattern of decreased activation on no-match trials compared with match trials (Fig. S2). Furthermore there were significant brain–behavior correlations with these 2 regions and behavioral performance. Both regions showed a positive correlation between RT switch cost and activation. This result is interesting, as a positive correlation between RT and activation would be expected for the DLPFC. However, in the ACC we also found a positive correlation, in that those who showed greater behavioral switch cost on no-match trials vs. match trials showed the smallest decrease in ACC for no-match versus match trials. An important note is that this finding of reduced ACC to negative feedback corresponds to similar findings usual matching tasks (McIntosh et al. 1996; Kronhaus and Willshaw 2006; Summerfield and Koechlin 2008) as well as social–cognitive paradigms (Somerville et al. 2006) and economic paradigms (Tomlin et al. 2006) suggesting a similar neural mechanism underlies outcome feedback processing in cognitive, emotion and social domains.
Main Effect of Retrieval-Load
We also found a main effect of retrieval load on RT and brain activation. There was a linear increase in RT with increasing load (Fig. 2). A number of brain regions exhibited this effect. The region that showed the largest effect of load was in the left DLPFC (Fig. S3). This result is consistent with a number of previous studies that have demonstrated an increase in activation in this region with increasing memory load using n-back (Braver et al. 1997; Owen et al. 2005) and Sternberg tasks (Rypma et al. 1999; Manoach et al. 2003). This region of left DLPFC also showed a significant positive correlation with RT. Our task slightly differed from the n-back and Sternberg paradigms in that it tapped purely retrieval and maintenance processes and not encoding processes.
An additional large cluster was located in the right cerebellum (Figs S4/S6). The cerebellum has also been activated during working memory performance (Kirschen et al. 2005). However the role of the cerebellum differed from that of the left DLPFC, as the cerebellum, unlike the left DLPFC, did not also show an effect of feedback. Therefore these results are consistent with forward-model theories (Wolpert and Miall 1996; Ramnani 2006) of cerebellar function, whereby the cerebellum predicts the sensory consequences of executed movement (O'Reilly et al. 2008). Additionally we found significant load-dependent activations in the left inferior temporal gyrus (Figs S4/S5), dACC and thalamus (Fig. S4). The left inferior temporal gyrus is likely to underlie holding representations of the target letters in mind (Vinckier et al. 2007). The dACC and thalamus are consistently reported in working memory and attention tasks and may reflect increases in attentional orienting, arousal and cognitive preparatory processes (Nagai et al. 2004; Marzinzik et al. 2008).
Main Effect of Anticipation-Interval
Underlying the effect of varying preparatory interval, we found a decrease in RT with increasing preparatory interval, hypothesized to be due to the certainty of the target stimulus presentation increasing with time (Niemi and Naatanen 1981; MacDonald and Meck 2004). This RT effect was likely diminished by using 2 intervals and should increase when a greater range of intervals are employed. At the neural level, we found an increase in the substantia nigra/ventral tegmental area (VTA) (Figs S9/S10) that increased in activity with delay interval and a cluster in medial prefrontal cortex (PFC) (Figs S7/S8) that decreased with delay interval. It is difficult to precisely localize activations to specific midbrain nuclei and so we are cautious in attributing the activation to either of these regions. But these results are consistent with our initial hypothesis, based on previously literature (Niemi and Naatanen 1981; MacDonald and Meck 2004) implicating the dopaminergic system. Previously activation in the substantia nigra in humans has been reported in a time reproduction task (Jahanshahi et al. 2006) and in a task comparing unpredictable and predictable timing (Dreher and Grafman 2002). Primate work has demonstrated dopamine neurons that increase their firing with increasing temporal delays (Fiorillo et al. 2008; Kobayashi and Schultz 2008).
The other activation cluster was located in the left superior medial prefrontal cortex, at approximately Brodmann area 9. Previous lesion studies have implicated the importance of the superior medial PFC in multiple attention and RT tasks (Stuss et al. 2005; Picton et al. 2006). Patients with superior medial PFC lesions consistently demonstrate RT slowing compared with other patients with lesions in other lesions of the PFC. In a recent review (Stuss and Alexander 2007), it has been proposed that lesions to the superior medial PFC results in impairment in “energization,” where this process is defined as the initiation and sustaining of any response. Further support for a role of the superior medial frontal cortex in timing and anticipation processes comes from single-cell studies in primates, where firing rates are greater for short delays compared with long delays (Roesch and Olson 2005; Genovesio et al. 2006), which is consistent with our observed BOLD responses.
It is important to note that we did not find any significant effect of preparatory interval on activation in the right DLPFC. This is in contrast to previous studies using TMS, fMRI, and lesion patients (Stuss et al. 2005; Vallesi, Mussoni, et al. 2007; Vallesi, Shallice, et al. 2007; Vallesi et al. 2009). The right DLPFC is thought to reflect temporal monitoring processes, and because of the additional memory retrieval processes and the factorial design of the task, it is likely participants were not performing any such monitoring processes.
Outcome-Feedback by Retrieval-Load Interaction
We found evidence of an outcome by load interaction in the left medial globus pallidus (GPi). We found that on match trials activity increased in this region with increasing load. However on no-match trials activity increased with loads 1 and 3 but dropped at load 5 (Figs 3/S11). This result appears unrelated to behavioral performance. The GPi, the main output structure of the basal ganglia is anatomically connected to anterior cingulate and DLPFC (Draganski et al. 2008) and so receives both load and feedback information. Lesions to this region result in feelings of apathy, emotional blunting, cognitive impairment and a more severe impairment termed an “auto-activation” deficit (Levy and Dubois 2006). The auto-activation deficit is primarily a deficit in self-activating thoughts or behavior. It is thought that lesions to different connectivity pathways between the PFC and basal ganglia affect the type of impairment (Levy and Dubois 2006). Lesions to the internal global pallidus, in particular can result in a severe form of apathy termed “athymhormia” (Habib 2004). It has been proposed that the auto-activation deficit results from a failure to reach the threshold of initiation/activation of thoughts or actions when subjects should behave on an internal basis but not in automatic response to perception (Levy and Dubois 2006).
Other evidence supports the role for this region in integrating cognitive and affective information to allow for action selection. A recent neuroimaging study found that activation in the left GPi predicted the extent that information was held in memory over a delay period (McNab and Klingberg 2008). Single-cell work has shown that GPi neurons are modulated by both motor commands and reward values, and that the tuning of these neurons changes dynamically with learning (Pasquereau et al. 2007). Recent work has shown the Gpi initiates reward-related signals through its effects on the lateral habenula, which then influences the dopaminergic and serotonergic systems (Hong and Hikosaka 2008).
Other work has also found this region activated in tasks measuring incentive motivation (Pessiglione et al. 2007; Schmidt et al. 2008) and novelty that enhances subsequent learning (Bunzeck and Duzel 2006). Indeed it has been proposed that a hippocampal novelty signal might be conveyed to the SN/VTA indirectly through the ventral striatum (nucleus accumbens) and the ventral pallidum (Lisman and Grace 2005). In addition it has been proposed following animal work that the ventral pallidum is a site for the generation of liking sensations (Berridge and Kringelbach 2008). It is unclear if this dorsal region of the pallidum is modulated by instrumental actions in the same manner to primary rewards studied in incentive motivation in the ventral pallidum.
Interestingly, deep brain stimulation of the GPi has been shown to induce brief periods of mania in humans (Miyawaki et al. 2000). In relation to the present work, we found that feedback modulated the brain activation on 5 load item trials, whereas for the other lower memory loads activation was higher in the GPi following negative feedback. This may be a marker of greater persistence following negative feedback similar to the “near-miss” effect shown in addiction-type behaviors (Kassinove and Schare 2001; Clark et al. 2009); whereas negative feedback at higher loads is associated with lower persistence. Thus this increased activation may represent a memory reinforcement mechanism to facilitate the retention of an uncertain action that produces positive feedback. This is similar to other proposals for the role of GPi in cognition (Frank and Claus, 2006).
An outcome by load by scan interaction was also found in the left DLPFC, but only when an additional “time” variable was taken into account. This is shown in Figure S14 and demonstrates that the left DLPFC processes information relating to both memory retrieval load and outcome feedback. It can be seen in Figure S6 that on “X” trials activity peaks early at scan 3. On these “X” trials participants know exactly what the probe outcome will be and therefore know exactly what response to make. Compare this to on the other trials where participants do not need to make until they receive the outcome feedback. Therefore the left DLPFC allows participants to maintain the potential responses (outcomes) until they receive the outcome feedback, and they can then select the appropriate response. It appears that if positive feedback occurs then the maintained letters are discarded. If negative feedback occurs then the letters continue to be maintained in memory. It therefore appears that the critical role of the left DLPFC is in response selection and maintenance (Rowe et al. 2000; Sakai, 2008); cognitive functions necessary for the temporal organization of behavior (Fuster 2001; Petrides 2005; Koechlin and Summerfield 2007). Furthermore this neural mechanism appears to be a correlate of the TOTE model of Miller et al. (1960), whereby the anterior cingulate carried out the test operation and the DLPFC the operate operation. It is only when there is a match between expectation and actual feedback that the exit operation can occur, and the DLPFC activity can return to baseline.
Outcome-Feedback by Anticipation-Interval Interaction
Using fMRI we were able to detect an outcome by interval interaction despite not finding one behaviorally. In this interaction we found greater activation in bilateral ventral inferior prefrontal cortex/caudalateral orbitofrontal cortex for no-match trials at the 1.5-s interval but no difference between outcome type at the 3-s interval (Figs 4/S12). This finding may be due to participants having shorter time to prepare for the upcoming target and so when a negative outcome occurs there is a bigger adjustment. This is analogous to driving a car, when one is going faster; one has less time to prepare. Therefore when one needs to move to avoid something, a bigger adjustment occurs. Lesions to this region result in impairment on reversal learning, and inhibition tasks (Iversen and Mishkin 1970; Robbins 2007), these findings are supported by fMRI studies (Cools et al. 2002; Hampshire and Owen 2006). Other studies have found activation in this region on tasks of involving negative feedback (Elliott et al. 2000; Lawrence et al. 2008; O'Doherty et al. 2001; Wolf et al. 2006).
Our results show that this region is not activated by negative feedback per se but rather only when it occurs in the context of fast pace. This is in agreement with a recent study that demonstrated that inferior lateral PFC activation was due to the steadiness of the outcomes and not merely to outcome valence (Windmann et al. 2006).
It is important to discuss these findings in light of the role of dopamine in timing models (MacDonald and Meck, 2004). Based on animal work and Parkinson's disease (PD) patients, dopaminergic manipulations have been shown to interact with reversal learning task performance. For example PD patients perform worse on reversal learning tasks when medicated compared with when unmedicated (Cools et al. 2001). Additionally, rats given methamphetamine similarly perform worse on reversal learning tasks (Cheng et al. 2007). This work then supports our results that anticipation and feedback processes interact, such that an increase in dopamine, causes an increase in anticipation, and speeds time perception, consequently enhances the response to negative feedback. Thus the bigger the expectation, the bigger the adjustment when the expected outcome does not occur.
Retrieval-Load by Anticipation-Interval Interaction
We found a significant load by interval interaction in the anterior midcingulate cortex (aMCC) explained by a differential effect of interval at the 1 load level (Figs 5 and S13). Again, interpretation of this result is not straightforward as there was no retrieval-load by anticipation-interval interaction on behavior. This region of the aMCC has been implicated in the processing of fear emotion processing (Vogt 2005) and this region receives input from the amygdala (Vogt and Pandya 1987). Additionally this region has been implicated in arousal and uncertainty from risk-related processes (Critchley et al. 2001). The Critchley et al. (2001) study used a gambling paradigm where participants had to maintain an expected outcome over a 8.5-s delay. The cognitive process of maintaining expected outcomes over a delay, that varied in the degree of uncertainty is similar to the present study paradigm. Therefore, the finding of increased activity in this region with increasing uncertainty between the 2 studies is consistent. Therefore if this region of the cingulate is modulated by uncertainty and arousal, a further interpretation of the current study is that there is a decrease in arousal at the 1 retrieval-load level following a 3-second delay compared with the 1.5-s delay. However the converse is also possible of an increase in arousal at a 1.5-s delay compared with a 3-s delay. In order to clarify this relationship future work is needed over a greater range of delay intervals. This region of the aMCC, approximately BA24, as detailed above is involved in the behavioral process of energization (Stuss and Alexander, 2007). Therefore greater energization may be required at a shorter delay, when the pace of the task is faster, than at a longer delay.
Outcome-Feedback by Retrieval-Load by Anticipation-Interval Interaction
There was a significant 3-way interaction in the right frontopolar cortex, giving evidence that this region processes information related to outcome feedback, retrieval load, and preparatory interval. This evidence is supported by anatomical studies showing strong reciprocal connections between frontal polar and orbital, medial and lateral prefrontal regions (Ramnani and Owen 2004; Petrides and Pandya 2007; Hagmann et al. 2008).
Deconstructing this interaction demonstrated that activation is significantly higher on no-match trials after a 3 s compared with a 1.5-s interval when one retrieves 3 letters; whereas activation is significantly reduced after a 3 s compared with a 1.5-s interval when one retrieves 5 letters from memory (Fig. 6). It is difficult to understand this interaction psychologically, and this difficulty relates to the great uncertainty of the role of the frontopolar cortex in human cognition.
Previous work has demonstrated a role for this region in memory retrieval (Rugg and Wilding 2000; Velanova et al. 2003), responses to feedback (Remijnse et al. 2005; Zanolie et al. 2008) and increased activity to reward trials (Pochon et al. 2002). Indeed a previous imaging study has shown that this region is activated by branching processes, that are an interaction of working memory delayed-response and attentional dual-task processes (Koechlin et al. 1999). Others have implicated the anterior medial PFC in recollection processes (Simons et al. 2005; Turner et al. 2008) and integration processes (Reynolds et al. 2006) and in the manipulation of internally generated information (Christoff et al. 2003).
Examining Figure 6, it is interesting that there is a similar effect of load at load 1 across both outcome and interval levels, but a different pattern at load 3 and a different pattern still at load 5. This suggests that there may be a memory capacity limit to the medial anterior prefrontal cortex (APFC). This finding may be related to work demonstrating that there is a maximum of 4 items that can be held in attention (Cowan 2001), but this interpretation is speculative. Related to this a recent attempt to model the function of APFC has suggested that there is a limited capacity of 2 behavioral plans or mental tasks (task sets) that can be currently maintained 2 items (Koechlin and Hyafil 2007). This model also shows that reward expectations associated with the active and pending task sets are continuously updated with respect to feedback signals related to current behavior. However, we are unable to draw firm conclusions on our data based on the current literature and theories of APFC function that do not make strong predictions for this finding. More research is needed to examine activation in APFC as a function of feedback at different retrieval loads and anticipation-interval levels.
Comparison of “X” and Fixation Contrasts
As a final comparison, we compared activation in the “X” control condition with the fixation blocks used as a baseline throughout the study. In the “X” > fixation comparison, greater activation was found predominantly in the putamen and cerebellum. These activations reflect the automatic motor response that occurred in this condition (Pastor et al. 2008). The reverse contrast (fixation > “X” trials) revealed activation in the anterior and posterior cingulate. Activation associated with the “default network.” Because there was no motor component associated with this condition, we argue that it reflects anticipatory processes.
Limitations
This study has a number of limitations that are important to discuss. One limitation is that during scanning we were unable to cover the whole brain due to the 1.5-s TR used. Future work should examine these interacting cognitive processes on parietal and occipital regions that were not covered in the present study.
Additionally with the scanning parameters used it is difficult to localize activations to particular basal ganglia nuclei, therefore we are cautious in attributing the interval factor activation to either the substantia nigra or VTA, and the outcome-feedback and retrieval-load interaction precisely to the GPi.
Another limitation is that compared with other studies that have used preparatory interval paradigms, we only used 2 time intervals. It will be important in future work to examine greater number of preparatory intervals on neural activity, to examine the full range of these anticipatory effects.
Due to the design of the study where we have used 3 experimental factors and their interactions, although some of our main effects survive whole-brain correction, we have reported interaction results that are uncorrected for multiple comparisons at the whole-brain level. We acknowledge this limitation but believe that the main effects findings are consistent with a large previous literature and the novel findings are of interest to others in the field. However we acknowledge that our findings should be considered preliminary until replicated.
Finally, in order to fully understand the 3-way interaction it is important in future work to use a greater range of retrieval loads (1–5 letters), in order to understand how regions such as the APFC operate at 2 and 4 letter loads. However, by including greater levels at each factor will have a significant impact on statistical power and increase the length of the experiment or the number of participants to be tested.
Implications and Future Directions
This work has a number of implications for our understanding of neurological and psychiatric disorders as it demonstrates that dysfunctional neural processing in one region may impact on information processing downstream from the site of actual impairment.
An important next step is to perform effective connectivity analyses, such as dynamic causal modeling (Friston et al. 2003) to see how one region implicated changes the information processing in other regions of the task network. Other methods such as TMS can also be used to test the causal role of connectivity among brain regions.
Additionally psychopharmacological studies can be performed to examine how different neurotransmitter systems (e.g., dopamine and serotonin) affect these information-processing networks. These studies then would then significantly extend the causal mechanisms that lead to dysfunctions in cognitive control in illness states.
Conclusions
In conclusion, we have shown how cognitive control, a multivariate phenomenon can be decomposed into a number of interacting subprocesses. This enables an understanding of how people retrieve prelearnt information from memory, use this information to guide responses for an impending decision, and adjust their responses on the basis of outcome feedback.
We found that the process of memory retrieval was associated with increasing activity in a distributed network of regions comprising lateral PFC, cerebellum, inferior temporal cortex, dorsal ACC and the thalamus. Brain regions important for anticipating upcoming responses were the medial PFC and VTA. Brain regions important for adjusting responses following outcome feedback were the ACC and lateral PFC.
We found a number of brain regions demonstrating interactions of these cognitive processes. A retrieval-load by outcome-feedback interaction was found in the left globus pallidus that may represent a memory reinforcement mechanism to facilitate the retention of an uncertain action that produces positive feedback. An outcome-feedback by anticipation-interval interaction in the inferior prefrontal cortex that we argue this is due to a bigger adjustment to negative outcome feedback for shorter compared with longer anticipation intervals. This interpretation is consistent with a role for this region in response reversal. A third interaction was a retrieval-load by anticipation-interval interaction in the midcingulate gyrus that we argue is due to “energization” or required effort to retrieve one item from memory in a shorter time interval.
Finally, a load by interval by outcome interaction was found in the right frontal pole. We argue that this region is involved in integrating the 3 psychological processes studied in the present experiment.
Taken together, these results build on previous findings and further advance our knowledge of how fundamental cognitive processes interact to enable flexible decision-making and behavioral control. It is also noteworthy that this study illustrates the use of functional neuroimaging to examine the underlying neurophysiological correlates of interacting cognitive processes that is not possible through behavioral study alone.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
NARSAD Independent Investigator Award to M.L.P.; and NIMH (R01 MH076971-01A2) to M.L.P.
Supplementary Material
Acknowledgments
The authors would like to thank Shi Qi for help programming the task. Thanks to Costin Tanase in particular and all colleagues at the Magnetic Resonance Research Center at the University of Pittsburgh Medical Centre for kind assistance during the study. Thanks to Natalia Lawrence, Owen O'Daly, and Graeme Fairchild for comments on earlier drafts. Conflict of Interest: None declared.
References
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelson P, Boons JP. Time uncertainty and choice reaction time. Nature. 1960;187:531–532. doi: 10.1038/187531a0. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. 8th international conference on functional mapping of the human brain. Sendai, Japan: Neuroimage; 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007;1186:255–266. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–90. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, Deichmann R, Ashburner J, Frackowiak RS. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci. 2002;16:1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x. [DOI] [PubMed] [Google Scholar]
- Driver J, Haggard P, Shallice T. Introduction. Mental processes in the human brain. Philos Trans R Soc Lond B Biol Sci. 2007;362:757–760. doi: 10.1098/rstb.2007.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ. Differential neural responses during performance of matching and nonmatching to sample tasks at two delay intervals. J Neurosci. 1999;19:5066–5073. doi: 10.1523/JNEUROSCI.19-12-05066.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis-I disorder (SCID-I), clinician version. American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex–an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95:3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neural networks underlying the encoding, maintenance, and retrieval phase in a delayed-match-to-sample task. Brain Res Cogn Brain Res. 2005;23:207–220. doi: 10.1016/j.cogbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Habib M. Athymhormia and disorders of motivation in basal ganglia disease. J Neuropsychiatry Clin Neurosci. 2004;16:509–524. doi: 10.1176/jnp.16.4.509. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Henson R. Efficient experimental design for fMRI. In: Friston KJ, Kiebel S, Nichols TE, Penny WD, editors. Statistical parametric mapping: the analysis of functional brain images. Academic Press; 2007. pp. 193–210. [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Dirnberger G, Frith CD. The substantia nigra pars compacta and temporal processing. J Neurosci. 2006;26:12266–12273. doi: 10.1523/JNEUROSCI.2540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassinove JI, Schare ML. Effects of the “near miss” and the “big win” on persistence at slot machine gambling. Psychol Addict Behav. 2001;15:155–158. doi: 10.1037//0893-164x.15.2.155. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Kronhaus DM, Willshaw DJ. The cingulate as a catalyst region for global dysfunction: a dynamical modelling paradigm. Cereb Cortex. 2006;16:1212–1224. doi: 10.1093/cercor/bhj062. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa gambling task. Cereb Cortex. 2008;19:1134–1143. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Los SA, Knol DL, Boers RM. The foreperiod effect revisited: conditioning as a basis for nonspecific preparation. Acta Psychol. 2001;106:121–145. doi: 10.1016/s0001-6918(00)00029-9. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Systems-level integration of interval timing and reaction time. Neurosci Biobehav Rev. 2004;28:747–769. doi: 10.1016/j.neubiorev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. Neuroimage. 2003;20:1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- Marzinzik F, Wahl M, Schneider GH, Kupsch A, Curio G, Klostermann F. The human thalamus is crucially involved in executive control operations. J Cogn Neurosci. 2008;20:1903–1914. doi: 10.1162/jocn.2008.20124. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Haxby JV, Ungerleider LG, Horwitz B. Changes in limbic and prefrontal functional interactions in a working memory task for faces. Cereb Cortex. 1996;6:571–584. doi: 10.1093/cercor/6.4.571. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter EG, Pribram KH. Plans and the structure of behavior. Holt, Rinehart and Winston, Inc; 1960. [Google Scholar]
- Miyawaki E, Perlmutter JS, Troster AI, Videen TO, Koller WC. The behavioral complications of pallidal stimulation: a case report. Brain Cogn. 2000;42:417–434. doi: 10.1006/brcg.1999.1113. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Fenwick PB, Trimble MR, Dolan RJ. Brain activity relating to the contingent negative variation: an fMRI investigation. Neuroimage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Niemi P, Naatanen R. Foreperiod and simple reaction time. Psychol Bull. 1981;89:133–162. [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Nadjar A, Arkadir D, Bezard E, Goillandeau M, Bioulac B, Gross CE, Boraud T. Shaping of motor responses by incentive values through the basal ganglia. J Neurosci. 2007;27:1176–1183. doi: 10.1523/JNEUROSCI.3745-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Macaluso E, Day BL, Frackowiak RS. Putaminal activity is related to perceptual certainty. Neuroimage. 2008;41:123–129. doi: 10.1016/j.neuroimage.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci USA. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: A new approach to brain activation experiments. Neuroimage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychol Bull. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Reynolds JR, McDermott KB, Braver TS. A direct comparison of anterior prefrontal cortex involvement in episodic retrieval and integration. Cereb Cortex. 2006;16:519–528. doi: 10.1093/cercor/bhi131. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity dependent on anticipated and elapsed delay in macaque prefrontal cortex, frontal and supplementary eye fields, and premotor cortex. J Neurophysiol. 2005;94:1469–1497. doi: 10.1152/jn.00064.2005. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends Cogn Sci. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Sakai K. Task set and prefrontal cortex. Annu Rev Neurosci. 2008;31:219–245. doi: 10.1146/annurev.neuro.31.060407.125642. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schmidt L, d'Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schupbach M, Hartmann A, Levy R, Dubois B, Pessiglione M. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Steinborn MB, Rolke B, Bratzke D, Ulrich R. Sequential effects within a short foreperiod context: evidence for the conditioning account of temporal preparation. Acta Psychol. 2008;129:297–307. doi: 10.1016/j.actpsy.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Borowiec A, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Koechlin E. A neural representation of prior information during perceptual inference. Neuron. 2008;59:336–347. doi: 10.1016/j.neuron.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tomlin D, Kayali MA, King-Casas B, Anen C, Camerer CF, Quartz SR, Montague PR. Agent-specific responses in the cingulate cortex during economic exchanges. Science. 2006;312:1047–50. doi: 10.1126/science.1125596. [DOI] [PubMed] [Google Scholar]
- Turner MS, Simons JS, Gilbert SJ, Frith CD, Burgess PW. Distinct roles for lateral and medial rostral prefrontal cortex in source monitoring of perceived and imagined events. Neuropsychologia. 2008;46:1442–1453. doi: 10.1016/j.neuropsychologia.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24:1123–1137. doi: 10.1076/jcen.24.8.1123.8375. [DOI] [PubMed] [Google Scholar]
- Vallesi A, McIntosh AR, Shallice T, Stuss DT. When time shapes behavior: fMRI evidence of brain correlates of temporal monitoring. J Cogn Neurosci. 2009;21:1116–1126. doi: 10.1162/jocn.2009.21098. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Mussoni A, Mondani M, Budai R, Skrap M, Shallice T. The neural basis of temporal preparation: Insights from brain tumor patients. Neuropsychologia. 2007;45:2755–2763. doi: 10.1016/j.neuropsychologia.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007;17:466–474. doi: 10.1093/cercor/bhj163. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional-anatomic correlates of sustained and transient processing components engaged during controlled retrieval. J Neurosci. 2003;23:8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kirsch P, Mier D, Stark R, Walter B, Gunturkun O, Vaitl D. On framing effects in decision making: linking lateral versus medial orbitofrontal cortex activation to choice outcome processing. J Cogn Neurosci. 2006;18:1198–1211. doi: 10.1162/jocn.2006.18.7.1198. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Walter H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia. 2006;44:2558–2563. doi: 10.1016/j.neuropsychologia.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Zanolie K, Van Leijenhorst L, Rombouts SA, Crone EA. Separable neural mechanisms contribute to feedback processing in a rule-learning task. Neuropsychologia. 2008;46:117–126. doi: 10.1016/j.neuropsychologia.2007.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.