Abstract
Although widespread neural atrophy is an inevitable consequence of normal aging, not all cognitive abilities decline as we age. For example, spoken language comprehension tends to be preserved, despite atrophy in neural regions involved in language function. Here, we combined measures of behavior, functional activation, and gray matter (GM) change in a younger (19–34 years) and older group (49–86 years) of participants to identify the mechanisms leading to preserved language comprehension across the adult life span. We focussed primarily on syntactic functions because these are strongly left lateralized, providing the potential for contralateral recruitment. In an functional magnetic resonance imaging study, we used a word-monitoring task to minimize working memory demands, manipulating the availability of semantics and syntax to ask whether syntax is preserved in aging because of the functional recruitment of other brain regions, which successfully compensate for neural atrophy. Performance in the older group was preserved despite GM loss. This preservation was related to increased activity in right hemisphere frontotemporal regions, which was associated with age-related atrophy in the left hemisphere frontotemporal network activated in the young. We argue that preserved syntactic processing across the life span is due to the shift from a primarily left hemisphere frontotemporal system to a bilateral functional language network.
Keywords: aging, compensation, language, laterlization, neuroimaging
Introduction
Normal healthy aging is accompanied by extensive tissue loss in both white and gray matter (GM; Good, Johnsrude et al. 2001; Resnick, Pham et al. 2003; Sowell et al. 2003). These changes are accompanied by a complex pattern of cognitive change, where some cognitive functions decline but others are preserved. The relationship between age-related neural and cognitive change has been studied using neuroimaging techniques, with the emphasis tending to be on the cognitive functions which show clear declines, such as memory and executive functions, and on the neural regions which have been shown to play an important role in these functions, such as prefrontal cortex (e.g., Raz et al. 1998; Grossman et al. 2002). This research shows that age-related changes in memory and executive function are associated with changes in patterns of neural activity including both increased and decreased activity for older compared with younger subjects. In some studies, older adults show underactivation of neural regions, which are activated in young adults (Logan et al. 2002), whereas in others, they activate regions which are not typically activated in the young (Morcom et al. 2003), perhaps indicating compensatory neural activity.
One of the key issues in understanding the relationship between age-related neural change and changes in cognition is how to explain the variability in this relationship that is, to explain why not all cognitive functions decline with age, in spite of extensive neural atrophy, and why not all individuals show the same degree of cognitive decline. This issue is usually addressed by taking advantage of individual variation within an age-group in performance on a cognitive function, which typically declines with age (such as episodic memory), and comparing activation patterns of better and worse performing older adults (e.g., Morcom et al. 2003). A different approach, and the one we adopt here, is to investigate a cognitive function—spoken language comprehension—which typically does not show age-related declines (for reviews, see Burke et al. 2000; Waters and Caplan 2005; Burke and Shafto 2008) and determine whether this preserved function is due to effective neural reorganization in the context of age-related increases in neural atrophy. One advantage of this approach is that behavioral performance can be equivalent across age-groups, which avoids problems associated with tasks where performance typically declines with age.
Examining age-related neural change in the context of spoken language comprehension has a number of advantages. First, although spoken language comprehension shows minimal age-related change, except when working memory demands increase (Waters and Caplan 2001; Waters and Caplan 2005), an absence of behavioral change does not necessarily mean an absence of neural change. Because age-related atrophy affects many neural regions critical for language processes, sentence comprehension may reflect effective neural reorganization, where performance remains intact because of successful neural compensation or cognitive strategies (Reuter-Lorenz 2002). Moreover, because the key regions involved in language comprehension, such as frontal cortex, are among the most vulnerable to age-related atrophy, preserved language function cannot be explained in terms of reduced neural atrophy in the relevant brain regions (demonstrated in, e.g., Raz 2000; Sowell et al. 2003). Under this account, a cognitive function would be preserved only if it involved neural regions which show less age-related neural change. Because this does not hold for areas related to language, the preservation of this function may be underpinned by compensatory changes in the neural substrate for these capacities (Reuter-Lorenz 2002). To understand these changes, we need to relate changes in brain structure and function to changes in functional activity and measures of cognitive function over the life span.
Second, many of the core aspects of language function are instantiated in a primarily left-lateralized frontotemporal neural system and lateralized processes provide an ideal opportunity for examining neural compensation (Cabeza 2002) because of the potential for contralateral recruitment. One component of language function—syntax—is a key example of strong left lateralization and is the primary focus of this study. Syntactic processing is thought to involve the online construction of grammatical strings based on information provided by the lexical category of each word, word-order constraints, and grammatical rules. These syntactic processes are thought to involve perisylvian regions of the left hemisphere (LH) including the left inferior frontal gyrus (IFG), left middle temporal gyrus (MTG), and posterior regions such as the angular gyrus and inferior parietal lobe (e.g., Dronkers et al. 2004; Humphries et al. 2006; Tyler and Marslen-Wilson 2008). The evidence for this LH system primarily comes from two sources: Neuropsychological studies have established that damage in left inferior frontal and/or middle temporal regions is associated with syntactic impairments, whereas damage to comparable regions in the right hemisphere (RH) is not (Caramazza and Zurif 1976; Berndt and Caramazza 1981; Caplan and Hildebrant 1988; Caplan et al. 1996; Dronkers et al. 2004). In a similar vein, neuroimaging evidence has shown that syntactic processing primarily involves frontotemporal regions within the LH (e.g., Caplan et al. 1998; Keller et al. 2001; Grossman et al. 2002; Friederici et al. 2003; Caplan et al. 2008).
However, most of these studies have used stimuli and tasks which make it difficult to separate the effects of the online construction of a syntactic representation from the contribution of variables that may not reflect the normal processes of comprehending language. For example, many studies aim to determine the neural regions involved in syntactic processing by manipulating syntactic complexity, which has the confounding side effect of increasing working memory demands (Just et al. 1996; Stromswold et al. 1996). Furthermore, end-of-sentence judgment tasks of various sorts—so called “off-line” tasks—tend to be used (e.g., Van der Linden et al. 1999; Kemper and Sumner 2001; Humphries et al. 2006), and these also increase working memory demands by requiring subjects to wait until the end of a sentence to make their judgment. This loading on working memory is problematic for studies designed to investigate the preservation of online syntactic processing in relation to neural changes across the life span because working memory is itself known to decline with age (Just and Carpenter 1992; Braver and West 2008). When syntactic processing is not confounded with working memory demands, it does not show age-related declines (Waters and Caplan 2005).
We avoided these problems by using a task—the word-monitoring task—which has been shown to reflect the online construction of different types of linguistic representations, while minimizing working memory demands (Marslen-Wilson and Tyler 1975, 1980; Tyler 1981; Tyler and Marslen-Wilson 2008). In the original version of this task, subjects listened to sentences and pressed a response key when they heard a prespecified target word in one of the spoken sequences—for example, LEAD in the examples below. Listeners were presented with 3 types of spoken stimuli: 1) Normal prose (NP) sentences which had normal syntactic, semantic, and pragmatic structure (The church was broken into last night. Some thieves stole most of the LEAD off the roof), 2) Anomalous prose (AP) sentences which had grammatical structure but lacked sentential meaning (e.g., The power was located in great water. No buns puzzle some in the LEAD off the text), and 3) Random word order (RWO), consisting of strings of words with no grammatical or sentential meaning (e.g., Into was power water the great located. Some the no puzzle buns in LEAD text the off). The position of the target word was varied from early to later word positions across the sentences and strings of words, and we found that word-monitoring response times (RTs) became increasingly faster at later word positions in both normal and AP but not random strings of words. We interpreted this pattern of “word position effects” as showing the online, word-by-word development of different kinds of linguistic representations. In NP, word position effects reflected the listener's ability to develop online meaningful representations spanning the sentence, whereas position effects in AP reflected the online construction of syntactic representations without the contribution of sentential meaning. This interpretation was reinforced by the absence of word position effects in random strings of words, for which there was neither a possible coherent syntactic nor semantic analysis (Marslen-Wilson and Tyler 1975, 1980). In subsequent studies with brain-damaged patients, we found that patients with extensive LH damage in inferior frontal and superior temporal gyrus (STG)/middle temporal gyrus (MTG) showed the typical word position effect in NP but not in AP (Tyler 1992). We interpreted this as indicating that word monitoring in NP sentences reflected the ability of listeners to carry out the task primarily on the basis of the combined meanings of individual words and their pragmatic implications and with a lesser contribution of syntax. In contrast, the processing of AP relies primarily on syntactic analyses because the meanings of the individual words cannot be combined into a meaningful sentential representation. Thus, the task differentially loads on syntactic processing, depending on whether the listener is hearing NP or AP sentences.
We used this paradigm in the present functional magnetic resonance imaging (fMRI) experiment to obtain a measure of the ability of listeners to construct semantic and syntactic sentential representations word by word as the sentence unfolds over time. We chose this task because it generates fast RTs and few errors in both young and older healthy subjects (Tyler 1992). It is particularly appropriate for studying age effects on spoken language processing because the pattern of word position effects—present in normal and AP sentences and absent in strings of unrelated words—is the important behavioral measure and not absolute RTs, which typically increase with age. Thus, we can differentiate between speed of responding and the ability to develop syntactic and semantic sentential representations. Moreover, because the to-be-monitored target word remains displayed throughout each trial, there are minimal working memory demands.
In the fMRI study, we asked 2 groups of healthy adults (a young group aged 19–34 years and an older group aged 49–86 years) to listen to the 3 types of spoken sequences described above: NP sentences, AP sentences, and sequences of unstructured lists of words (random word order, RWO), which were generated by reordering words in the normal and anomalous sentences. We obtained measures of the word position effect for the 3 prose types during scanning and related these to neural activity associated with each prose type and to measures of GM integrity within each group. By combining behavioral measures, functional activation, and age-related neural change, we aimed to identify the conditions and mechanisms leading to preserved language comprehension across the adult life span. Specifically, we predict 1) that on the basis of previous studies (Caplan et al. 1998; Friederici et al. 2006; Tyler and Marslen-Wilson 2008), the young group will show LH frontotemporal activation for anomalous sentences, where syntactic analysis is dominant, whereas left frontal activation may be diminished in simple NP sentences, where the semantic and pragmatic interpretation of the utterance, cued by the meanings of the words in the sentences, dominates over syntactic factors. For quite different reasons, we expect no LH frontal activation for random word-order sentences, where no higher order analysis, whether syntactic or semantic, is possible. We further predict 2) that patterns of word position effects will remain stable across the life span, reflecting preserved sentence comprehension but that 3) we will see these in the context of age-related deterioration of neural structures critical for comprehension in younger adults, so that 4) preserved sentence comprehension in old age will require some form of neural compensation. Given the left-lateralized nature of the syntax-comprehension system, this is likely to not only include contralateral (RH) recruitment but could also include the recruitment of other cognitive processes supported by other neural regions.
Methods
Participants
We recruited 58 healthy right-handed adults from the Cambridge community (27 female, 31 male). These included 14 younger adults aged 19–34 (M = 23.9, standard deviation [SD] = 4.1), to establish the baseline young adult neural system, and an older group of 44 participants aged 49–86 (M = 67.4, SD = 8.0), in order to sample a wide age range of older adults. All gave informed consent, and the study was approved by the Suffolk Local Research Ethics Committee. No participants had audiometer results indicating severe hearing impairment, and their scores on the Mini Mental State Exam (MMSE; Folstein et al. 1975) averaged 28.7 (SD = 1.2). Major exclusion criteria included bilingualism, left-handedness, MR contraindications, neurological or hormonal disorders, recent treatment (within one year) for psychiatric disorders, major head trauma, stroke, or dyslexia. All volunteers were screened to exclude neurological or psychiatric illness and had not been taking psychoactive medication for at least 5 months before scanning.
Materials
We generated 3 types of spoken language stimuli—normal sentences (NP), anomalous sentences (AP), and unstructured strings of words (RWO), with 30 items in each condition. In addition, we constructed 24 baseline items, which we refer to as musical rain (MuR; see below).
The NP stimuli consisted of sentences with normal grammatical structure and sentential meaning, in which a short introductory sentence was followed by a longer sentence which contained the prespecified target word (NECK in the following example). “Jane didn't enjoy herself very much. Her NECK was stiff because she had a bad cold and she couldn't lift anything properly.” The AP stimuli were grammatically correct but had no overall sentential meaning. They were closely modeled on the NP sentences, having the same type of syntactic structure and length, but with no discernible meaning. For example, “Stephen didn't catch himself very much. Her TOOTH was driven because he had a weak nail and she couldn't heat anyone properly.” RWO stimuli were generated by scrambling words in the NP or AP sentences so that they had neither grammatical structure nor overall meaning. For example, “Very Stephen catch much himself didn't. Her NOSE because properly had anyone couldn't he and nail weak a heat driven was.” In all, 50% of the random sentences were derived from the normal sentences and 50% from the anomalous sentences.
Participants monitored for target words, which occurred either early or late in the spoken sequences, matched across the 3 types of stimuli. Differences between early and late RTs provide a measure of semantic and syntactic analysis for NP and syntactic analysis in AP, as noted in the Introduction (Marslen-Wilson and Tyler 1975, 1980; Tyler 1992). Target words in the early position occurred on average 3 words into the second sentence in each sequence, whereas those occurring late were placed on average 12 words into the second sentence. Target words across the 3 prose types were also matched on a number of relevant psycholinguistic variables (e.g., lemma and wordform frequency, familiarity, imageability, number of phonemes, and number of syllables) as shown in Table 1. Univariate analyses of variance (ANOVAs) with 2 independent factors—prose type (NP, AP, and RWO) and target position (early and late)—were conducted on each of these variables. The target words in each of the 3 prose types did not differ on any of these variables (all F values < 1). Target words were presented in written form, accompanied by black and white line drawings, mostly taken from the Snodgrass and Vanderwart (1980) set, to ensure rapid access to the meaning of the word.
Table 1.
Descriptive statistics of the stimuli
| Sentence type & Target position | |||||||||
| Lemma frequency | Word form frequency | Familiarity | Imagabillity | Letters | Phonemes | Syllables | Target onset (ms) | Length (ms) | |
| Normal | |||||||||
| Early | |||||||||
| Mean | 131 | 87 | 558 | 604 | 4.3 | 3.5 | 1.1 | 3003 | 6948 |
| SD | 189 | 119 | 50 | 32 | 1.1 | 1.4 | 0.4 | 429 | 844 |
| Median | 76 | 56 | 558 | 610 | 4.0 | 3.0 | 1.0 | 2958 | 6586 |
| Late | |||||||||
| Mean | 137 | 100 | 582 | 589 | 5 | 3.3 | 1.2 | 5608 | 7448 |
| SD | 133 | 119 | 30 | 34 | 1 | 1.2 | 0.4 | 813 | 917 |
| Median | 87 | 48 | 588 | 597 | 5 | 3.0 | 1.0 | 5450 | 7812 |
| Anomalous | |||||||||
| Early | |||||||||
| Mean | 122 | 79 | 565 | 600 | 5 | 3.5 | 1.1 | 3070 | 7299 |
| SD | 161 | 124 | 47 | 39 | 1 | 1.1 | 0.4 | 596 | 815 |
| Median | 86 | 37 | 579 | 601 | 4.5 | 3.0 | 1.0 | 3019 | 7137 |
| Late | |||||||||
| Mean | 136 | 81 | 580 | 589 | 5 | 3.2 | 1.2 | 5520 | 7428 |
| SD | 155 | 76 | 43 | 22 | 1 | 1.1 | 0.4 | 835 | 953 |
| Median | 69 | 60 | 567 | 587 | 4.5 | 3.0 | 1.0 | 5459 | 7542 |
| Random | |||||||||
| Early | |||||||||
| Mean | 140 | 109 | 568 | 591 | 5 | 3.5 | 1.1 | 3120 | 7557 |
| SD | 200 | 189 | 42 | 35 | 1 | 0.6 | 0.4 | 610 | 779 |
| Median | 81 | 68 | 575 | 598 | 4.5 | 3.0 | 1.0 | 3343 | 7812 |
| Late | |||||||||
| Mean | 151 | 104 | 578 | 587 | 5 | 3.3 | 1.2 | 5830 | 7824 |
| SD | 151 | 125 | 39 | 28 | 1 | 0.8 | 0.4 | 800 | 896 |
| Median | 77 | 37 | 589 | 593 | 4.5 | 3.0 | 1.0 | 5792 | 8121 |
In addition to these 3 prose types, we included a baseline condition which consisted of 24 acoustic stimuli which were constructed to share complex auditory properties of speech without triggering phonetic interpretation, in order to allow the separation of lower level auditory processing bilaterally from lexical processing. We used envelope-shaped MuR for this purpose as described by Uppenkamp et al. (2006). In MuR the long-term spectrotemporal distribution of energy is matched to that of the corresponding speech stimuli, and the temporal envelope of each segment is modulated by the temporal envelope extracted from the corresponding speech segment. We also matched the root mean squared amplitude of the MuR to that of the speech. Despite the similarities in the distribution of energy over frequency and time, MuR does not sound like speech. Because of the absence of continuous formants in the signal, it cannot be interpreted as speech generated by a vocal tract. MuR does, however, produce a similar level of BOLD activation to that of vowels in all centers of the auditory pathway up to and including the primary receiving areas of auditory cortex in Heschl's gyrus and planum temporale (Uppenkamp et al. 2006). Beyond the primary receiving areas, in secondary auditory regions such as the anterior superior temporal sulcus and superior temporal gyrus, it produces much less activation than the corresponding speech. MuR was chosen over previously used baseline stimuli such as spectrally rotated speech, nonwords, foreign, or reversed speech (Blesser 1972; Binder et al. 2000; Scott et al. 2000; Narain et al. 2003) because all these baselines can give the percept of speech, even if unintelligible, and can potentially engage higher level lexical interpretation processes.
The MuR items were developed directly from the prose stimuli used in the experiment, so that they were matched in duration. To make the task demands comparable in MuR and the prose conditions, we added a burst of white noise (1000 ms) to the MuR stimuli and instructed participants to press a response key as soon as they heard it.
Stimuli were recorded onto a digital tape by a female native speaker of British English who was naive to the purposes of the experiment and who did not know the identity of the target word. The lists of unrelated words were spoken with an approximation to a normal prosodic contour. Stimuli were recorded at 44, 100 Hz and then downsampled to 22, 050 Hz, and each item was saved in .wav format. Stimuli were presented in the scanner via pneumatic insert earphones (ER3, Etymotic Research Inc., Elk Grove, IL). To further reduce interference from scanner noise, ear protectors were placed over the earphones. To compensate for earphone-related changes in sound frequency profile, stimuli were pre-emphasized (http://www.mrc-cbu.cam.ac.uk/∼rhodri/headphonesim.htm). Visual stimuli were presented using an LCD projector, and participants viewed the screen via a mirror inside the MRI head coil. Auditory and visual stimulus presentation was cued using CAST (http://www.mrc-cbu.cam.ac.uk/∼maarten/CAST.htm) running on a PC. Responses were collected using an MRI-compatible button box.
Task
Participants heard the auditory stimuli played over headphones. Before each language stimulus, they saw a target word and a picture (denoting the same concept; e.g., DOG) presented simultaneously on a computer screen. Their task was to press a response key when they heard the target word in the spoken stimulus. Monitoring RTs were measured from the onset of each target word in the spoken stimulus. The target word and picture stayed on the screen throughout the trial in order to reduce memory demands. In the baseline condition, participants pressed a response key when they heard a period of white noise within sequences of MuR.
Imaging Methods and Analysis
We used a sparse imaging method to avoid scanner noise while participants were listening to spoken language (Hall et al. 1999). Visual target words were presented 1.1 s before the onset of each spoken stimulus and lasted the duration of the trial. Spoken stimuli were presented in a 9-s silent period that occurred between each 2-s scan such that scanning started 8.9 s after sentence onset, thus ensuring that scans were maximally sensitive to the different types of linguistic representations. Given that sentences varied in duration, this method ensured variability in the point at which the hemodynamic response was sampled, in order to increase the likelihood of sampling at the peak of the hemodynamic response. The stimuli were presented in 2 sessions with half of the items in each session. Stimuli within each prose type were presented in a blocked design to avoid increased activity due to frequent task switching which is known to be sensitive to aging (Kramer and Madden 2008). In each session, trials were presented in the following order: 15 trials NP, 12 trials silence, 15 trials RWO, 12 trials MuR, and 15 trials AP.
Participants were scanned at the MRC Cognition and Brain Sciences Unit, Cambridge, with a Siemens 3-T Tim Trio MRI scanner (Siemens Medical Solutions, Camberley, UK). Each functional volume consisted of 32 oblique axial slices, 3 mm thick with interslice gap of 0.75 mm and in-plane resolution of 3 mm. Slices were angled such that those covering middle temporal gyrus passed superior to the eyes, to prevent eye motion from obscuring activation in language areas. Field of view = 192 × 192 mm, repetition time = 11 s, acquisition time = 2 s, time echo = 30, and flip angle 78°.
The fMRI data were preprocessed and analyzed using SPM5 software (SPM5, Wellcome Institute of Cognitive Neurology, London, UK). Preprocessing comprised within-subject realignment, spatial normalization of images to a template in standard space, and spatial smoothing using an 8-mm Gaussian kernel. Unified normalization was used, which improves upon standard normalization by correcting for magnetic field inhomogeneity and fitting to the template using only brain tissue.
Following preprocessing, the data for each subject were modeled using a voxel-wise general linear model. The model comprised predicted RT series for each stimulus type, generated by convolving stimulus onset times with a canonical hemodynamic response. In addition, the 6 movement parameters calculated during realignment were included to reduce the probability of obtaining false positives that could be attributed to residual movement-related artifacts. We removed low-frequency noise by applying a high-pass filter with a period of 660 s. The relative contributions of each stimulus were used to calculate contrasts of interest, and the resulting contrast images were entered into group random-effects analyses. One-sample t-tests were used to assess the group-level significance of each contrast. Results were subject to voxel-level thresholds of P < 0.001, within this we report clusters that yielded corrected cluster-level significance of P < 0.05. Montreal Neurological Institute coordinates are reported. In order to identify anatomical regions within clusters and cluster maxima the Montreal Neurological Institute coordinates were converted to Talairach equivalent coordinates (Brett 2001). Anatomic labels and Brodmann areas (BA) were identified using the Talairach atlas (Talairach and Tournoux 1988) and confirmed using the template developed by the van Essen lab as implemented in MRIcron (http://www.MRicro.com/MRicron). Whole-brain analyses included all voxels across the brain, and the contrasts between each prose type and MuR showed small clusters of bilateral occipital activity due to the presence of a picture of the target word throughout each prose trial and the absence of a picture in the baseline MuR trials. Because these activations are not relevant to language processing, they are not reported.
We used correlational methods to examine the relationship between activity, age, and performance. We first identified significant clusters from the separate whole-brain random-effects analyses carried out on the young subjects’ data and the data from the group of older subjects. For each significant cluster, we extracted mean activation values for each participant averaged across the active voxels using the MarsBaR toolbox (http://marsbar.sourceforge.net/). These values were obtained from the contrast (weighted parameter estimate) images, which are constructed by summating the weighted sum of the parameter estimate images. This provided a mean measure of activity for each participant across all significant voxels from the analysis within each group; these values were entered into correlational analyses with age and performance measures as covariates.
Similarly, we used correlational methods to relate activity and performance to age-related changes in GM. We first obtained measures of GM density for each significant cluster from the fMRI analyses by taking values from GM probability images, which we obtained using optimized voxel-based morphometry. Individual structural images were segmented to produce GM probability maps by alternating steps of image bias correction, tissue classification, and tissue class-based normalization. Template tissue probability maps were based on the International Consortium for Brain Mapping Tissue Probabilistic Atlases (http://www.loni.ucla.edu/ICBM/ICBM_TissueProb.html). Segmentation was based on overall intensity distribution and used 2 Gaussians per tissue class and 4 Gaussians for nonbrain tissue. Standard regularization was used. The resulting GM probability images were smoothed using a Gaussian full width half maximum of 12 mm and entered into multiple regression analyses using age or behavioral measures as covariates.
Results
Behavioral Data
RTs to monitor for the target word in each spoken sequence were measured from target word onset. For each subject, RTs were inverse transformed (to reduce the effect of outliers without the need to remove reaction time data points; Ratcliff 1993), and the mean for each condition was calculated. Our main behavioral measures were RTs (mean inverse transformed) and proportional word position effect (WPE) for each of the 3 prose types, calculated by dividing the difference between early and late position RTs by mean RT. This calculation enabled us to look at the effect of age on the pattern of word position effects, controlling for any general effects of age on speed.
We first conducted an ANOVA on the mean inverse transformed RTs for the young and the older subjects separately. There were 2 factors: prose type (NP, AP, or RWO) and position (early or late). For the subjects analysis (F1), these factors were repeated measures, and for the items analysis (F2), these were independent measures. For the young subjects, there was a main effect of prose type (F12,26 = 108.76, P < 0.001, F22,84 = 63.06; P < 0.001) due to the faster RTs for NP (256 ms) compared with AP (342 ms), which in turn was faster than RWO (420 ms; all Newman–Keuls P < 0.05). A very similar pattern of results was found for the older group of subjects. There was a main effect of prose type (F12,86 = 333.45; P < 0.001; F21,84 = 51.97; P < 0.001), with NP (277 ms) faster than AP (361 ms), which was faster than RWO (440 ms, P < 0.05).
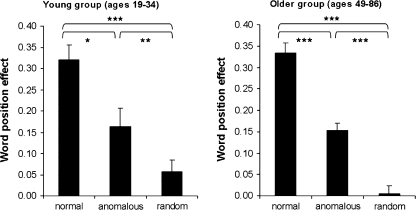
We next entered WPE scores into a 2 (age-group: young vs. older) × 3 (prose type: normal, anomalous, or random) ANOVA. There was a significant main effect of prose type, F12,112 = 92.62; P < 0.001. This reflected a larger WPE for NP (M = 0.33) than for AP items (M = 0.15, F11,56 = 49.78, P < 0.001), which in turn had a larger WPE than RWO items (M = 0.01, F11,56 = 56.65; P < 0.01). The WPE in both AP and NP was due to faster RTs in the later compared with the earlier word positions. There were no interactions with age (both F values < 1.10). Finally, we correlated the proportion WPE with age for the older group. There were no correlations between age and performance, as shown in Figure 1. These analyses establish that there were no behavioral differences between the young and older subjects in the ability to develop syntactically and semantically coherent sentential representations, consistent with previous findings (Tyler 1992).
Figure 1.
Behavioral results: showing the WPE (RTearly − RTlate)/RTmean) for each of the 3 prose types: NP, AP, and RWO for the young and older groups. *P < 0.05, **P < 0.01, ***P < 0.001.
Imaging Data
Young Group
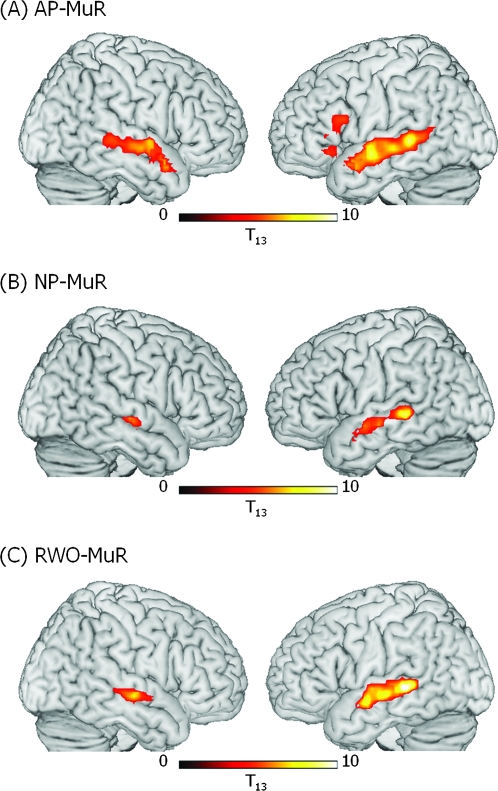
We first analyzed the data obtained from the young group of subjects and used their results as the baseline against which to evaluate the effects of age-related changes. The analyses focussed on the differences between each prose type and the MuR baseline in order to examine activity for different types of linguistic representations. Given our focus on syntax, we first compared activity for the sentences, which were grammatically coherent but lack sentential meaning (AP) against the baseline (MuR) to remove nonspeech auditory components of the stimuli. We predicted, on the basis of previous studies (Caplan et al. 1998; Friederici et al. 2006; Tyler and Marslen-Wilson 2008), that this contrast would elicit activity in LIFG and LSTG/MTG with smaller activation in RSTG/MTG. This is exactly the pattern we obtained (see Fig. 2a, b): 2 frontal clusters of activity, one in LIFG/Brodmann Area 44/45 and the other in LIFG/BA 47, significant at corrected voxel level, a large swathe of activity in left superior and middle temporal gyrus (LMTG; BA 21/22) which extended posteriorly to the posteriorMTG (pMTG; BA 37) and anteriorly to the border of the temporal pole (BA 38), and a smaller cluster of activity extending along the RSTG/MTG which, unlike the LSTG/MTG activation, did not extend into pMTG (see Table 2 and Fig. 2a).
Figure 2.
Significant clusters of activation in the young group (ages 19–34) for (A) AP–MuR, (B) NP–MuR, and (C) RWO–MuR. Activation is rendered on the surface of a canonical brain image. Color scale indicates T value of contrast. Voxel-level threshold P < 0.001, cluster size threshold P < 0.05 (see text).
Table 2.
Young participants (ages 19–34 years); regions showing significant activation for contrasts of each prose type minus MuR
| Contrast | Region | BA | Cluster |
Peak voxel |
MNI coordinates (mm) |
||||
| Pcorrected | Extent | PFDR | Z | x | y | z | |||
| NP–MuR | LMTG | 21 | <0.001 | 175 | 0.005 | 5.06 | −60 | −36 | 0 |
| LMTG | 21 | 0.029 | 4.17 | −60 | −15 | −9 | |||
| LMTG | 21 | 0.032 | 4.05 | −60 | −3 | −9 | |||
| RMTG | 21 | 0.042 | 42 | 0.027 | 4.23 | 57 | −18 | −3 | |
| AP–MuR | LIFG | 47 | 0.065 | 40 | 0.010 | 3.84 | −39 | 27 | −6 |
| LIFG | 47 | 0.015 | 3.62 | −48 | 24 | −9 | |||
| LIFG | 44 | 0.003 | 83 | 0.010 | 3.82 | −51 | 15 | 18 | |
| LIFG | 45 | 0.025 | 3.35 | −54 | 30 | 6 | |||
| LMTG | 21 | <0.001 | 524 | 0.004 | 5.12 | −63 | −36 | 0 | |
| LMTG | 21 | 0.004 | 4.99 | −60 | −6 | −9 | |||
| LMTG | 21 | 0.005 | 4.55 | −54 | −24 | −6 | |||
| RMTG | 21 | <0.001 | 319 | 0.005 | 4.58 | 54 | 9 | −18 | |
| RMTG | 21 | 0.005 | 4.44 | 63 | −3 | 0 | |||
| RMTG | 21 | 0.006 | 4.31 | 63 | −9 | −6 | |||
| RWO–MuR | LMTG | 21 | <0.001 | 324 | <0.001 | 5.88 | −56 | −35 | 2 |
| LMTG | 21 | 0.001 | 4.86 | −60 | −6 | −9 | |||
| LMTG | 21 | 0.001 | 4.80 | −63 | −21 | −6 | |||
| RMTG | 21 | <0.001 | 125 | 0.002 | 4.58 | 62 | −15 | −4 | |
| RMTG | 21 | 0.010 | 3.80 | 48 | −33 | 0 | |||
| RMTG | 21 | 0.019 | 3.52 | 63 | −3 | −9 | |||
Note: Extent, 3 × 3 × 3 mm voxels; PFDR, peak voxel statistical significance corrected for multiple comparisons using false discovery rate. Bold type: statistics for whole cluster and peak voxel. Normal type: voxel-level statistics for local maxima > 8mm apart.
Processing sentences that can be semantically interpreted (NP) compared with baseline (MuR) generated bilateral STG/MTG activity, regions known to be involved in semantic analysis (Rissman et al. 2003; Dronkers et al. 2004; Indefrey and Cutler 2004). The lack of significant frontal activity in NP (see Table 2 and Fig. 2a) reflects the shift in the processing demands involved in the word-monitoring task, where the processing of NP sentences loads on semantic and pragmatic factors and AP loads on syntactic analysis. We obtained a similar pattern of bilateral MTG activity for lists of structurally unrelated words (RWO, Table 2 and Fig. 2c), reflecting the loading on lexical semantic analysis for RWO strings in this task.
Further analyses confirmed that LIFG was recruited more strongly for syntactic processing. In these analyses, we compared activation between the 3 prose types. To exclude differences not related to language processing, each comparison included only voxels with a significantly larger response to speech than to MuR in at least one prose type. Responses in LIFG were greater in AP than in either NP or RWO (clusters significant at P < 0.05 corrected, voxel-level P < 0.01). No regions showed the reverse effect, and there were no significant differences between NP and RWO. The comparison between AP and RWO shows activation for syntax over and above that accruing from lexical semantic analysis; this generated greater activity in a frontal cluster comprising BA 44, 45, and 47 (along with deep frontal operculum), and in 2 separate LMTG clusters—one in left anterior MTG (LaMTG; BA 21, at the border of BA 38) and another in LpMTG (BA 21, inferior to the border of angular and supramarginal gyri). Comparing AP with NP also revealed activity in LIFG BA 44/45 and LpMTG, extending to the border of supramarginal gyrus. This contrast also activated RposteriorMTG, slightly anterior to the cluster on the left.
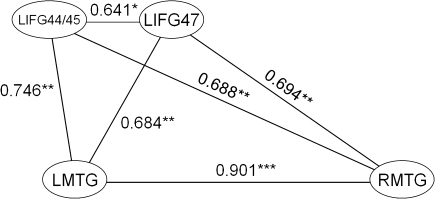
To further investigate the relationship between the neural regions involved in different linguistic analyses, we examined the intercorrelations of mean activity (see Methods) in the activated regions for each prose type separately. For syntactic processing (AP), mean activity in frontotemporal regions was highly intercorrelated (Fig. 3): Activity in two LIFG clusters (LIFG BA 44/45 and LIFG BA 47) correlated with each other (r = 0.641, P < 0.05) and each correlated with activity in the LMTG (LIFG BA 47: r = 0.684, P < 0.01; LIFG BA 44/45: r = 0.746, P < 0.01) and right middle temporal gyrus (RMTG; LIFG BA 47, r = 0.694, P < 0.01; LIFG BA 44/45, r = 0.688, P < 0.01), and activity in the LMTG was strongly correlated with activity in the RMTG (r = 0.901, P < 0.001).
Figure 3.
Significant (at P < 0.01.) correlations between activity in significantly activated regions for the contrast AP–MuR in the young group. *P < 0.05, **P < 0.01, ***P < 001.
Older Group
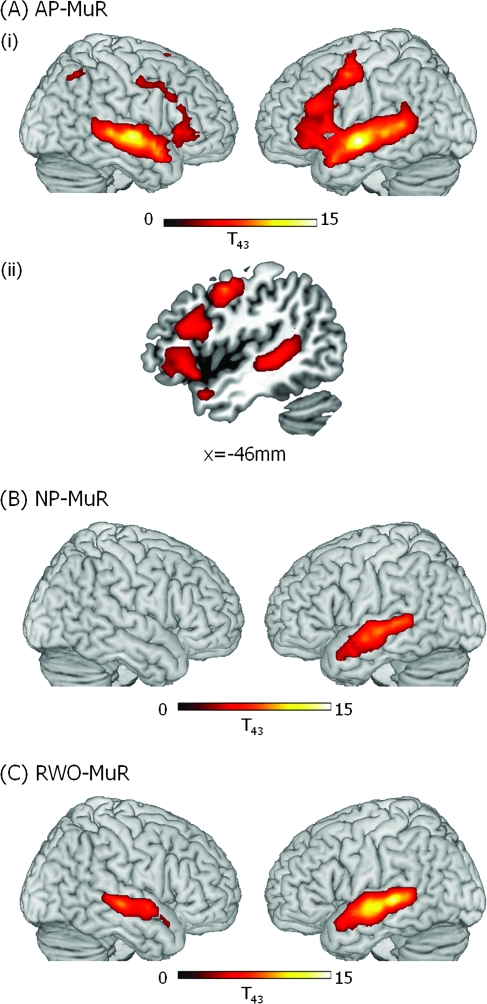
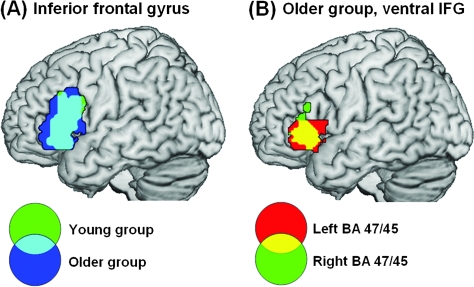
An analysis of all older participants (aged 49–86) comparing syntactic processing (AP) with baseline (MuR), showed a shift from a left frontal/bilateral STG/MTG network, typical of the younger group, to a more bilateral network for syntactic processing. The older group showed a similar, although more extensive, left frontotemporal network of activity as seen in the young and, in addition, large clusters of frontotemporal activity in the RH (see Figs 4a and 5a). This included an extensive LH frontotemporal cluster (peak voxel: x = −60, y = −9, z = 6, Z > 8.00, 1046 voxels) and a largely homologous RH frontotemporal cluster (peak voxel: x = 63, y = −12, z = −3, Z > 8.00, 1819 voxels; see Fig. 5b). In order to identify functionally relevant clusters of activity for AP, we subdivided the large frontotemporal clusters, separating frontal from temporal activity, and identified separable clusters of frontal activity (see Table 3). First, because frontal activity in both hemispheres abutted activation in middle temporal gyri, we separated activity spanning the frontal and temporal lobes by means of frontal and temporal masks defined using the WFU Pick Atlas based on the Talairach and Tournoux stereotactic atlas (Talairach and Tournoux 1988). Second, although the older group showed a similar pattern of LIFG activity for syntax (AP) as seen in the young (Figs. 4a and 5a), their activation was much more extensive: Activity in LIFG extended posteriorly and superiorly along the middle frontal gyrus (MFG) to the border of the precentral sulcus. This large cluster consisted of 3 distinct subclusters joined by narrow “bridges” of suprathreshold voxels. We divided the subclusters from each other in the axial plane where these bridges were minimized. We identified these minima objectively by averaging voxel T scores in successive axial planes along the extent of the larger cluster. This revealed local minima in activation at z = 12 mm and z = 36 mm that were used to define 3 subclusters. From ventral to dorsal, one cluster peaked in BA 47 and included a large portion of BA 45, a second involved BA 44 and the dorsal part of BA 45, and a third cluster primarily included the LMFG BA 6 (see Table 3 and Fig. 4a(ii)). Finally, just like the young group, the older group showed significant activity in both LSTG/MTG (BA 21/22) and RSTG/MTG (BA 21/22) which, in the LH, extended along the gyrus from LaMTG to LpMTG. In addition to more extensive activity, the older group generated additional regions of activity, which were absent in the young group. These included bilateral intraparietal sulci (IPS, BA 7), bilateral MFG (BA 6) and right inferior frontal gyrus (RIFG BA 47/45), and supplementary motor area (SMA).
Figure 4.
Significant clusters of activation in older participants (ages 49–86) for (A) AP–MuR, (i) activations rendered onto the surface of a canonical brain image, (ii) LH frontotemporal activity in saggital slice at x = −53; (B) Normal prose–musical rain (NP–MuR) activations rendered onto the surface of a canonical brain image; and (C) RWO (RWO–MuR) activations rendered onto the surface of a canonical brain image. Color scale indicates T value of contrast. Voxel-level threshold P < 0.001, cluster size threshold P < 0.05.
Figure 5.
Common activation to AP–MuR in LIFG between young and older groups (A) and left and right hemisphere in the older group (B). (A) The young and older groups activate overlapping regions within LIFG. Activation is shown in LIFG only and involves BA 45, 47, and 44. Clusters significant at P < 0.05 corrected with voxel-level P < 0.001 in the older group and lowered to P < 0.005 in the young group to show the full extent of the overlap. (B) The older group activate homologous regions in left and right BA 45/47. Activation shown in BA 45/47 only, clusters significant at P < 0.05 corrected, voxel-level P < 0.001.
Table 3.
Older participants (ages 49–86 years); regions showing significant activation for contrasts of each prose type minus MuR
| Contrast | Region | BA | Cluster |
Peak voxel |
MNI coordinates (mm) |
||||
| Pcorrected | Extent | PFDR | Z | x | y | z | |||
| AP–MuR | LIFGa | 47 | <0.001 | 262 | <0.001 | 5.92 | −48 | 21 | −12 |
| LIFG | 47 | <0.001 | 5.90 | −54 | 15 | 0 | |||
| LIFG | 47 | <0.001 | 4.92 | −42 | 30 | −3 | |||
| LIFGa | 45 | <0.001 | 321 | <0.001 | 5.86 | −51 | 24 | 24 | |
| LIFG | 44 | <0.001 | 5.45 | −39 | 9 | 27 | |||
| LIFG | 44 | <0.001 | 5.23 | −54 | 12 | 33 | |||
| RIFGa | 45 | 0.001 | 188 | 0.026 | 4.81 | 54 | 27 | 0 | |
| RIFG | 47 | <0.001 | 4.21 | 39 | 27 | 0 | |||
| RIFG | 47 | 0.001 | 3.98 | 45 | 27 | −12 | |||
| LMFGa | 6 | <0.001 | 191 | <0.001 | 7.05 | −48 | 0 | 48 | |
| LMFG | 6 | <0.001 | 6.31 | −45 | −6 | 54 | |||
| LMFG | 6 | <0.001 | 6.08 | −51 | −9 | 48 | |||
| RMFG | 6 | 0.024 | 64 | <0.001 | 4.32 | 54 | −6 | 45 | |
| RMFG | 9 | <0.001 | 4.30 | 54 | 21 | 30 | |||
| RMFG | 9 | 0.001 | 3.97 | 51 | 12 | 39 | |||
| LMTGa | 21 | <0.001 | 983 | <0.001 | >8.00 | −60 | −9 | −6 | |
| LMTG | 21 | <0.001 | 7.57 | −63 | −30 | 0 | |||
| LMTG | 21 | <0.001 | 7.42 | −57 | −45 | 6 | |||
| RMTGa | 21 | <0.001 | 856 | <0.001 | >8.00 | 63 | −12 | −3 | |
| RMTG | 21 | <0.001 | 7.72 | 60 | −30 | 0 | |||
| RMTG | 21 | <0.001 | 7.44 | 57 | 6 | −12 | |||
| LIPS | 7 | 0.023 | 65 | 0.001 | 4.00 | −27 | −48 | 45 | |
| LIPS | 7 | 0.001 | 3.97 | −24 | −66 | 39 | |||
| LIPS | 7 | 0.001 | 3.95 | −33 | −57 | 49 | |||
| RIPS | 7 | 0.008 | 83 | <0.001 | 4.90 | 33 | −54 | 45 | |
| RIPS | 7 | 0.004 | 3.35 | 33 | −69 | 33 | |||
| LSMA | 6 | <0.001 | 152 | <0.001 | 6.43 | −6 | 3 | 63 | |
| LSMA | 6 | <0.001 | 4.06 | 0 | 12 | 48 | |||
| NP–MuR | LMTG | 21 | <0.001 | 567 | <0.001 | 6.71 | −60 | −18 | −6 |
| LMTG | 21 | <0.001 | 6.70 | −60 | −33 | 3 | |||
| LMTG | 21 | <0.001 | 6.63 | −54 | −39 | 3 | |||
| RWO–MuR | LMTG | 21 | <0.001 | 727 | <0.001 | >8.00 | −63 | −30 | 0 |
| LMTG | 21 | <0.001 | 7.83 | −63 | −18 | −6 | |||
| LMTG | 21 | <0.001 | 5.03 | −51 | 12 | −18 | |||
| RMTG | 21 | <0.001 | 349 | <0.001 | 6.95 | 60 | −24 | −3 | |
| RMTG | 21 | <0.001 | 6.00 | 60 | 0 | −6 | |||
| RMTG | 21 | <0.001 | 5.34 | 66 | −12 | −3 | |||
Frontal and temporal clusters identified by segmenting from large continuous LH or RH frontotemporal clusters (see Results for segmentation details).
Note: Extent, 3 × 3 × 3 mm voxels; PFDR, peak voxel statistical significance corrected for multiple comparisons using false discovery rate; IPS, intraparietal sulcus. Bold type: statistics for whole cluster and peak voxel. Normal type: voxel-level statistics for local maxima > 8mm apart.
As with the younger group, comparisons between prose types reinforced the findings from the comparisons of each prose type against baseline. AP produced stronger responses than RWO in bilateral IFG and RMTG. Responses in bilateral IFG were in separate clusters in BA 47/45 and BA 6/44 at voxel-level P < 0.001 and at P < 0.01 formed a large swathe of activation from BA 47 extending superiorly and posterior through BA 45 and 44 to 6. Activation in RMTG extended from BA 38 anteriorly to the posterior border of BA 42 posteriorly. There were no significant clusters showing stronger responses to NP or RWO than to AP and no significant differences between NP and RWO.
As in the analyses of the younger group, we examined the relation between activity and other measures by computing correlations with activation in different regions for the AP condition. We first correlated age within the older group (49 to 86 years) with activity in the bilateral frontal and STG/MTG clusters and found that activity increased as a function of increasing age in all the frontal regions (all r values > 0.3, P < 0.05) but in neither of the STG/MTG clusters. In the other activated clusters, although activity within the left inferior parietal sulcus (LIPS) and SMA significantly positively correlated with age (LIPS: r = 0.379, P < 0.05; SMA: r = 0.308, P < 0.05), activity in the RIPS was not age sensitive (r = 0.221, P = 0.149).
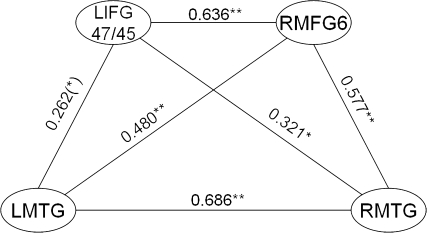
We next correlated activity between the significant clusters in the AP condition and found that activity within each cluster was correlated with activity in a wide range of other clusters. Of these, the main correlations were within the 3 LH frontal clusters (all r values > 0.5, P < 0.001), the 2 RH frontal clusters (r = 0.431, P < 0.01), the RMTG and LMTG (r = 0.686, P < 0.001), and between the RIPS, LIPS, and SMA (all r values > 0.38, P < 0.01). Because the important difference between the older and young groups centers on the relationship between the left BA 44/45 and bilateral MTG—regions whose interaction is considered to be central in the processing of syntactic structure (Dronkers et al. 2004; Rodd et al. 2004; Caplan et al. 2008; Tyler and Marslen-Wilson 2008)—we focussed primarily on correlations within these regions. In the present study, the younger group (see Fig. 3) showed strongly correlated activity between the two LIFG regions (BA 44/45 and BA 47) between both LIFG regions and bilateral MTG (all r values > 0.6, P < 0.001), and between left and right MTG (r = 0.901, P < 0.001). A similar pattern of left frontal/bilateral temporal correlation was also seen in the older group with correlated activity between LIFG BA 44/45 and LIFG BA 47/45 (r = 0.642, P < 0.001), LIFG BA 47/45 and bilateral temporal regions (LMTG: r = 0.262, P = 0.086; RMTG: r = 0.321, P < 0.05), and between LMTG and RMTG (r = 0.686, P < 0.001). The hub of the left frontal system seems to be the BA 47/45 cluster because it was only for this region that activity correlated with bilateral MTG activity. However, the strong correlations in activity between the 2 left frontal regions suggests that both contribute to the construction of a syntactic representation.
In addition, in the older group, activity in each of these left frontal/bilateral temporal regions correlated with activity in RMFG BA 6 (LIFG BA 47/45: r = 0.636, P < 0.001; LIFG BA 44/45: r = 0.424, P < 0.01; LSTG/MTG: r = 0.480, P < 0.01; RSTG/MTG: r = 0.577, P < 0.001; see Fig. 6), and activity in right BA 47/45 marginally correlated with activity in left BA 47/45 (r = 0.256 P = 0.093). In addition to the bilateral frontotemporal network, syntactic analysis also activated bilateral IPS and the SMA in the older group. Activity in all these regions correlated with each other (all r values > 0.38, P < 0.01) and with LH frontal regions (all r values > 0.3, P < 0.05, except RIPS), but not with LSTG/MTG or RSTG/MTG. Also, activity in IPS regions did not correlate with activity in any of the RH frontal regions, although there were significant correlations between activity in SMA and RH frontal clusters (all r values > 0.3, P < 0.05).
Figure 6.
Significant (at P < 0.01) correlations between activity in significantly activated regions for the contrast AP–MuR in the older group. (*)P < 0.1, *P < 0.05, **P < 0.01.
This extensive pattern of correlated activity in the AP condition suggests that a similar left frontal/bilateral temporal neural system underpinned syntactic processing in both the young and the older subjects, with the older group showing additional RH involvement as well as activity within bilateral parietal regions and the SMA. Although bilateral parietal activity, which is typically associated with task-related attentional demands (Rushworth et al. 2001; Corbetta and Shulman 2002), was highly correlated with activity in some other regions, it was not convincingly correlated with the bilateral network of regions involved in language.
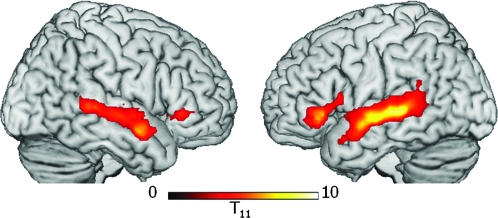
To investigate further the possibility that increased attentional demands in AP for older subjects may have affected activity due to language processing, we carried out a further experiment with a subset of the older group. Twelve subjects (aged 56–81 years) who had participated in the word-monitoring study were scanned 12–14 months later in a passive listening study, using exactly the same stimuli as in the word-monitoring experiment. We found a similar pattern of bilateral frontotemporal activity in passive listening to AP sentences as we had seen in the word-monitoring task, but without the bilateral parietal activity seen in the word-monitoring study. Activation for AP included bilateral MTG and bilateral left BA 47/45, extending into BA 44 on the left (see Fig. 7), which clearly paralleled the bilateral frontal (BA 47/45) and temporal activation for AP seen in the word-monitoring study. These parallels and differences in the activation patterns seen in the two tasks, together with the absence of a correlation between bilateral parietal and bilateral frontotemporal activations in the word-monitoring task, strongly suggest that the increased language-related activations seen bilaterally in the older adults were language-specific and not generated by age-related increases in attentional demands.
Figure 7.
Activation to AP–MuR in the absence of a task in a subset of the older group (ages 56–81). Participants passively listened to stimuli identical to those used in the word-monitoring task 12–14 months after their first scan. Activation is shown only in voxels active during word monitoring, with threshold lowered to voxel-level P < 0.01, cluster size >60 to maximize sensitivity to common effects. Clusters in LIFG BA 47/45, RIFG BA 47/45, and bilateral MTG were significant at P < 0.05 corrected, except RIFG (P < 0.05 uncorrected). No effects were seen in MFG or parietal lobe.
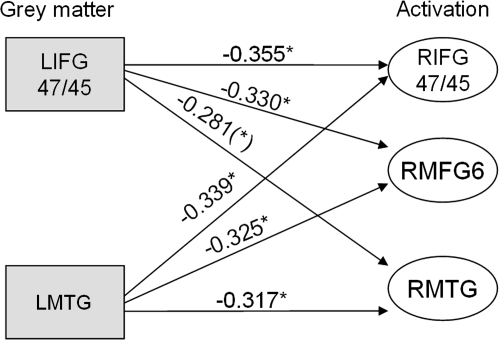
We next asked whether age-related decreases in GM density within the older group were associated with changes in activity by correlating activity in each region with extracted measures of mean GM density (see Methods). First, we established that mean GM densities in activated regions were positively correlated with each other (all r values > 0.3, P < 0.05) and GM density in all activated regions correlated negatively with age (all |r values| > 0.3, P < 0.05). Second, we examined the relationship between GM density and activity, focusing on the left hemisphere frontotemporal language networks seen in both the older and the young groups in the AP condition. Decreasing GM density in LMTG was associated with increased activity in RIFG BA 47/45, RMFG BA 6, and RMTG (r = −0.339, P < 0.05; r = −0.325, P < 0.05; r = -0.317, P < 0.05, respectively; see Fig. 8). In LIFG BA 47/45—a key region in the LH frontotemporal correlational analyses—decreasing GM density was associated with increased activity in homologous RH regions (RIFG BA 47/45: r = −0.355, P < 0.05; RMFG BA 6: r = −0.330, P < 0.05; and RMTG: r = −0.281, P = 0.065), and it correlated marginally with activity in the same region (r = −0.273, P = 0.073). Decreases in GM density in LBA 44/45 did not correlate with increases in activity. In contrast, activity in left frontotemporal correlated regions did not change in response to RH or LH GM decreases. This pattern of correlations invites the inference that RH frontotemporal activity reflects compensation for decreases in GM density in the LH frontotemporal language network. Outside of the language network, there was little evidence for similar patterns of compensation: GM decreases in RIPS and the SMA were both associated with increased activity in RIFG BA 47, whereas GM decreases in LIPS had no functional consequences.
Figure 8.
Correlations between measures of GM integrity and neural activity in the older group. Gray squares represent GM changes and white ovals represent significant clusters of activity in specified regions. The graph shows that decreases in GM in left frontotemporal regions are associated with increases in right frontotemporal activation (*)P < 0.1, *P < 0.05.
This age-related shift to a bilateral frontotemporal system can be largely localized to the processing of syntactic structure. When comprehension can be supported by semantics and pragmatics, as in the case of processing normal sentences (NP–MuR), the older group showed essentially the same network as the younger group, which was dominated by STG/MTG activity, with frontal cortex less involved. The only difference in activity between the young and old was the small cluster of activity in the RSTG/MTG in the young which was absent in the old. Likewise, older adults’ processing of random word strings (RWO–MuR) involved the same pattern of bilateral MTG activity seen with younger adults (see Table 3).
The age-related shift to bilateral processing of syntax largely relied on the recruitment of RH frontal regions. We confirmed that RIFG recruitment is the locus of this change in laterality by conducting age by hemisphere interactions on regional activity. First, we extracted mean activation values from clusters in bilateral IFG BA 47/45. Because the younger group did not have significant activation for syntax in the RIFG, we extracted activation from an RIFG region homologous to the observed activation in LIFG BA 47/45. Bilateral clusters from the young group were located largely within the clusters from the mature groups (proportion of young cluster overlapping mature in LIFG: 82%, in RIFG: 70%). We then ran a 2-way ANOVA with the within-subject factor of hemisphere and the between-subjects factor of age-group. Consistent with the activation in each age-group separately, the ANOVA showed a significant interaction of age-group and hemisphere (F1,56 = 10.3, P < 0.01). This interaction reflected similar levels of activation in the LIFG between age-groups but stronger activation in the RIFG for the older compared with the younger group. We confirmed that these age-related changes were specific to processing of syntax in IFG by running similar analyses in the MTG for all 3 prose types. Activity levels in the MTG showed no age-group by hemisphere interaction in AP or RWO (AP: F1,56 = 2.1, P = 0.15, RWO: F1,56 = 0.007, P = 0.9). The mature group did not activate RMTG during NP, so as above, we extracted activation from a region homologous to activation in LMTG. Further confirming the specificity of our findings for syntax, processing of NP in MTG showed the opposite effect, with more left-lateralized activity in the mature than young group (F1,56 = 5.7, P < 0.05).
Discussion
Our results demonstrate a complex interplay of neural structure, neural activity, and performance, which support our main predictions: First, we found no evidence for age-related performance differences in the comprehension of syntax with or without semantic support or in more general sentence comprehension capacities. Performance was preserved in the older group despite age-related GM loss in the network of neural regions typically involved in syntactic aspects of language comprehension. Accounting for preserved performance, we found evidence for age-related neural compensation, where increased RH frontotemporal activity was associated with age-related neural atrophy in the LH frontotemporal network activated in the younger group. We argue that this shift toward a bilateral functional language network underpins preserved syntactic processing functions in the older group.
Syntactic Processing in Younger Adults
The pattern of results across the 3 types of prose supports the general claim that spoken language processing is carried out within a primarily left-lateralized frontotemporal system which is modulated by different types of linguistic analysis, such as syntax, morphology, and semantics (Caplan et al. 1998; Friederici et al. 2003; Marslen-Wilson and Tyler 2007; Tyler and Marslen-Wilson 2008). Processing spoken words involves bilateral STG/MTG, with stronger and more extensive activity in the LH compared with the RH (e.g., Indefrey and Cutler 2004). The LIFG—especially BA 45—is primarily involved in processing the syntactic aspects of language, in conjunction with LMTG (Caplan et al. 1996). We see this in the present study by the enhanced activity in the LIFG when syntactic structure is the primary source of information available. Activity in the LIFG is modulated by the linguistic properties of the spoken input such that it is maximally engaged when sequential processing is primarily based on syntactic analysis (in AP) without semantic support. There is reduced activity in the LIFG when comprehension can be supported by the combination of word meanings and their pragmatic implications, as in the case for NP. It is important to note that the increased frontal activity for syntactic analysis (AP) cannot be simply attributed to the increased processing difficulty for these materials in the context of the demands of the task (Kaan and Schwaab 2002; Crinion et al. 2003) because RWO, where processing difficulty was greatest (as reflected in longer RTs), showed only bilateral MTG activity, with no increased activation of left frontal cortex, arguing against a general effect of task difficulty.
Syntactic Processing in Older Adults
Two main findings emerge from younger and older adults’ neural activity during sentence processing: First, older adults employ a similar network of regions during sentence comprehension as younger adults—this is evident from the activity in response to NP sentences and also the LH frontotemporal network activated in response to AP items. However, age seems to adjust the relationship between frontal and temporal regions, with reorganization involving changes in interregional connectivity. Although activity within both frontal clusters—left BA 44/45 and 47/45—were highly correlated in the older group, only the latter was correlated with MTG activity, suggesting that although aging may weaken some frontotemporal connections, functionality is preserved by means of strong connectivity between functionally related regions.
Second, when processing syntax without semantic support (AP), older adults recruit a more extensive left frontotemporal network. In addition, they recruit a RH frontotemporal network that works in conjunction with the LH network to support preserved performance. Note that the measure of syntactic and semantic processing that we employed here—WPE—directly reflects the online construction of syntactic and semantic representations. This differs from previous studies which have reported age-related declines in comprehension using off-line measures, which make it difficult to dissociate age-related changes in language processing from age-related changes in other cognitive task components, such as memory (for review, see Burke and MacKay 1997). Our results are in keeping with online comprehension measures which typically do not support age-related deficits in either semantic or syntactic aspects of sentence processing (e.g., Light et al. 1991; Waters and Caplan 2001, 2005).
Evidence that RH activity is “compensatory” comes both from performance across the life span and from the relationship between structure and function across the hemispheres. First, we found preserved performance across the life span, with older subjects showing WPEs which are indistinguishable from the young group. Moreover, within the older group (aged 49–86), there were no age-related changes in the WPEs for each of the 3 prose types, and the pattern of WPEs across the 3 prose types did not change as a function of age, which is important for establishing that performance is maintained across the life span. Both NP and AP showed significant WPEs in both the young and the older groups, replicating previous behavioral data (Tyler 1992), and there was no WPE in RWO for either age-group, establishing that the WPE in NP and AP measures the online word-by-word construction of syntactic and semantic representations. There is no suggestion in these behavioral data that older subjects engaged in processes that were different from the young while processing spoken language.
Perhaps, the most compelling evidence that RH activity is compensatory comes from hemispheric differences in the relation between GM density and activity. Although age-related atrophy impacted all of the neural regions which were activated during syntactic processing, only atrophy in a subset of these regions was associated with increased activity. Atrophy in left BA 47/45 was associated with increased atrophy in that region, and increases in functional activity in the RH frontotemporal regions were only associated with atrophy in those left frontotemporal regions which were similar to the LH regions associated with syntactic processing in the younger group. In contrast, neural atrophy in the RH was not related to neural activity in the LH. Age-related atrophy was, additionally, accompanied by changes in the functional connectivity between regions. Apart from correlated activity in left BA 47/45 and LSTG/MTG, in the older group, there was also correlated activity in the RH, but this did not involve inferior frontal cortex; rather activity in RMFG (BA 6) correlated with activity in RMTG.
These variations in LH and RH correlations, together with the finding that activity in RIFG BA 45/47 correlated with activity in LH BA 47/45, suggest that right frontal activation contributes to syntactic processing by supporting the activity of the LIFG under conditions where the integrity of the LIFG becomes increasingly compromised during aging. It is unlikely that the right frontotemporal regions which are correlated (right BA 6 and RMTG) form a network which are directly involved in syntactic analysis because this would be inconsistent with existing data and neural models of syntactic function which are thought to involve the LH.
Finally, syntactic analysis was also associated with activity in bilateral parietal regions which are typically associated with increased attentional demands (Rushworth et al. 2001; Corbetta and Shulman 2002). However, parietal activation had almost no direct effects on the frontotemporal language network: Activity in the LIPS only correlated with activity in LBA 47 and activity in the RIPS did not correlate with activity in any region other than the LIPS. In addition, GM loss in these parietal regions did not correlate with increases in activity in any of the regions involved in the frontotemporal language network nor did GM loss in any of the language network regions correlate with increased activity in bilateral parietal regions. These results suggest that parietal activity did not significantly contribute toward preserved language function or toward the shift to a bilateral frontotemporal language network in the older group, a conclusion supported by the results of the passive listening study. This established that, although middle frontal and parietal regions are only activated in the word-monitoring task and not during passive listening, nevertheless the same bilateral frontotemporal regions are activated in both.
Recruitment of RH Regions: Syntactic Processing or General Resources?
Our results support the conclusion that during syntactic processing, older adults recruit a right frontotemporal network homologous to the left-lateralized frontotemporal language network. However, previous neuroimaging studies of cognitive aging have focussed on the role of frontal cortex and its impact on executive function, suggesting that increased frontal activity may reflect the recruitment of cognitive resources which make a “general” contribution to preserved performance (Reuter-Lorenz 2002). According to this account, the RH frontal activity observed in the older but not the younger group reflects the recruitment of some general (or at least nonlinguistic) processes which provide cognitive support to older adults to perform the task. This stands in contrast to the claim that recruitment of a RH frontotemporal network, homologous to that in the LH, is involved—albeit indirectly—in syntactic processing. The current study was not designed to directly test between these possibilities, but some of our findings are relevant.
Support for the claim that increased frontal activity indicates the additional recruitment of general processing resources primarily comes from the finding that, although bilateral IFG activity increased with age within the older group, temporal activity did not. This finding is broadly in keeping with the frontal lobe hypothesis (West 1996) and with other cognitive/behavioral models of aging which posit that cognitive changes in old age reflect a decline in a general cognitive resource such as working memory capacity, which is linked by some researchers to the early and substantial drop in frontal cortex function (e.g., West 1996). The word-monitoring task minimizes memory requirements by making the target word available throughout the trial and measuring comprehension online. However, it is possible to argue that compared with the NP condition, the AP condition taxed working memory more because syntactic structures are constructed without semantic support.
However, a number of findings are problematic for an interpretation of the current data in terms of the general resource decrement hypothesis. First, measures of working memory (digit span forward and backward) taken during cognitive screening of our participants did not correlate with our performance measures (WPE score) or with activity in any of the frontal regions activated in syntactic processing (all |r| < 0.23, P > 0.1). Also problematic is the finding that older adults do not only recruit RH frontal regions but also MTG regions. These results are in keeping with models of cognitive aging, which suggest “specific” rather than general age-related mechanisms, under which changes to language reflect linguistic factors rather than reflecting a general cognitive resource limitation (e.g., Burke et al. 2000).
Summary and Conclusions
Our results support behavioral research demonstrating that language comprehension is preserved in old age and are consistent with a complex picture in which language comprehension is spared in the context of declining aspects of language production (for reviews, see Burke et al. 2000; Burke and Shafto 2004; Shafto et al. 2007; Burke and Shafto 2008). Because language production and comprehension largely engage the same sets of neural regions, this pattern of preservation and decline raises interesting questions, such as why does neural change affect language production more than comprehension, and if neural compensation preserves comprehension, why does it not preserve language production (e.g., Shafto et al. 2007)? The answer to these questions is beyond the scope of our current findings, but we believe that differences in the patterns of connectivity within the neural networks involved in language comprehension and production may help to clarify these issues.
More generally, our results support models of cognitive aging in which age does not result in the inexorable decline of all cognitive functions, contradicting cognitive and neurocognitive models of aging which attempt to identify universal factors underpinning general cognitive declines in old age. Our results underscore the importance of identifying abilities (such as online syntactic processing) that do not decline behaviorally with normal aging; they are as important for characterizing normal aging as abilities that decline. First, language is a critical everyday cognitive skill, and real and perceived performance declines are a major concern for older adults (e.g., Lovelace and Twohig 1990) and a part of what drives negative stereotypes and condescending treatment of older adults (e.g., Ryan et al. 1986; Kemper and Harden 1999). To counteract the assumption of universal cognitive decline, it is important to highlight the fact that many complex cognitive processes, such as language comprehension, remain stable in old age. Preserved abilities also play a crucial role in the development of cognitive aging theories because successful theories must account for both spared and impaired abilities. This is particularly apparent in neuroimaging results such as those reported in the current study, where preserved abilities present an important context for understanding the relevance of age-related changes in neural activity, which have become the focus of much recent research (e.g., Reuter-Lorenz et al. 2001; Reuter-Lorenz 2002). Our results indicate that older adults’ bilateral activity is compensatory and helps preserve performance. Critically, these results indicate that not only is language comprehension preserved across the life span but so is the neural plasticity required for compensatory recruitment. The question that remains for future research is to understand more specifically the cognitive and neural contexts associated with neural compensation and whether recruitment is always associated with improvements in performance.
Funding
This research was supported by an MRC programme grant (G0500842 to L.K.T.); a Strategic Promotion of Ageing Research Capacity grant (to L.K.T. and M.A.S.); Research into Ageing Fellowship (to M.A.S.).
Acknowledgments
We thank Phillip Kellmeyer for his help with this study and Marie Dixon for her extensive administrative assistance. Conflict of Interest: None declared.
References
- Berndt RS, Caramazza A. Syntactic aspects of aphasia. In: Sarno MT, editor. Acquired aphasia. New York: Academic Press; 1981. pp. 157–181. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Blesser B. Speech perception under conditions of spectral transformation. 1. Phonetic characteristics. J Speech Hear Res. 1972;15:5–41. doi: 10.1044/jshr.1501.05. [DOI] [PubMed] [Google Scholar]
- Braver TS, West RF. Working memory, executive control, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York: Psychology Press; 2008. pp. 311–372. [Google Scholar]
- Brett M. Using the Talairach atlas with the MNI template. Neuroimage. 2001;13:S85. [Google Scholar]
- Burke DM, MacKay DG. Memory, language and ageing. Philos Trans R Soc Lond B Biol Sci. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, James LE. Theoretical approaches to language and aging. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford: Oxford University Press; 2000. pp. 204–237. [Google Scholar]
- Burke DM, Shafto MA. Aging and language production. Curr Dir Psychol Sci. 2004;13:21–24. doi: 10.1111/j.0963-7214.2004.01301006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Shafto MA. Language and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York: Psychology Press; 2008. pp. 373–443. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci. 1998;10:541–552. doi: 10.1162/089892998562843. [DOI] [PubMed] [Google Scholar]
- Caplan D, Chen E, Waters G. Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex. 2008;44:257–275. doi: 10.1016/j.cortex.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Hildebrant N. Disorders of syntactic comprehension. Cambridge (MA): MIT Press; 1988. [Google Scholar]
- Caplan D, Hildebrant N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119:933–949. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in language comprehension—evidence from aphasia. Brain Lang. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’ a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Fiebach CJ, Schlesewsky M, Bornkessel ID, von Cramon DY. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, De Vita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: an fMRI study. Neuroimage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. ”Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Cutler A. Pre-lexical and lexical processing in listening. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge (MA): MIT Press; 2004. pp. 759–774. [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension—individual-differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Schwaab T. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6:350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cereb Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kemper S, Harden T. Experimentally disentangling what's beneficial about elderspeak from what's not. Psychol Aging. 1999;14:656–670. doi: 10.1037//0882-7974.14.4.656. [DOI] [PubMed] [Google Scholar]
- Kemper S, Sumner A. The structure of verbal abilities in young and older adults. Psychol Aging. 2001;16:312–322. [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. New York: Psychology Press; 2008. pp. 189–250. [Google Scholar]
- Light LL, Valencialaver D, Zavis D. Instantiation of general terms in young and older adults. Psychol Aging. 1991;6:337–351. doi: 10.1037//0882-7974.6.3.337. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lovelace EA, Twohig PT. Healthy older adults’ perceptions of their memory functioning and use of mnemonics. Bull Psychon Soc. 1990;28:115–118. [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Processing structure of sentence perception. Nature. 1975;257:784–786. doi: 10.1038/257784a0. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. The temporal structure of spoken language understanding. Cognition. 1980;8:1–71. doi: 10.1016/0010-0277(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Morphology, language, and the brain: the decompositional substrate for language comprehension. Philos Trans R Soc Lond B Biol Sci. 2007;362:823–836. doi: 10.1098/rstb.2007.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Narain C, Scott SK, Wise RJS, Rosen S, Leff A, Iversen SD, Matthews PM. Defining a left-lateralized response specific to intelligible speech using fMRI. Cereb Cortex. 2003;13:1362–1368. doi: 10.1093/cercor/bhg083. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychol Bull. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition—II. Mahwah (NJ): Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Marshuetz C, Jonides J, Smith EE, Hartley A, Koeppe R. Neurocognitive ageing of storage and executive processes. Eur J Cogn Psychol. 2001;13:257–278. [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE. An event-related fMRI investigation of implicit semantic priming. J Cogn Neurosci. 2003;15:1160–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Longe OA, Randall B, Tyler LK. Syntactic and semantic processing of spoken sentences: an fMRI study of ambiguity. J Cogn Neurosci. 2004;16:C89. [Google Scholar]
- Rushworth MFS, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J Neurosci. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EB, Giles H, Bartolucci G, Henwood K. Psycholinguistic and social psychological components of communication by and with the elderly. Lang Commun. 1986;6:1–24. [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Burke DM, Stamatakis EA, Tam PP, Tyler LK. On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. J Cogn Neurosci. 2007;19:2060–2070. doi: 10.1162/jocn.2007.19.12.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardised set of 260 pictures: norms for name agreement, familiarity and visual complexity. J Exp Psychol Learn Mem. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S. Localization of syntactic comprehension by positron emission tomography. Brain Lang. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart (Germany): Georg Thieme Verlag; 1988. [Google Scholar]
- Tyler LK. Children's processing of spoken language. Journal of Verbal Learning and Verbal Behaviour. 1981;20:400–416. [Google Scholar]
- Tyler LK. Spoken language comprehension: an experimental approach to disordered and normal processing. Cambridge (MA): MIT Press; 1992. [Google Scholar]
- Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans R Soc Lond B Biol Sci. 2008;363:1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson W, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–1296. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Hupet M, Feyereisen P, Schelstraete MA, Bestgen Y, Bruyer R, Lories G, El Ahmadi A, Seron X. Cognitive mediators of age-related differences in language comprehension and verbal memory performance. Aging Neuropsychol Cogn. 1999;6:32–55. [Google Scholar]
- Waters GS, Caplan D. The relationship between age, processing speed, working memory capacity, and language comprehension. Memory. 2005;13:403–413. doi: 10.1080/09658210344000459. [DOI] [PubMed] [Google Scholar]
- Waters GS, Caplan D. Age, working memory, and on-line syntactic processing in sentence comprehension. Psychol Aging. 2001;16:128–144. doi: 10.1037/0882-7974.16.1.128. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]