Abstract
Tissue engineering is becoming increasingly ambitious in its efforts to create functional human tissues, and to provide stem cell scientists with culture systems of high biological fidelity. Novel engineering designs are being guided by biological principles, in an attempt to recapitulate more faithfully the complexities of native cellular milieu. Three-dimensional (3D) scaffolds are being designed to mimic native-like cell environments and thereby elicit native-like cell responses. Also, the traditional focus on molecular regulatory factors is shifting towards the combined application of molecular and physical factors. Finally, methods are becoming available for the coordinated presentation of molecular and physical factors in the form of controllable spatial and temporal gradients. Taken together, these recent developments enable the interrogation of cellular behavior within dynamic culture settings designed to mimic some aspects of native tissue development, disease, or regeneration. We discuss here these advanced cell culture environments, with emphasis on the derivation of design principles from the development (the biomimetic paradigm) and the geometry-force control of cell function (the biophysical regulation paradigm).
Keywords: STEM CELLS, BIOPHYSICAL REGULATION, SCAFFOLDS, BIOREACTORS, TISSUE ENGINEERING
Cellular processes involved in regeneration of some adult human tissues are similar to those in early development. It has been proposed that tissue regeneration (in contrast to the repair which is a “quick fix” of the injury) recapitulates developmental events [Caplan and Bruder, 2001; Stocum, 2001]. Tissue engineering—which attempts to build functional grafts in vitro or to induce tissue regeneration in vivo—thus also needs to recapitulate development. If stem cells are to be induced to form the right cells in the right places and the right time, they need to be subjected to the signals that drive the native development. Therefore, lessons learned from early development (and adult tissue regeneration) should ideally guide the design of tissue engineering systems.
During early development, the newly formed zygote undergoes rapid cell division until the formation of a homogeneous sphere of undifferentiated cells—morula. Fluid begins to enter the morula leading to the formation of a single cell layer—trophoblast—around the fluid-filled blastocyst cavity—blastocoel. Inside the trophoblast resides the inner cell mass that contains the cells that will give rise to all three germ layers and ultimately form the embryo [Sarraf, 2007].
The formation of the blastocyst constitutes the first structural division during the developmental process where the trophoblast separates the totipotent stem cells of the inner cell mass from the outside environment. The blastocyst will eventually be implanted in the endometrium and undergo further transformation. It is in this location where gastrulation takes place by transforming the bilaminar embryonic disc (consisting of the epiblast and hypoblast) into the three primary germ layers: ectoderm, mesoderm, and endoderm. Gastrulation involves the rearrangement and migration of cells and the formation of a groove on the dorsal surface of the epiblast—the primitive streak—establishing the long axis (proximal vs. distal) of the embryo. Further structural changes follow, such as the invagination of the epiblast forming the endoderm. Stated differently, the formation of the gastrula is characterized by the conversion of a simple and loosely organized group of undifferentiated cells to a more complex and organized collection of cells with highly defined boundaries extracellular matrix. The underlying cell migration is a result of molecular gradients and the early establishment of cellular fate along the embryo’s axis of differentiation (i.e., proximal vs. distal) [Sarraf, 2007].
From this point on, morphogenesis and organogenesis proceed through a complex sequence of events that include sorting, positioning, and differentiation of cells. A two-dimensional trilaminar embryonic disk is being converted into a three-dimensional cylinder via embryonic folding, to position endoderm in the center, ectoderm on the outside, and mesoderm in between. During this phase, the cross talk between the germ layers is synchronizing the cellular activities and up-regulating genes such as fibroblast growth factor (FGF), retinoic acid, Sonic hedgehog, bone morphogenetic protein (BMP), Wnt, and β-catenin. The cell–cell and cell–matrix interactions also regulate cell movement, proliferation, and adhesion [Sarraf, 2007].
Formation of the primitive heart is an example of the series of changes leading to the development of a functioning organ. The primitive heart begins to develop from the cardiogenic area of the mesodermal layer of the gastrula, with regulatory signals coming from the underlying endoderm. The cells form endocardial tubes, which then fuse into a single heart tube with its distinct myocardium and the endocardial surfaces [Abu-Issa and Kirby, 2007]. This primitive heart tube undergoes a complex set of elongating, bending, and twisting motions resulting in the formation of the four chambers of the heart. Remarkably, the heart—a marvel of “engineering by nature”—is the first functioning organ that forms during development. The efforts to derive cardiac cell populations from human embryonic stem cells (hESCs) [Yang et al., 2008] and to develop effective tissue engineering systems [Radisic et al., 2004b; Zimmermann et al., 2006; Tandon et al., 2009] have been guided by these developmental “blueprints.”
Within the complexity of the developmental process, one common denominator is biophysical control. From fluid-induced tension experienced by the cells in the trophoblast to the compression forces experienced by rapid proliferating cells within a confined space, mechanical forces are present during the entire embryogenesis. The same can be said about molecular gradients and structural complexity. Mechanical forces even play a role in maintaining orientation via cilia generated movement [Patwari and Lee, 2008]. The complexity of the biophysical forces grows with the embryo, evolving from relatively simple gradients and traction/ compression forces to complex set of forces and extracellular signals that are site-specific and are essential for the survival of the embryo.
LEARNING FROM DEVELOPMENT
The goal of a tissue engineer is to grow tissue grafts ex vivo (by using exogenous cells and specialized scaffolds and bioreactors) or induce tissue formation in situ (by providing the necessary environments for tissue regeneration). Ideally, the resulting tissues should be customized to the needs of the patient and specific clinical situation, and have at least some immediate function. It is believed that in order to create such functioning tissues one needs to unlock the full potential of stem cells, by mobilizing some of the factors involved in early development, and controlling factors leading to scar formation. Of note, injured fetal tissues heal without scarring by processes that involve a large pool of stem/progenitor cells and a reduced immune response. Fetal healing results in full reconstruction of the tissue architecture and function. In contrast, adult healing leads to fibrous scar that is formed by a much smaller population of progenitor cells and with strong involvement of the immune response, by processes that stop bleeding and restore homeostatis without infection, but do not restore the original tissue [Ferguson and O’Kane, 2004]. This is true for a range of tissues, from skin to heart, and suggests that tissue engineering approaches should mimic early development rather than adult tissue repair.
A multitude of cellular, biochemical, and biophysical mechanisms takes place in a highly orchestrated spatial and temporal manner to give rise to a functioning organ. This complexity needs to be considered and recapitulated, at least to some extent, when engineering tissues. Our attempts to mimic the necessary conditions in a laboratory setting are limited by how much is known about the combinations and timing of regulatory factors involved in cell differentiation. From a tissue engineer’s perspective, it is important to understand how uncommitted cells behave in response to external and internal signals and stressors, and what are the most important cellular interactions and biophysical factors regulating these interactions. Importantly, any information derived from developmental biology will help build better tissue engineering systems, and these systems will in turn help interrogate the additional factors of interest.
DESIGNING THE RIGHT CONTEXT FOR IN VITRO CELL CULTURE
For just over 100 years, since the first-ever tissue culture study performed by Harrison [1907], mammalian cells have been cultured in 2D settings, either on tissue culture plastics or on various types of coatings, from extracts of native matrix to cell feeders and purified proteins. These experiments resulted in seminal findings that constitute our knowledge of molecular regulation of stem cell biology. Recently, several groups of investigators used these simple culture settings to interrogate geometric control of cell life and death in culture [Chen et al., 1993], and the control of cell differentiation by substrate stiffness [Engelmayr et al., 2008; Discher et al., 2009]. These studies go beyond the traditional focus on molecular regulatory factors and open a new era of utilization of physical factors mediating cell function, with direct implications to tissue engineering and regenerative medicine.
At the same time, there is a growing notion that the cell confinement to 2D culture is an inherently unnatural situation (due to the attachment of one side of the cell to the substrate, and limited contact with other cells and matrix) that in many cases results in unnatural cell responses. Examples include major differences in growth patterns [such as the tumorogenic growth in 2D and normal cell growth in 3D settings, Petersen et al. [1992], and phenotype expression [a classical demonstration that dedifferentiated chondrocytes re-express their phenotype when transferred from monolayers into 3D culture, Benya and Shaffer [1982]].
The conventional culture settings provide environmental control only through periodic exchange of culture medium, and fundamentally lack the capability for coordination of molecular and physical regulatory signals. This is far from the in vivo milieu where cells reside in precisely controlled environment, and are subjected to spatial and temporal gradients of multiple factors. Overall, the classical culture falls short of providing a realistic model for studies of cells at various hierarchical levels, and in response to dynamic changes of regulatory factors.
We will discuss in this article some recent advances that enable probing of cellular behavior using tightly controlled, dynamic culture settings designed to mimic the native cell and tissue milieu. We will first describe three important developmental events: (i) proliferation and migration of cells, (ii) structural changes that lead to separation of layers and axis formation, and (iii) mechanical loading of fully functional organs and tissues, to identify some of key developmental factors. Then, we will discuss the use of advanced technologies to study these factors and utilize their effects for engineering functional tissues. Throughout, our focus will be on the derivation of design principles from native development (the biomimetic paradigm) and the geometry-force control of cell function (the biophysical regulation paradigm).
DEVELOPMENTAL STAGES AND THEIR REGULATORY FACTORS
PROLIFERATIVE AND MIGRATORY PHASE
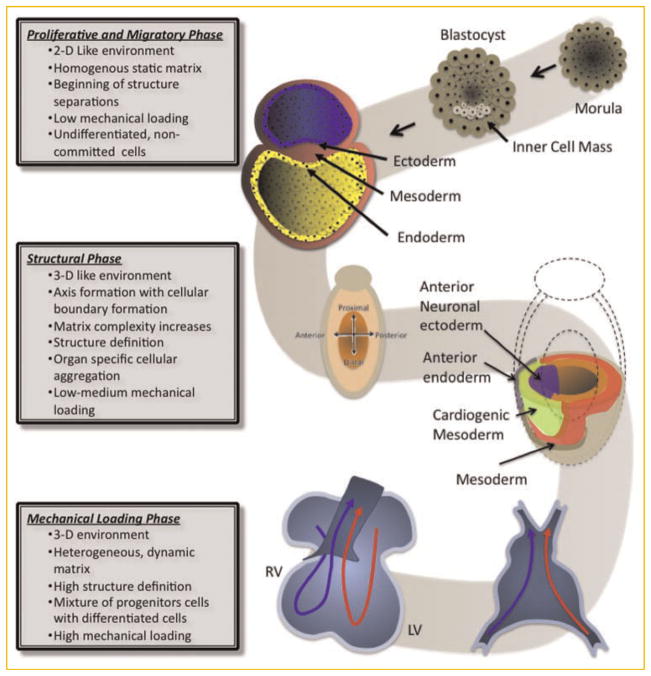
During development, cells first proliferate and start to migrate in response to gradients, the processes that lead to cell positioning and the division of the germ layers. Further migration leads to cell accumulation on the sites specific for the organ that will be created, and is the precursor to structure formation as it leads to the segmentation of the embryo and the establishment of posterior, anterior, ventral, and dorsal sides and ultimately the locations of all future organs. During this phase, the extracellular matrix is being formed, and its complexity increases with the maturity of the embryo. Laminin is the first ECM molecule found in the ventral surface of the epiblast and in the hypoblast, and known to assist in cell adhesion and migration during gastrulation. The fibrilar nature of the ECM found during embryogenesis also helps the migration of cells via contact guidance. As development progresses, the extracellular matrix becomes more heterogenous and starts to express properties specific to the type of organ that is being generated. Among the ECM molecules that appear during development are collagen type IV, hyaluronate, perlecan, entactin/ nidogen, tenascin, and fibronectin [Zagris, 2001]. With changes in matrix composition come the changes in traction forces and structural stiffness. This is when a dynamic interplay between the cells and their extracellular matrix starts and continues throughout development and the whole life of an organism. These processes are nicely illustrated by the series of events leading to the migration and folding of the matrix to form the primitive streak and endocardial tubes (Fig. 1).
Fig. 1.
Schematic presentation of the stages of heart development. Beginning from the blastocyst and ending with a functioning organ (heart), the embryo’s complexity increases substantially. Starting from the undifferentiated cells, the embryo begins to form via a series of coordinated migratory patterns and rearrangements of the cells establishing physical boundaries and the formation of the three germ layers: endoderm, ectoderm, and mesoderm. The complexity increases as the differentiation of the cells (both spatial and phenotypical) progresses establishing the orientation of the embryo (e.g., anterior vs. posterior), the location of future organs, the matrix, and the beginning of the vascular network. Mechanical loading also increases in complexity during organogenesis, leading to a completely competent and functioning heart.
STRUCTURAL FORMATION PHASE
The proliferation–migration phase continues into the phase of structure formation, exemplified by the involution and folding of cellular layers involved in the formation of the heart (Fig. 1). For most organs, the structure formation is achieved by cell guidance and the modulation of the extracellular space. The matrix plays an increasingly important developmental role, and its properties become more differentiated. Using heart development as an example of the growing complexity of the ECM, chondroitin slufate, collagen I and IV, laminin, fibronectin, fibrilin, and fibulin can be found within the primitive heart [Little and Rongish, 1995]. Fibronectin plays an important role in cell migration in the heart forming regions, while fibrilar proteins such as collagen I and IV assume structural roles. The ECM continues to evolve as the cardiac tubes form and fuse moving from the cardiac jelly into a load bearing tubular structure. The four-chamber heart becomes a functioning organ with a complex ECM arranged in a specific 3D ultra-structure consisting primarily of collagen types I and III, collagen IV in the basement membrane, fibronectin, laminin, entactin, dermatan sulfate, chondroitin slufate, and hyaluronic acid [Little and Rongish, 1995]. During this phase, ECM becomes an integral part of the mechanical stabilization of the developing structure, while serving as a reservoir of signals, both soluble and immobilized, that are transmitted to the cell. The dynamic interplay between the matrix and the cells—known as dynamic reciprocity [Nelson and Bissell, 2005]—largely determines further differentiation and specification of the cells and their ECM.
The ECM is considered an active component of tissues that interacts with the cells in many ways [Bornstein et al., 1978]. While cells directly secrete their matrix and thereby determine the matrix properties, the cells themselves are regulated by secreted matrix proteins [Bissell and Aggeler, 1987; Roskelley and Bissell, 1995]. In a more general sense, the physical and biochemical connections exist between the extracellular matrix, the cell cytoskeleton, and the cell’s nucleus that lead to regulation of cellular processes and gene expression at multiple levels [Lin and Bissell, 1993; Roskelley et al., 1994, 1995]. This two-way communication between the cells and their matrix is very important during organogenesis and structure formation.
MECHANICAL LOADING PHASE
For many organs, the final phase of organogenesis includes mechanical loading and mechanical function. This is exemplified during heart development where the heart tube loops into a rightward spiral paving the way for the four chambers of the mammalian heart (Fig. 1). During this entire processes blood flow is maintained. This is an example of organogenesis under multiple mechanical loads such as shear stresses on the lumen of the heart tube, tension on the heart tube wall, and cellular contraction during the looping process. These forces are transmitted to the cells by direct contact (in the case of shear stresses), cell–matrix contact, or cell–cell interactions. Cells respond according to the type of flow and tensional forces by secreting factors or up-regulating and/or down-regulating genes specific to the cell’s location.
CONTROL OF CELLULAR FUNCTION: MOLECULES, STRUCTURE, AND FORCES
The differentiation of cellular function highly depends on the microenvironment—sometimes termed as a “niche”—in which the cells reside during tissue growth. As already described, cells can actively modify their niches by synthesizing or degrading the ECM, secreting cytokines, and communicating with other cells and matrix by molecular and physical signals. The “dynamic reciprocity” of cell–cell and cell–matrix signaling takes place in a 3D environment, and at many different hierarchical levels—from membrane channels to cells, tissues and eventually whole organs. At each level, there are specific readouts, and these change from one level to another, and from one cell or tissue type to another.
The diffusible factors directing cellular function are either secreted by the cells themselves (autocrine), or produced by other cell types (paracrine), or transported through bloodstream (endocrine). Additional molecular factors are incorporated into the extracellular matrix, and presented to the cells either in ligand-immobilized form, or through sustained release. The multiple chemokines and cytokines act in concert with biomechanical signals to regulate cell function and tissue assembly. For example, the precise positional control of cell differentiation during tissue looping and branching is attributed to the local biomechanical environment of the cells and their matrix. The overall complexity of cell regulation during development is further increased by the dynamic nature of regulatory signals, changing in space and time, within a 3D setting, and in ways that are not entirely known.
The study of the individual and combined effects of multiple regulatory signals, via precise spatiotemporal control of signal type and magnitude, is not a trivial task and certainly not one that is achievable by using the traditional well plate cultures. Some recent developments of microarray and microfluidic technologies offer the opportunities of singling out one factor of interest from other systematic signals, and superimposing this factor by other, also well-defined signals [see reviews Chen et al., 2004; Whitesides, 2006; Bettinger et al., 2009]. These systems cannot capture the enormous complexity of the actual regulatory pathways, but they allow, for the first time, to conduct controllable studies of multiple factors regulating developmental processes. The high throughput nature of microtechnologies allows screening of many different combinations and levels of possible factors, towards the selection of the conditions of interest for regulating cellular behavior. Most importantly, testing platforms are now becoming available that enable tight control of the cellular environment, that could be used to maintain cell phenotype or direct stem cell differentiation. In the following sections, we review some representative studies of the regulation of cell function using well-controlled, relatively simple yet biologically relevant culture systems.
REGULATION OF MOLECULAR SIGNALS
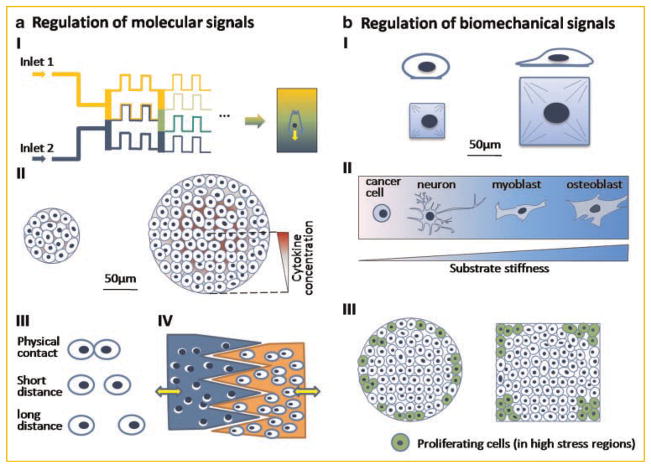
Molecular regulatory signals are transmitted to the cells by dissolution, diffusion, and as an immobilized form. In each case, the spatial and temporal profiles of biological activity are essential for morphogenesis, both in vivo and in vitro. The microfluidic systems provide a simple and direct tool to deliver citokynes at certain well-defined concentrations, via laminar flow within narrow channels (Fig. 2a-I). Using such a system, the primary neutrophils were shown to migrate towards the higher concentration of interleukin-8 (IL-8). Interestingly, there was a distinct difference in cellular responses to sharp and gradual changes in spatial IL-8 concentration [Li Jeon et al., 2002]. Other microfluidic studies suggested that cellular chemotactic responses depended on the shape of the concentration profile (linear vs. polynomial) of the cytokine [Wang et al., 2004]. As the precision with which the microfluidic systems are fabricated go up to 0.1 μm, these systems allow us to study cell polarity and signal propagation at sub-cellular levels [Sawano et al., 2002]. Efforts have also been taken to fabricate 3D microfluidic systems from various hydrogels such as collagen, agarose, and alginate that generate controlled molecular gradients within the scaffolds [e.g., Choi et al., 2007]. Precise control of chemical environment could potentially aid in engineering complex tissues and studies of tissue interfaces, by allowing researchers to mimic chemical gradients found during morphogenesis.
Fig. 2.
Micromanipulation of cellular function. a: Regulation of molecular signals (I) biochemical gradients generated from microfluidic devices influence cell polarization and migration; (II) the concentration level of growth factors secreted by cells depends on colony size and mediates the commitment of embryonic stem cells; (III) micropatterning controls cell–cell contact and distance; (IV) microfabricated devices regulate the distance and timing of cell–cell interaction. b: Regulation of Biomechanical signals (I) the control of cell shape regulates stem cell differentiation. Large substrate size leads to osteogenesis and small size adipogenesis; (II) substrate stiffness directs stem cell differentiation. High stiffness directs stem cells into osteoblasts, medium stiffness promotes myogenic differentiation, and low stiffness promotes neurogenesis. Only cancer cells can survive on an extreme soft substrate; (III) shape control of multicellular system affects local cell proliferation. High cell proliferation rates are in the regions with high mechanical stresses, such as edge of circles and corns of squares.
The biochemical signals that cells experience can also be regulated via paracrine signaling by manipulating the shape and size of cell colonies (Fig. 2a-II). The mammary epithelial cell colonies in micromolded collagen gels were shown to branch out at specific positions within the gel consistent with the pattern of a diffusible inhibitor secreted by the cells [Nelson et al., 2006]. The cellular colony size is known to be critical for the maintenance and expansion of hESCs. The size and shape of colonies can be precisely controlled using 3D microwells, and the hESCs can be maintained in their undifferentiated state for long periods of time [Mohr et al., 2006]. The control of hESC differentiation via colony size can be established quite elegantly by using micropatterned cell culture substrates [Peerani et al., 2007]. The colony size dependent differentiation is mediated by the level of Smad1 inside hESCs. As the colony size increases, the level of smad1 antagonist growth differentiation factor-3 (GDF3) also increases. The increase in smad1 antagonist GDF3 occurs while the level of bone morphogenetic protein-2 (BMP2) remains constant resulting in a decrease of pSmad1 level and increase in pluripotency in large cell colonies.
With micropatterning technology, the distance and contact area between cells can also be precisely controlled, enabling studies that can distinguish the effects of direct cell contact from those of the diffusing signals on cell–cell communication (Fig. 2a-III). Using an elegant and highly tunable microfabricated device (Fig. 2a-IV), the effects of cell contact and transmission of soluble factors were systematically studied for co-cultures of hepatocytes and supportive stromal cells [Hui and Bhatia, 2007]. The minimum direct contact time was determined for the maintenance of the hepatocellular phenotype, and the effective diffusion distance of soluble signal was estimated to be less than 400 μm. Indeed, microtechnologies are expected to have substantial impact on future advances in cell culture and tissue engineering.
REGULATION OF BIOMECHANICAL SIGNALS
Biomechanical signals that the cells sense in vivo are associated with cellular deformation (due to compressibility, shear), mechanical stress (in response to pressure, shear force), and also the deformation of cell nucleus (elongation in response to tension). Among them, cell shape (or, more precisely, the aberration from “normal” cell shape) appears to be the most obvious indicator and regulator of physical effects on cell function. First, all cells have their unique morphology: chondocytes are small and round, myoblasts are medium-sized and spindle-like, and osteoblasts are large and polygonal. Second, the variation in cellular function (gene expression leading to matrix synthesis and expression of surface markers) is often associated with the changes in cellular morphology. The cell shape can be regulated by osmotic pressure, by micro/nanotopological features of the cell attachment substrate, or by the adhesiveness and stiffness of the substrate.
The 2D micropatterning is a direct way to control the cell shape without introducing additional side effects such as changes in the solute concentration associated with osmotic swelling (Fig. 2b-I). By microcontact printing, the extracellular proteins are deposited onto specific locations of the substrate, and the cells can spread only over the protein-patterned geometry. The control of cell size was demonstrated to switch cell functions between apoptosis, proliferation, and differentiation [Chen et al., 1997; Dike et al., 1999]. In particular, human mesenchymal stem cells tended to undergo adipogenic differentiation on small adhesion islands (forcing the cells to take their round configuration), and osteogenic differentiation on larger adhesion islands (which in turn allowed cell spreading) [McBeath et al., 2004]. Such differences are believed to result from the geometric control of cell cytoskeleton via control of cell shape.
The modification of substrate topology is another easy way to regulate cell function. Just like naturally occurring topographic structures within the extracellular matrix that can influence cell migration, polarization, and other functions, scaffold topography containing micro and nanofeatures can affect cell morphology, alignment, adhesion, migration, proliferation, and differentiation. Oriented topological features incorporated into a substrate to serve as contact guidance dramatically changed the cytoskeletal arrangement and focal adhesions in cultured hESCs [Gerecht et al., 2007]. Many studies have demonstrated that the nanopatterned substrate can promote the elongation of stem cells and their subsequent differentiation into bone cells [e.g., Oh et al., 2009]. Interestingly, the randomness (as compared to order) in the distribution of nanodots enhanced the osteogenic differentiation of hMSCs [Dalby et al., 2007].
A series of groundbreaking studies demonstrated that the cell shape and cytoskeletal tension can be effectively manipulated by varying the substrate stiffness [Engler et al., 2006, 2008]. These studies show that the cells prefer the mechanical environment they normally experience in vivo, to the extent that substrate stiffness alone can direct cell differentiation [Engler et al., 2006]. For example, embryonic cardiomyocytes beat best on a substrate with the stiffness close to native heart tissues, which is optimal for transmitting contractile work to the matrix, and only poorly on stiffer substrates matching the stiffness of scar tissue formed following cardiac infarction [Engler et al., 2008]. A stiff substrate (comparable to an osteoid) leads to osteogenic differentiation into bone cells, medium substrate (comparable to the heart matrix) supports myogenic differentiation into muscle cells, while soft substrate (matching the soft fat tissue) leads to neurogenesis (Fig. 2b-II). On extremely soft gels, only cancer cells can survive, and this feature can be exploited in some cases to select cancerous cells from mixed populations [Discher et al., 2005].
The changes in cell mechanics in response to substrate stiffness have been related to the cell–matrix interactions via actin-myosin motors. Cell contraction at integrin-based adhesion sites is resisted by the underlying substrate, which leads to the recruitment of additional adhesion molecules. Therefore, the variation of substrate stiffness can affect the buildup of the cytoskeletal tension and thereby regulate multiple cellular functions.
The mechanical stresses in single cells can also be regulated through direct cell–cell interactions. When cells are patterned onto substrates in defined geometric shapes and sizes (such as circles or rectangles), cell proliferation was observed mostly in regions with highest traction forces—at the edges of circles and in the corners of rectangles (Fig. 2b-III) [Nelson et al., 2005]. The mechanical stresses generated by cell–cell interactions can be measured using micro-posts [Tan et al., 2003] or traction force microscopy [Dembo and Wang, 1999], providing important information on the mechanical status of the individual cells and cellular monolayers.
Finally, the cell function can also be affected by other physical signals such as temperature, pH, oxygen level, electrical field, and extracellular matrix properties. The embryonic patterning network in Drosophila was perturbed with the temperature gradient generated using a microfluidic system in a spatiotemporal manner [Lucchetta et al., 2005].
REGULATION OF STRUCTURAL SIGNALS
In general, cells behave more naturally (i.e., the cell responses measured in vitro are closer to those in vivo) when cultured in 3D environments. For a long time, 3D scaffolds were designed to provide a biocompatible structural template for cell attachment that would be permissive for the exchange of nutrients, metabolites, and cytokines and allow cell seeding. Numerous studies [see excellent reviews Langer and Tirrell, 2004; Lutolf and Hubbell, 2005; Tibbitt and Anseth, 2009] have convincingly demonstrated that the cell phenotype depends on the entire context of the cellular environment.
Consequently, passive scaffolding materials (permissive and conducive to exogenous signals, but without specific bioactive roles) are now being replaced with “cell-instructive” materials designed to mimic the native matrix and actively interact with the cells. Importantly, these new scaffolds are engineered to be functional at multiple length and time scales: molecular (by incorporation of integrin-binding ligands, regulation of availability of growth factors), cellular (mediation of cell–cell contacts, stiffness as a differentiation factor), and tissue levels (directed migration, establishment of boundaries and interfaces, structural anisotropy). The enormous variation of cell/tissue properties has led to “designer scaffolds” instead of scaffolds universally suitable for a range of applications. We provide here a couple illustrative examples for the new generation of scaffolds being used for studies of cells and engineering of tissues.
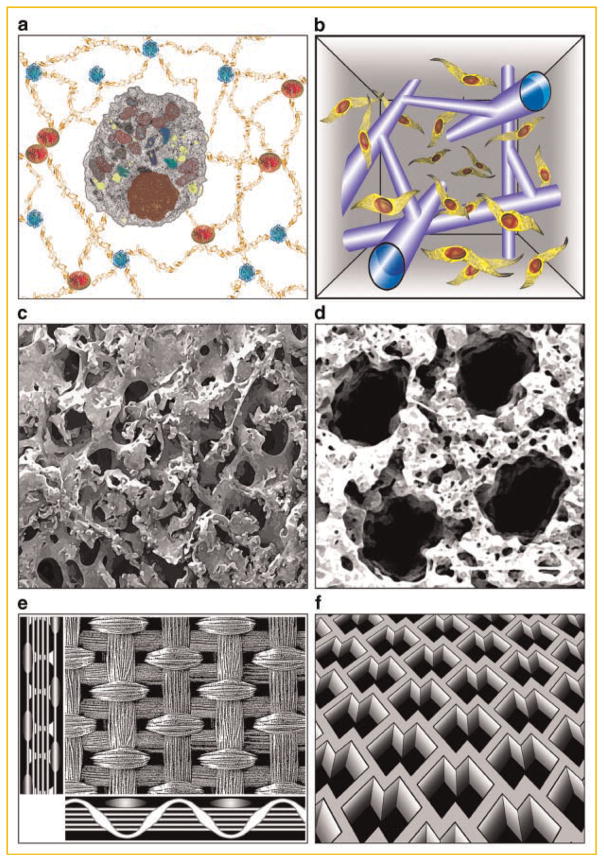
Hydrogels are a particularly suitable material for highly hydrated scaffolds with tunable molecular, mechanical, and degradation properties [Richardson et al., 2001; Lutolf and Hubbell, 2005; Wang et al., 2007; Benoit et al., 2008; Tibbitt and Anseth, 2009]. Hydrogels found applications for culture of hESCs [Elisseeff et al., 2006; Gerecht et al., 2007], and engineering of a variety of soft tissues—cartilage [Hwang et al., 2008], cardiac muscle [Zimmermann et al., 2006], and many others—largely based on the ability to incorporate specific molecular and physical cues for directing cell behavior (Fig. 3a). A recent development of methods for post-gelation modifications of hydrogel properties by laser light enable hydrogel modifications “on the go,” and after the cells have been encapsulated [Kloxin et al., 2009]. For the first time it is possible to induce geometrically precise degradation of hydrogel, for example, to form channels for cell migration (Fig. 3a) or to modulate the hydrogel functionality. This kind of post-gelation 3D patterning may have major implications on engineering of hierarchically structured tissues.
Fig. 3.
Structural signals provided through scaffold design. a: Hydrogels with tunable molecular, mechanical, and degradation properties. b: Post-gelation modification of hydrogel scaffold by laser light enables geometrically precise degradation of hydrogel, to form channels for cell migration [Kloxin et al., 2009]. c: Mechanically strong, highly porous, mineralized silk scaffold for bone tissue engineering [Wang et al., 2006]. d: Soft, highly porous, channeled elastomer scaffold for engineering vascularized cardiac muscle [Radisic et al., 2006]. e: Knitted matt-gel composite scaffold with structural and mechanical anisotropy for cartilage tissue engineering [Moutos et al., 2007]. f: Accordion-like elastomer scaffold with structural and mechanical anisotropy designed for cardiac tissue engineering [Engelmayr et al., 2008].
For engineering bone and cardiac muscle, the common structural requirements include the optimization of scaffold pores (to provide the right balance between the pore size determining cell migration and pore curvature determining cell attachment), and the establishment of hierarchical structure (orientation, anisotropy, channels for vascular conduits). Bone tissue development can be largely directed by the scaffold design. Silk is a scaffold material with tailorable molecular, structural, and mechanical properties that induce and promote the formation of human bone [Meinel et al., 2004; Wang et al., 2006]. For example, flat bone forms on scaffolds with small pores, trabecular bone on scaffolds with large pores, and transient bone on scaffolds with a gradient of structure [Uebersax et al., 2006]. Because bone is anisotropic, it would be of great interest to develop scaffolds of this kind with oriented, and elongated pores (Fig. 3c). An entirely different material—sebasic acid based elastomer—has been used as a scaffold for cardiac tissue engineering [Radisic et al., 2006]. The pores, stiffness, and channel geometry in this scaffold have been designed to enable engineering of vascularized cardiac tissue with its unique structural and mechanical features. Again, the structural and mechanical properties of native tissue have guided the scaffold design (Fig. 3c,d).
Cartilage is another example of a structurally and mechanically anisotropic load-bearing tissue. Such tissue poses many challenges to the rapid and complete restoration of its quite unique structural and biomechanical features. A recent development of a composite scaffold specifically tailored for cartilage tissue engineering provides an integrated approach to structure-function relationships [Moutos et al., 2007]. The scaffold is a composite of an anisotropic woven structure and cell-loaded hydrogel, engineered to replicate to great extent the multidirectional viscoelastic and tension-compression nonlinear properties of cartilage (Fig. 3e). This elegant study is likely to advance our efforts to engineer cartilage with load-bearing capability, and potential for integration and remodeling. Presumably, this “proof of concept” scaffold, constructed using polyesther fibers and simple agarose or fibrin gel, can be further optimized by using a custom-designed hydrogel with added functional features such as control of bioactive molecules and scaffold degradation. Also, one can imagine that the same concept could be extended to other tissues that are too complex to be “templated” by using any single, spatially and mechanically isotropic material.
Another “designer scaffold” was engineered to mimic the anisotropic structure and biomechanics of cardiac muscle (Fig. 3f) [Engelmayr et al., 2008]. The scaffold material was a highly porous, degradable elastomer extensively used for cardiac tissue engineering, also shown in Figure 3d. The curing time of this polymer was adjusted to achieve an effective tensile stiffness matching that of native rat myocardium. The material was processed by micro-fabrication into an accordion-like honeycomb scaffold with geometric properties adjusted to mimic the structural and biomechanical anisotropy of native heart muscle. When cultured with neonatal heart myocytes, the scaffold induced cell alignment and coupling, and resulted in direction-dependent contractile behavior, a situation much closer to native heart tissue properties than it can be achieved with isotropic scaffolds. It will be interesting to see if these scaffolds will also support the development of vascular networks, and be compatible with the use of perfusion bioreactors, both of which are needed for creating thick and compact tissue grafts.
GEOMETRY-FORCE CONTROL OF CELLS IN ENGINEERED TISSUES
Mechanical forces play similar developmental roles in native and engineered tissues. During development, mechanical manipulation of embryo causes abnormal axis formation [Belousov and Ermakov, 2001], abnormal blood flow impairs the formation of heart chambers and valves [Hove et al., 2003], and microgravity results in increased bone loss [Lang et al., 2004]. In tissue engineering, physical regulation during cultivation of mechanically active tissues has emerged as a new paradigm of “functional tissue engineering” [Butler et al., 2000]. We provide here several examples of methods that can be used to control the mechanical microenvironments around the cells, study of the mechanical regulators of cell behavior, and enhance functional tissue engineering through the application of mechanical forces.
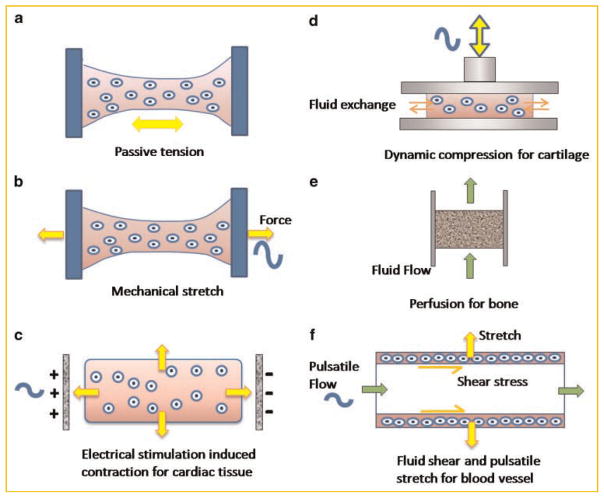
For cells seeded in collagen scaffolds fixed at both ends (Fig. 4a), mechanical forces are generated inside the gel, as cells are rearranging, aligning, and actively pulling collagen fibrils along the scaffold axis [Vandenburgh et al., 1996; Vandenburgh et al., 2009]. Tensile forces could also be generated by external mechanical tension (Fig. 4b). When applied to cardiac tissue engineering, this concept induced rapid formation of interconnected, longitudinally oriented cardiac muscle bundles with morphological features resembling those of adult native tissue [Zimmermann et al., 2002]. Moreover, the cellular tension can be induced via excitation-contraction coupling by applying cardiac-like electrical stimulation of cultured cells on scaffolds (Fig. 4c) [Radisic et al., 2004a]. Similar to the application of mechanical stretch, the electrically stimulated cells undergo electromechanical coupling, conduct electrical pacing signals over macroscopic distances, and beat synchronously at the frequency of stimulation. Both the mechanical and electrical stimulation enhanced cellular, ultra-structural, and functional properties of engineered myocardium.
Fig. 4.
Physical regulation of engineered tissues. a: Constructs are fixed at two ends, such that the cellular contraction generates tensile forces in the tissue; (b) active dynamic stretch provides mechanical regulation of the cells; (c) electrical stimulation paces the beating of cardiac constructs and cardiomyocytes apply tensile forces on the scaffold. d: Dynamic compression stimulates chondrocytes in engineered constructs and facilitates nutrient transport. e: Medium perfusion through engineered constructs promotes cell proliferation and matrix production. f: Pulsatile flow exerts shear forces to endothelial cells along the inner wall of engineered blood vessel and stretches the vessel in the circumferential direction.
While mechanical stretch benefits the maturation of engineered cardiac tissues, other tissues may be more responsive to other types of signals such as compression and fluid shear. Indeed, the same tissue can respond quite differently to different signals. For instance, tensile stress promotes the growth of immature animal growth plate while compressive stress inhibits tissue growth [Stokes et al., 2006]. Dynamic compression has been successfully used to promote the formation of mechanically functional engineered cartilage (Fig. 4d) [Mauck et al., 2000]. The effects were attributed to a combination of mechanical stimulation and fluid transport that increased the availability of nutrients and growth factors to the cells by mechanisms similar to those intrinsic to loading-enhanced transport in articulating joints. When optimal combinations and timing of application of growth factors and mechanical loading were implemented, the engineered cartilage had composition and compressive properties comparable to native cartilage.
For bone tissue, with its high density of metabolically active cells, direct perfusion generating interstitial fluid flow is a preferred and most physiologically relevant option (Fig. 4e). Perfusion supports cell viability (by local control of mass transport of nutrients and, most critically, oxygen between the culture medium and the cells) and enhances osteogenesis (by subjecting the cells to hydrodynamic shear) [Sikavitsas et al., 2003; Grayson et al., 2008].
For some tissues, a combination of physical signals may be necessary. One example is the application of pulsatile flow within the lumens of engineered small caliber arteries (Fig. 4f). The resulting dynamic strain in the circumferential direction improved the structural organization and mechanical strength of the smooth muscle wall, whereas the fluid flow enabled the vessels to remain open and provided shear forces to the endothelial cells on internal vessel walls [Niklason et al., 1999]. Another example is the application of multiparametric mechanical stimulation to engineered human ligaments. To mimic the combination of mechanical strains that an anterior cruciate ligament experiences in vivo, human mesenchymal stem cells cultured in collagen gel (or on silk scaffolds) were subjected to a combination of dynamic axial tension and torsion, both applied at a physiologic amplitude and frequency. Again, the resulting ligaments had substantially improved collagen fibril organization, tissue morphology and composition, and expression of collagen types I and III and tenascin-C [Altman et al., 2002].
In summary, the last decade resulted in the development of a new generation of culture systems of high biological fidelity that are finding applications in fundamental biological research, engineering of functional tissue grafts, and studies of disease. These engineering designs are inspired and guided by “biomimetics”—an approach that aims to recapitulate in vitro some of the aspects of the native cellular milieu associated with tissue development and regeneration. Of note, there is increased recognition that physical regulatory signals involved in tissue development and function act in concert with molecular factors, in most cases in form of spatial and temporal gradients. Such highly sophisticated environments, in which cells establish dynamic relationship with the matrix and other cells, are enabling the biological fidelity and levels of control not achievable in the past. We discussed here the new possibilities of the control of cell function by structural (geometric) and physical (force-related) factors, in the context of the biomimetic approach to cell culture and tissue engineering.
Acknowledgments
National Institutes of Health; Grant numbers: R01 DE16525, R01 HL076485, P41-EB002520, T 32 HL087745.
We gratefully acknowledge the research support of NIH (R01 HL076485, R01 DE16525, P41-EB002520 to GVN), and the NIH Postdoctoral Fellowship (to DF, from T 32 HL087745). We also thank Nebojsa Mirkovic for his outstanding help with illustrations.
References
- Abu-Issa R, Kirby ML. Heart field: From mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- Belousov LV, Ermakov AS. Artificially applied tensions normalize development of relaxed Xenopus Laevis embryos. Ontogenez. 2001;32:288–294. [PubMed] [Google Scholar]
- Benoit DS, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- Bornstein P, Duksin D, Balian G, Davidson JM, Crouch E. Organization of extracellular proteins on the connective tissue cell surface: Relevance to cell-matrix interactions in vitro and in vivo. Ann NY Acad Sci. 1978;312:93–105. doi: 10.1111/j.1749-6632.1978.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: The role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Bruder SP. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- Chen H, Feyereisen M, Long XP, Fitzgerald G. Stability, bonding, and geometric structure of Ti8C12, Ti8N12, V8C12, and Zr8C12. Phys Rev Lett. 1993;71:1732–1735. doi: 10.1103/PhysRevLett.71.1732. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisseeff J, Ferran A, Hwang S, Varghese S, Zhang Z. The role of biomaterials in stem cell differentiation: Applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295–303. doi: 10.1089/scd.2006.15.295. [DOI] [PubMed] [Google Scholar]
- Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, O’Kane S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Bhumiratana S, Cannizzaro C, Chao PH, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14:1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. Observations on the living developing nerve fiber. Anat Rec. 1907;1:116–118. [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci USA. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- Li Jeon N, Baskaran H, Dertinger SK, Whitesides GM, Van de Water L, Toner M. Neutrophil chemotaxis in linear and complex gradients of interleukin-8 formed in a microfabricated device. Nat Biotechnol. 2002;20:826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Little CD, Rongish BJ. The extracellular matrix during heart development. Experientia. 1995;51:873–882. doi: 10.1007/BF01921738. [DOI] [PubMed] [Google Scholar]
- Lucchetta EM, Lee JH, Fu LA, Patel NH, Ismagilov RF. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature. 2005;434:1134–1138. doi: 10.1038/nature03509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extra-cellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Meinel L, Karageorgiou V, Fajardo R, Snyder B, Shinde-Patil V, Zichner L, Kaplan D, Langer R, Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008;103:234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, Kumacheva E, Zandstra PW. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004a;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004b;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Bissell MJ. Dynamic reciprocity revisited: A continuous, bidirectional flow of information between cells and the extracellular matrix regulates mammary epithelial cell function. Biochem Cell Biol. 1995;73:391–397. doi: 10.1139/o95-046. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf C. Cell and tissue organisation. 2007 westminsterresearch.wmin.ac.uk.
- Sawano A, Takayama S, Matsuda M, Miyawaki A. Lateral propagation of EGF signaling after local stimulation is dependent on receptor density. Dev Cell. 2002;3:245–257. doi: 10.1016/s1534-5807(02)00224-1. [DOI] [PubMed] [Google Scholar]
- Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100:14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocum DL. Stem cells in regenerative biology and medicine. Wound Repair Regen. 2001;9:429–442. doi: 10.1046/j.1524-475x.2001.00429.x. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res. 2006;24:1327–1334. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebersax L, Hagenmüller H, Hofmann S, Gruenblatt E, Müller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006;12:3417–3429. doi: 10.1089/ten.2006.12.3417. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H, Del Tatto M, Shansky J, Lemaire J, Chang A, Payumo F, Lee P, Goodyear A, Raven L. Tissue-engineered skeletal muscle organoids for reversible gene therapy. Hum Gene Ther. 1996;7:2195–2200. doi: 10.1089/hum.1996.7.17-2195. [DOI] [PubMed] [Google Scholar]
- Vandenburgh H, Shansky J, Benesch-Lee F, Skelly K, Spinazzola JM, Saponjian Y, Tseng BS. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts. FASEB J. 2009 doi: 10.1096/fj.09-134411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Saadi W, Lin F, Minh-Canh Nguyen C, Li Jeon N. Differential effects of EGF gradient profiles on MDA-MB-231 breast cancer cell chemotaxis. Exp Cell Res. 2004;300:180–189. doi: 10.1016/j.yexcr.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Zagris N. Extracellular matrix in development of the early embryo. Micron. 2001;32:427–438. doi: 10.1016/s0968-4328(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]