Abstract
Numerous functional neuroimaging studies have observed lateral parietal lobe activation during memory tasks: a surprise to clinicians who have traditionally associated the parietal lobe with spatial attention rather than memory. Recent neuropsychological studies examining episodic recollection after parietal lobe lesions have reported differing results. Performance was preserved in unilateral lesion patients on source memory tasks involving recollecting the context in which stimuli were encountered, and impaired in patients with bilateral parietal lesions on tasks assessing free recall of autobiographical memories. Here, we investigated a number of possible accounts for these differing results. In 3 experiments, patients with bilateral parietal lesions performed as well as controls at source recollection, confirming the previous unilateral lesion results and arguing against an explanation for those results in terms of contralesional compensation. Reducing the behavioral relevance of mnemonic information critical to the source recollection task did not affect performance of the bilateral lesion patients, indicating that the previously observed reduced autobiographical free recall might not be due to impaired bottom-up attention. The bilateral patients did, however, exhibit reduced confidence in their source recollection abilities across the 3 experiments, consistent with a suggestion that parietal lobe lesions might lead to impaired subjective experience of rich episodic recollection.
Keywords: metamemory, neuropsychology, recall, recollection, source memory
Introduction
The role played by the human parietal lobe in episodic memory has recently provoked a great deal of controversy amongst neuroscientists (see Cabeza et al. 2008; Simons and Mayes 2008, for reviews). This interest arose after numerous reports in functional neuroimaging studies of significant lateral parietal lobe activation during episodic memory tasks, particularly those involving the recollection of previously encountered information (Wagner et al. 2005). Activity in parietal regions is often observed during stimulus recognition tasks (Konishi et al. 2000), which may involve recollection and/or a sense of familiarity, and during tasks that contrast recollection against familiarity, emphasizing processes involved in retrieving details of the context in which stimuli were previously encountered. For example, lateral parietal activity has often been observed during performance of source memory (Dobbins et al. 2002) and remember/know (Henson et al. 1999) tasks, which both involve recollection of context details concerning prior occurrence (Tulving 1985; Johnson and Raye 2000). Indeed, a recent analysis indicated that lateral parietal cortex exhibited significant activity more consistently across studies of recollection than other brain regions generally considered more important for memory, such as prefrontal and medial temporal cortices (Simons et al. 2008). The remarkable consistency of these parietal lobe findings has been difficult to explain because they contradict the traditional view that lateral parietal lesions typically impair visual and spatial attention, but do not result in amnesia (Critchley 1953; Mesulam 1999). Despite this clinical impression, the recurrent neuroimaging results raise the possibility that previously unidentified recollection deficits may be present in patients with parietal lesions.
Three recent studies sought to address this issue using tests of source recollection, autobiographical recall, and remember/know judgments. Simons et al. (2008) used a source memory task that evoked significant activity in lateral parietal cortex when healthy volunteers recollected the context in which stimuli were previously encountered. When the same task was administered to patients with unilateral parietal lobe lesions that overlapped closely with the regions activated in the healthy volunteers, no significant impairment was observed in the patients. In another study, however, Berryhill et al. (2007) tested autobiographical recollection in patients with bilateral parietal lobe lesions, asking them to recall significant events from different periods of their lives. During free recall, the patients exhibited significantly diminished vividness and amount of detail in their spontaneous autobiographical recollections. Finally, Davidson et al. (2008) compared source memory and remember/know judgments concerning previously presented word-definition pairings in patients with unilateral parietal lesions, observing normal source recollection performance, consistent with the results of Simons et al. (2008), but reduced numbers of “remember” responses.
The present study investigates a number of possible accounts for these differing results. One potential explanation is that the preserved source recollection observed in the patients with unilateral parietal lesions in the studies by Simons et al. (2008) and Davidson et al. (2008) might be attributable to compensation from the patients’ intact contralesional parietal lobe. If this were the case, the prediction in the present study, which involves patients with unilateral and bilateral parietal lesions, would be that significant source recollection impairments should be observed in the bilateral patient group. Another possibility is that the parietal lobe may support attentional processes recruited in the service of episodic memory (Cabeza et al. 2008), and that the reduced free recall performance observed by Berryhill et al. (2007) might be due to a deficit in the capture of attention by mnemonic representations. This hypothesis is examined in the present study by manipulating the behavioral relevance (Corbetta and Shulman 2002) of mnemonic information during a source memory task which, if this view is correct, should have an impact on the patients’ recollection performance. An alternative account for the patient findings is that parietal lesions may impair the subjective experience of confidence in the richness or vividness of one's memories, leading to impoverished autobiographical recall (Ally et al. 2008; Davidson et al. 2008). If reduced subjective recollection occurs following parietal lobe lesions, then patients may exhibit normal source memory performance, but reduced ratings of subjective confidence in their recollections (Lyle and Johnson 2006, 2007).
Materials and Methods
Experiment 1
The first experiment investigated the ability of patients with unilateral and bilateral parietal lobe lesions to recollect whether sentences had been read aloud to them during a previous study phase by either a male or female speaker. The gender of the speaker was made particularly behaviorally salient by using an orienting task during the study phase that focused participants’ attention specifically towards whether the speaker was male or female. To additionally investigate the effect of parietal lesions on subjective recollection, participants were also asked to rate how confident they were in recollecting the speaker's gender for each trial.
Participants
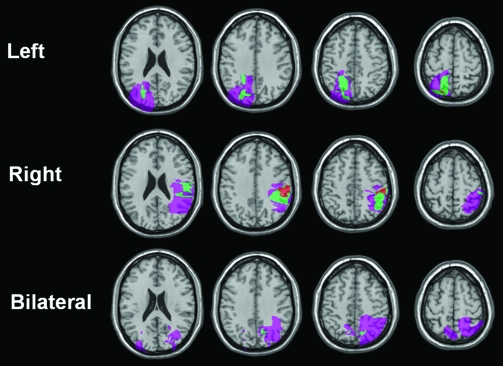
Six patients with unilateral parietal lobe lesions (3 left, 3 right, mean age 60.2 years), and 3 patients with bilateral parietal lobe lesions (mean age 49.0 years), participated in the first experiment, along with an equal number of healthy control volunteers, individually age-matched with the patients (mean age for unilateral controls: 60.0 years, bilateral controls: 49.7 years). Two of the bilateral patients were also involved in the study by Berryhill et al. (2007). Patients were recruited without regard for behavioral profile, on the basis of their lesion record indicating stable, nontraumatic brain injury affecting unilateral or bilateral parietal cortex. Lesions were the result of infarcts or surgical resection of meningiomas. Lesion overlay diagrams for the patients with unilateral and bilateral parietal lobe lesions are displayed in Figure 1. The unilateral and bilateral patient groups were matched for overall lesion volume (mean volume 43 075 mm3 for the unilateral patients and 34 996 mm3 for the bilateral patients), t (7) = 0.49, P = 0.64, and for volume of parietal cortex involvement (mean volume 13 727 mm3 for the unilateral patients and 14 512 mm3 for the bilateral patients), t (7) = 0.08, P = 0.94. Lesions in all patients involved lateral parietal (Brodmann areas [BAs] 5, 7, 39, 40) and occipital cortices (BAs 17–19), sparing retrosplenial and posterior cingulate areas (BA 29, 30). In addition, there was variable involvement of somatosensory areas (BAs 1–4) in some of the patients. Informed consent was obtained from all participants in a manner approved by the Cambridge Psychology Research Ethics Committee and the Institutional Review Boards at the University of Pennsylvania and Temple University. Participants received honoraria for their participation.
Figure 1.
Lesion overlay diagrams of the patients with unilateral and bilateral parietal lobe lesions, manually traced on a structural MRI scan of their brain, normalized to MNI space, and displayed on axial slices of a canonical structural image.
Procedure
The stimuli consisted of 144 “trivia” sentences, previously used by Simons et al. (2004), and designed such that subjects would be unlikely to know whether they were true or false (e.g., “Al Capone's business card said he was a used furniture dealer.”). Digital recordings were made of 4 speakers, 2 male and 2 female, reading out each of the sentences.
In the study phase, 72 of the sentences were presented auditorily to participants through headphones or loudspeaker. Half the sentences were spoken by one of the male speakers, and the other half by one of the female speakers, pseudorandomly intermixed such that no more than 4 consecutive trials were spoken by the same speaker. After hearing each sentence, participants were oriented towards the gender of the speaker by being asked to judge whether the sentence had been read aloud by a male or female speaker. They were also asked to judge whether the speaker believed that the sentence was true or false. For each judgment, participants responded verbally and the experimenter pressed an appropriate key on the computer keyboard.
Following the study phase, participants were administered a surprise memory test. One hundred and forty-four sentences were presented auditorily to participants, comprising the 72 previously studied old sentences randomly intermixed with 72 new sentences. The sentences were read aloud by different speakers to those encountered in the study phase, half by a male speaker and half by a female speaker. For the old sentences, half were spoken by a speaker of the same gender as previously, whereas the other half were spoken by a speaker of different gender. Participants were asked to judge whether each sentence was old or new and, if they thought a sentence was old, to recollect whether the sentence had previously been read aloud by a male or female speaker. Following each memory judgment, participants were asked to rate how confident they were in their decision on a scale of 1–9 (1 being extremely unsure and 9 being extremely sure); thus 2 confidence ratings were made. As before, participants responded verbally and the experimenter recorded each response with a key press.
In both phases, participants had as long as they wanted to make their judgments. Different versions of the task were created to allow old/new status and speaker gender to be counterbalanced between subjects.
Experiment 2
In the second experiment, we examined the effect of a study phase manipulation that was aimed at reducing the behavioral relevance of the mnemonic information critical to the source recollection judgment. The recollection task used corresponded to that from Experiment 1 except that the study phase did not focus participants’ attention specifically towards the gender of the speaker. If it is the case that the reduced free autobiographical recall demonstrated by Berryhill et al. (2007) is attributable to impaired capture of bottom-up attention by memory representations, the bilateral lesion patients in the current experiment should exhibit reduced subsequent source recollection compared with their matched controls.
Participants
Two of the patients with bilateral parietal lobe lesions who took part in Experiment 1 (the same 2 patients who were also involved in the study by Berryhill et al. 2007; mean age 44.5 years) participated in this second experiment, undertaken several months later. Ten age-matched healthy control volunteers (mean age 44.9 years) years also participated in the experiment.
Procedure
The stimuli consisted of 72 new sentences, similar in construction to those used in the previous experiment. Digital recordings were made of 4 new speakers, 2 male and 2 female, reading out each of the sentences. In the study phase, 36 of the sentences were presented auditorily to participants and, after hearing each sentence, participants were asked to judge whether the speaker believed that the sentence was true or false. There was no instruction to attend to the speaker's gender. The test phase, which comprised the 36 previously studied old sentences randomly intermixed with 36 new sentences, proceeded exactly as in Experiment 1, with judgments about old/new status, recollection of speaker gender, and ratings of confidence.
Experiment 3
If lateral parietal cortex supports attention or subjective awareness processes that can be recruited in the service of memory, it follows that the effects observed in Experiments 1 and 2 should occur regardless of the modality or stimulus-type of the information being remembered. To address this question, Experiment 3 involved assessment of the replicability of the findings from the previous experiments with a different modality of input (visual) and a different kind of stimuli (colored drawings of everyday objects).
Participants
The same 2 patients with bilateral parietal lobe lesions participated in Experiment 3, along with 18 age-matched healthy control volunteers (mean age 44.4 years).
Procedure
The stimuli comprised 80 colored and shaded line drawings of everyday items like fruit, appliances, utensils, and furniture, taken from the picture set of Rossion and Pourtois (2004). In the study phase, 40 of the drawings, half of which were of items that would be found in a kitchen, were presented visually and participants were cued to make either a semantic judgment (“would this item be found in a kitchen?”) or a pleasantness judgment (“do you find the image pleasant?”). For each judgment, participants responded by pressing a key on the computer keyboard. In the test phase, 80 drawings were presented, 40 of which were the previously studied old drawings randomly intermixed with 40 new drawings, of which half were items that would be found in a kitchen. Similar to the previous experiments, participants were instructed to judge whether each drawing was old or new and, if they thought a drawing was old, to recollect which of the 2 judgments they had previously made about it (semantic or pleasantness). Following each judgment, participants rated how confident they were in their decision on a scale of 1–9 (1 being extremely unsure and 9 being extremely sure).
Statistical Analysis
To provide as informative an analysis of the patient performance as possible, statistical comparisons between patient groups and controls are presented using both parametric and nonparametric tests, following our previous practice (e.g., Simons et al. 2008). This strategy allows results to be fully evaluated while bearing in mind the relative possibility of falsely significant results in parametric tests of small patient groups (Type I error) and false null results that might occur in the more conservative nonparametric tests (Type II error).
Results
Experiment 1
During the study phase, all participants performed at ceiling when reporting whether the speaker of each sentence was male or female, indicating no auditory perceptual deficits that might confound performance in the memory task.
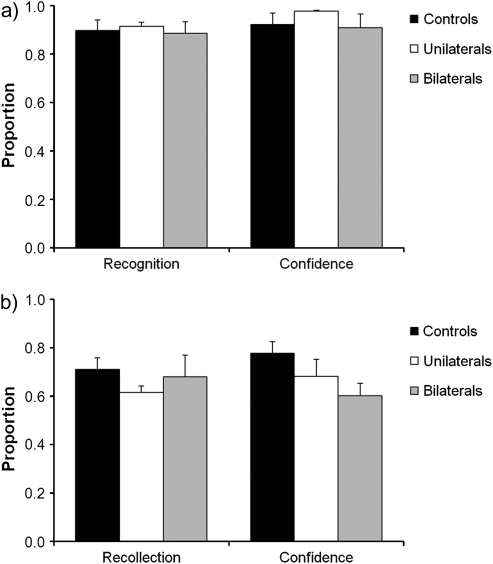
Performance in the auditory sentence memory test phase is shown in Figure 2. Statistical comparisons for the differently aged unilateral and bilateral patient groups were conducted against separate age-matched control groups. Corrected old/new recognition (hits minus false alarms) was high for both patient groups. Using nonparametric Mann–Whitney Z tests, no impairment was apparent for unilateral patients, Z = 0.0, P = 1.0, or bilateral patients, Z = 0.22, P = 0.83. Even using more powerful, but less robust, parametric t-tests, no significant differences emerged, unilaterals: t (10) = 0.61, P = 0.55; bilaterals: t (4) = 0.50, P = 0.64. Patients’ confidence in their old/new recognition responses was also unimpaired. The unilateral patients showed a slight, although not significant, tendency for higher old/new confidence than their control group, Z = 1.31, P = 0.19; t (10) = 1.74, P = 0.11. There was no difference between the bilateral patients and their controls, Z = 1.11, P = 0.27; t(4) = 1.24, P = 0.28.
Figure 2.
Performance of the patients with unilateral and bilateral parietal lobe lesions and the combined matched control participants on (a) the recognition and recognition confidence, and (b) the recollection and recollection confidence, components of the auditory source memory task in Experiment 1. Error bars indicate standard error of the mean.
Turning to the source recollection component of the memory test, neither patient group showed a significant impairment in recollecting the gender of the speaker. This was demonstrated using both nonparametric tests, unilaterals: Z = 0.72, P = 0.47; bilaterals: Z = 0.22, P = 0.83, and less conservative parametric tests, unilaterals: t (10) = 1.62, P = 0.14; bilaterals: t (4) = 0.15, P = 0.89. Interesting group differences did emerge when patients were asked to rate their confidence in their recollection responses. Patients with unilateral parietal lesions showed no significant deficit, Z = 0.40, P = 0.69; t (10) = 0.48, P = 0.64. However, patients with lesions affecting bilateral parietal cortex showed significantly reduced confidence in their recollection, Z = 1.96, P = 0.05; t (4) = 4.01, P = 0.02.
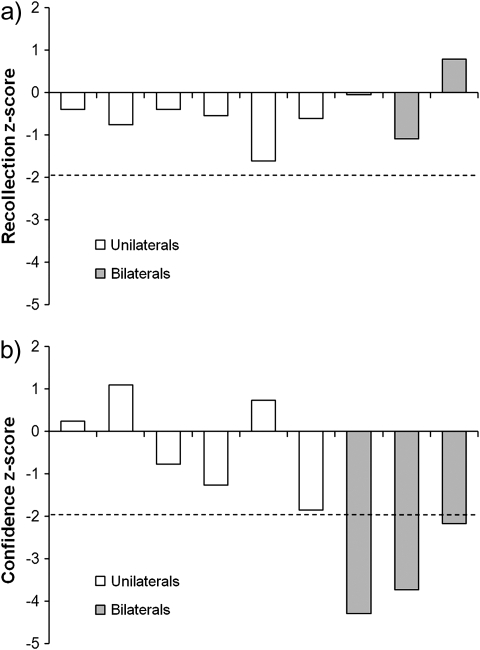
To assess the reliability of the group recollection results across individual patients, standardized Z-scores were calculated for each patient relative to their control group mean. As shown in Figure 3a, none of the unilateral or bilateral patients obtained recollection scores that were 2 or more standard deviations below controls. There was also no significant effect of laterality in the unilateral patient group, Z = 1.11, P = 0.4; t (4) = 1.12, P = 0.33. Turning to recollection confidence (Fig. 3b), the patients with unilateral parietal lesions all performed within 2 standard deviations of controls (again with no significant laterality effect, Z = 1.11, P = 0.4; t (4) = 1.03, P = 0.36), whereas all 3 patients with bilateral parietal damage exhibited significantly reduced confidence in their recollection responses (Z = −4.29, −3.73, and −2.18). As such, confidence Z-scores were significantly lower in the bilateral patients than the unilateral patients, Mann–Whitney Z = 2.32, P = 0.02; t (7) = 3.81, P = 0.007.
Figure 3.
Standardized Z-scores enabling comparison between the differently aged unilateral and bilateral patients on (a) recollection, and (b) recollection confidence, in Experiment 1. The dashed line indicates 2 standard deviations below the patients’ control group mean.
Experiment 2
Poor old/new discrimination in 2 of the control participants resulted in a trend towards lower corrected old/new recognition (mean = 0.73, SD = 0.25) than in the 2 patients with bilateral parietal lesions (mean = 0.97, SD = 0.04). This was apparent using nonparametric Mann–Whitney Z tests, Z = 1.73, P = 0.08, although was not so obvious using parametric t-tests, t (10) = 1.29, P = 0.23. There was no significant difference in old/new recognition confidence (control mean = 0.92, SD = 0.11; patient mean = 0.99, SD = 0.11), Z = 0.11, P = 0.91; t (10) = 0.87, P = 0.41.
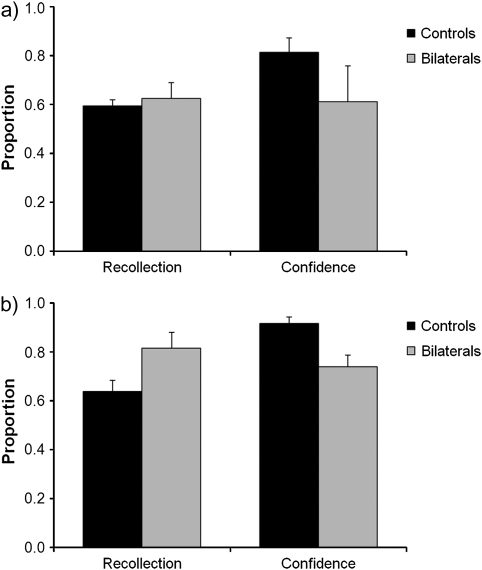
Turning to source recollection (Fig. 4a), reducing the behavioral relevance of speaker gender resulted in significantly lower source recollection across participants than was observed in the previous experiment, t (22) = 2.30, P = 0.03, indicating that the behavioral relevance manipulation did have its expected impact on overall performance. However, directly comparing patient and control performance, there was no evidence that the behavioral relevance manipulation had elicited a disproportionate impairment in the ability of the bilateral parietal patients to recollect speaker gender, Z = 0.54, P = 0.59; t (10) = 0.50, P = 0.63 (patient standardized Z-scores = −0.43 and 1.21). This result held even if the 2 control participants with poor old/new recognition were excluded from the analysis, Z = 0.66, P = 0.51; t (8) = 0.49, P = 0.64. Recollection confidence was numerically lower in the patients than controls (mean 0.61 versus 0.81), but variability between the patients meant that confidence was not significantly impaired in this experiment, Z = 1.40, P = 0.16; t (10) = 1.34, P = 0.21. Although the rated confidence of one patient was considerably reduced relative to controls (standardized Z-score = −1.83), the other patient's confidence was within the control range on this occasion (Z-score = −0.29).
Figure 4.
Performance of the patients with bilateral parietal lesions and matched control participants on the recollection and recollection confidence components of (a) the auditory source task with reduced behavioral relevance in Experiment 2, and (b) the visual source task in Experiment 3. Error bars indicate standard error of the mean.
Experiment 3
Performance of the patients and matched control participants on the visual source recollection task is shown in Figure 4b. Old/new recognition performance was at similarly high levels in both groups (patient mean = 0.82, SD = 0.02; control mean = 0.71, SD = 0.19), Z = 0.51, P = 0.61, t (18) = 0.80, P = 0.44, as was recognition confidence (patient mean = 0.86, SD = 0.12; control mean = 0.93, SD = 0.1), Z = 1.15, P = 0.25, t (18) = 0.91, P = 0.38. Consistent with the previous experiments, there was no significant impairment in the patients when it came to recollecting which of 2 judgments they had made about the drawings (in fact, the patients scored numerically higher than the matched control participants), Z = 1.40, P = 0.17, t (18) = 1.25, P = 0.23 (patient standardized Z-scores = 0.58 and 1.25). Also echoing the earlier findings, a patient deficit was apparent when participants were asked to rate their confidence in their recollection responses (patient mean 0.74 vs. 0.92 for controls). This reduction in recollection confidence trended towards significance using a nonparametric test, Z = 1.71, P = 0.087, and exceeded the threshold for significance using a parametric t-test, t (18) = 2.14, P = 0.046 (standardized Z-scores = −1.15 and −1.99).
Combining Across Experiments
To assess the reliability of the observed difference between source recollection and rated confidence in recollection following bilateral parietal lobe lesions, standardized Z-scores of the bilateral patients were combined across the 3 experiments. Both nonparametric tests, Wilcoxon Z = 2.20, P = 0.03, and parametric tests, t (6) = 4.81, P = 0.003, confirmed the significance of the dissociation between recollection accuracy and recollection confidence. Additionally, the specificity of the patients’ confidence reduction to recollection was confirmed by a significantly greater impairment in source recollection confidence than old/new recognition confidence, Z = 2.37, P = 0.02, t (6) = 5.02, P = 0.002.
Discussion
In 3 experiments, patients with bilateral parietal lobe lesions performed as well as matched healthy controls in recollecting the context in which previous events were experienced. Their source recollection ability held firm despite variations in task requirements across experiments, confirming previous results involving patients with unilateral parietal lesions (Davidson et al. 2008; Simons et al. 2008), and arguing against an explanation for those previous results in terms of compensation from the patients’ contralesional hemisphere. A manipulation during the study phase, aimed at reducing the behavioral relevance of mnemonic information critical to the source recollection task, did not have a disproportionate impact on performance of the bilateral lesion patients, suggesting that the reduced autobiographical free recall previously observed in the same patients by Berryhill et al. (2007) might not be due to impaired capture of bottom-up attention by mnemonic representations. The bilateral patients did, however, exhibit reduced confidence in their source recollection abilities across the 3 present experiments, supporting the proposal that parietal lobe lesions might lead to impaired subjective recollection (Ally et al. 2008; Davidson et al. 2008).
The primary finding, observed consistently across the 3 present experiments, was that patients with bilateral parietal lesions obtained source recollection scores that could not be distinguished from those achieved by matched control participants or, in Experiment 1, by patients with unilateral lesions. This result replicates and extends previous reports in unilateral parietal patients (Davidson et al. 2008; Simons et al. 2008). Simons et al. (2008) found that patients with left and right parietal lesions performed normally when recollecting the context in which words and faces were previously encountered, despite the patients’ lesions overlapping closely with the regions activated by healthy volunteers during the same source recollection task. Similarly, Davidson et al. (2008) observed normal performance in unilateral parietal patients during source recollection of previously presented word-definition pairings, although the patients were impaired when asked to make remember/know judgments. The present results extend the findings of intact source memory for visually presented word and face stimuli to an auditory source recollection task involving discrimination between different voices, and demonstrate that the previous results in patients with unilateral lesions cannot be attributed to possible compensation from the patients’ contralesional parietal lobe. The potential for a compensation explanation arose from meta-analyses of functional imaging studies of memory, which noted that memory-related processing may be supported by the parietal cortex in both hemispheres, implying some level of functional redundancy (e.g., Vilberg and Rugg 2008). However, in Simons et al.’s (2008) neuroimaging study of source recollection involving words and faces, material-specific activity lateralization was observed in parietal cortex. Furthermore, studies of spatial awareness and attention in unilateral parietal patients have demonstrated functional differentiation between the hemispheres (e.g., Robertson et al. 1998; Peers et al. 2006).
Ruling out contralesional compensation means that another explanation must be sought for the differing results previously reported in patients with parietal lesions by Simons et al. (2008), Berryhill et al. (2007), and Davidson et al. (2008). Those studies demonstrated intact source recollection in unilateral lesion patients (Davidson et al. 2008; Simons et al. 2008), but impaired free recall of autobiographical narratives in patients with bilateral lesions (Berryhill et al. 2007). In the latter study, Berryhill et al. asked the 2 patients with bilateral parietal lesions who also participated in the present study to recall events from their past lives. Both patients produced spontaneous narratives that lacked richness and specificity compared with controls, although the patients were unimpaired when asked specific probe questions concerning particular memories.
Looking beyond a compensation account, 2 other possible explanations for the differing source recollection and autobiographical free recall results have been proposed. One hypothesis, drawing on previous theories of attention (Corbetta and Shulman 2002), suggests that the parietal lobe supports the attentional control of memory (Cabeza et al. 2008). According to this view, the impaired autobiographical free recall observed by Berryhill et al. (2007) might be due to deficits in the capture of attention by behaviorally relevant mnemonic information. If this is the case, then introducing a manipulation during the study phase, aimed at reducing the behavioral relevance of mnemonic information key to the source recollection task, might result in impaired subsequent recollection performance in patients with parietal lesions. An alternative view is that the parietal lobe might be responsible for the subjective experience of richness, vividness, and confidence in one's recollections, and that the impoverished autobiographical memory observed by Berryhill et al. could be due to impaired subjective experience of memory (Ally et al. 2008; Davidson et al. 2008). If this hypothesis is correct, patients with bilateral parietal lesions might be expected to report reduced confidence in their source recollection abilities (Lyle and Johnson 2006, 2007).
The results of the present study appear to provide greater support for the subjective recollection hypothesis. Reducing the behavioral relevance of the mnemonic information critical to the auditory source recollection task, by not instructing participants to attend to speaker gender during the study phase, reduced subsequent source recollection in all participants, indicating that the manipulation may have resulted in gender being encoded as a less salient feature of the memory representation that was thus less likely to capture bottom-up attention at retrieval (Corbetta and Shulman 2002). However, the behavioral relevance manipulation crucially did not disproportionately impair the ability of patients with bilateral parietal lobe lesions to recollect the context in which sentences were previously heard, which suggests that the degree to which mnemonic representations capture bottom-up attention at retrieval may not be reduced following parietal lobe damage. It should be borne in mind that the present study examined just one manipulation of attention; further studies involving additional attentional factors are needed before a thorough assessment of the attention-to-memory hypothesis can be provided.
Evidence presented here was, however, consistent with the alternative subjective recollection hypothesis. Across the 3 experiments, the patients exhibited significantly reduced rated confidence in their source recollection responses, both relative to controls and, in Experiment 1, to patients with unilateral lesions. This effect of reduced recollection confidence in the bilateral patients could not simply be due to larger lesions than in the unilateral patients, because lesion volume was matched between the 2 groups. Moreover, an account in which the bilateral patients might simply have been generally less confident in their cognitive abilities is inconsistent with the observed normal levels of confidence in patients’ recognition memory responses. The dissociation between intact source recollection and reduced recollection confidence indicates that the bilateral patients retained the ability to recollect contextual information accurately, but that their experience of recollection may have been deficient in the rich episodic detail typical of healthy participants, resulting in lower ratings of confidence (Lyle and Johnson 2006, 2007). Reduced subjective recollection is consistent with anecdotal observations by Davidson et al. (2008), also noted in other studies (Ally et al. 2008; Simons et al. 2008), of parietal patients reporting diminished confidence in their memory abilities when faced with an episodic recollection task, and remarking that their recollection of events lacks richness or vividness. This view can also explain the distinction in Davidson et al.’s study between preserved source recollection but impairment when the patients were asked to make remember/know judgments about their subjective experience of remembering previously presented stimuli. The normal levels of “know” responses observed by Davidson et al. are consistent with the finding in the present study that the patients’ confidence impairment was specific to recollection, as they had preserved confidence in their old/new recognition abilities.
A role for the parietal lobe in supporting subjective aspects of recollection is also consistent with data from functional neuroimaging studies. Although parietal activity has been observed in studies that have used subjective recollection tasks, such as remember/know judgments (e.g., Henson et al. 1999), and more objective measures of recollection, such as source memory (e.g., Simons et al. 2008), studies that have examined both subjective and objective memory within participants have indicated that parietal cortex may be particularly important for subjective memory. For example, Chua et al. (2006) demonstrated that parietal activity was greater when participants made subjective memory confidence assessments than when they made objective recognition memory decisions. Moreover, Duarte et al. (2008) linked parietal cortex activity to subjective “remember” responses but not objective source judgments in young and high-functioning older adults, and showed that this parietal activity was significantly reduced in low-functioning older adults who were impaired at the subjective remember/know task. Similarly, Olson and colleagues recently found that patients with bilateral parietal damage express an unusually low number of remember responses, but a normal number of know responses, on a false memory task (Drowos et al. 2009). Thus, patients with parietal lesions appear to have deficits in the assessment and monitoring processes that contribute to subjective aspects of recollection, resulting in low confidence (as seen in the present data), diminished detail in spontaneous autobiographical narratives and in reported mnemonic vividness and richness (Berryhill et al. 2007; Davidson et al. 2008; Simons et al. 2008), as well as reduced “remember” responses (Davidson et al. 2008; Drowos et al. 2009).
The subjective memory processes described above may contribute towards objective measures of recollection such as source memory, as evidenced by parietal activity during source memory in healthy volunteers (e.g., Simons et al. 2008), but they appear in many instances not to be necessary for accurate objective recollection to occur, as demonstrated by the intact source recollection observed in the present study and in previous reports (Davidson et al. 2008; Simons et al. 2008). Further studies are required to understand the conditions under which reduced subjective recollection may influence objective memory accuracy. For example, it is possible that patients with parietal lobe lesions may be particularly susceptible to experimental manipulations of response bias (e.g., Fortin et al. 2004).
Drawing on cognitive theories of episodic retrieval (e.g., Norman and Bobrow 1979; Burgess and Shallice 1996; Johnson and Raye 2000), there are a number of different component processes that, if damaged, could cause difficulties with subjective memory. First, it is possible that mental imagery problems could impair subjective memory states due to reduced ability to visualize rich and vivid detail in the mind's eye. This explanation is weakened by the finding that the 2 bilateral patients tested here do not have general visual imagery deficits (Berryhill et al. 2007). Second, it is possible that problems with assessing memory strength or making decisions about recollected memory strength could diminish subjective memory. Perceptual decision making has been strongly linked to cellular activity in portions of the intraparietal sulcus (IPS) in nonhuman primates (Shadlen and Newsome 2001) and a small number of functional imaging studies have reported higher-order decision making activity in the IPS and inferior parietal lobe (Vickery and Jiang 2009). If this is the case, such an account predicts that lateral parietal lesions might cause deficits on decision making tasks that require recollection, and on memory tasks requiring a recollection-based decision such as those that elicit linear receiver operating characteristics (Yonelinas 2002).
One acknowledged weakness of all the episodic memory accounts of parietal lobe function is that they cannot easily explain the full spectrum of memory performance observed after lateral parietal damage. For instance, deficits have been reported on tasks assessing visual working memory (Berryhill and Olson 2008b) and iconic visual short-term memory (Peers et al. 2005). It is unclear how the subjective recollection hypothesis, or any of the other proposed accounts (see Simons and Mayes 2008, for a recent summary of the various hypotheses that have been proposed), could explain both the short-term and long-term memory impairments, or the observation that working memory deficits are most evident on old/new recognition tasks with nearly normal performance apparent on recall tasks (Berryhill and Olson 2008a). Although it is not necessary to assume that the processes involved in working memory and episodic memory are wholly identical, a full account of parietal lobe memory mechanisms will need to accommodate both short-delay and long-delay memory deficits.
To conclude, the present study demonstrated a dissociation between intact source recollection and impaired recollection confidence in patients with bilateral parietal lesions. Contralesional compensation and differential attentional capture were ruled out as accounts for previous findings of preserved source memory in unilateral lesion patients and impaired autobiographical free recall in patients with bilateral lesions. Instead, reductions in rated recollection confidence observed across the 3 present experiments support the alternative hypothesis that parietal cortex may play a particular role in the subjective experience of recollection.
Acknowledgments
Conflict of Interest: None declared.
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: Electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46:1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008a;46:1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008b;46:1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: Neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Critchley M. The parietal lobes. New York: Hafner; 1953. [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Drowos DB, Berryhill ME, Andre JM, Olson IR Forthcoming. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. J Cogn Neurosci. 2009 doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL. Cognitive and brain mechanisms of false memories and beliefs. In: Schacter DL, Scarry E, editors. Memory, brain, and belief. Cambridge (MA): Harvard University Press; 2000. pp. 35–86. [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Lyle KB, Johnson MK. Importing perceived features into false memories. Memory. 2006;14:197–213. doi: 10.1080/09658210544000060. [DOI] [PubMed] [Google Scholar]
- Lyle KB, Johnson MK. Source misattributions may increase the accuracy of source judgments. Mem Cognit. 2007;35:1024–1033. doi: 10.3758/bf03193475. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. Descriptions: An intermediate stage in memory retrieval. Cogn Psychol. 1979;11:107–123. [Google Scholar]
- Peers PV, Cusack R, Duncan J. Modulation of spatial bias in the dual task paradigm: Evidence from patients with unilateral parietal lesions and controls. Neuropsychologia. 2006;44:1325–1335. doi: 10.1016/j.neuropsychologia.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJH, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, Driver J, Antoun N, Duncan J. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;15:1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–172. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Simons JS, Dodson CS, Bell D, Schacter DL. Specific- and partial-source memory: effects of aging. Psychol Aging. 2004;19:689–694. doi: 10.1037/0882-7974.19.4.689. [DOI] [PubMed] [Google Scholar]
- Simons JS, Mayes AR. Editorial: what is the parietal lobe contribution to human memory? Neuropsychologia. 2008;46:1739–1742. doi: 10.1016/j.neuropsychologia.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can Psychol. 1985;26:1–12. [Google Scholar]
- Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cereb Cortex. 2009;19:916–925. doi: 10.1093/cercor/bhn140. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]