Abstract
Purpose
To estimate the independent relationships of running intensity with antihypertensive, LDL-cholesterol–lowering, and antidiabetic medication use when adjusted for running volume (km/d).
Methods
Self-reported medication use was compared cross-sectionally to running pace (m/s during usual run) in 25,552 male and 29,148 female National Runners’ Health Study participants.
Results
The men ran a mean ± SD of 5.2 ± 3.1 km/d at 3.3 ± 0.5 m/s (8.3 ± 1.4 min/mile) and the women 4.7 ± 2.9 km/wk at 3.0 ± 0.4 m/s (9.2 ± 1.8 min/mile). When adjusted for kilometers per day, each meter-per second increment in intensity in men and women reduced the odds for antihypertensive drug use by 54% and 46%, respectively, reduced the odds for LDL-cholesterol–lowering medication use by 55% and 48%, respectively, and reduced the odds for antidiabetic medication use by 50% and 75%, respectively (all P < 0.0001). Compared with men who ran slower than 10 min/mile, the odds for medication use in those who ran or exceeded a 7-min/mile pace were 72% less for antihypertensive, 78% less for LDL-cholesterol lowering, and 67% less for antidiabetic medications (the corresponding odds reductions in women were 61%, 64%, and 87%, respectively, for 8 min/mile or faster versus slower than 11 min/mile). Although usual running pace correlated significantly with a 10-km performance (male, r = 0.55; females, r = 0.49), usual pace remained significantly related to lower use of all three medications in men and antihypertension and antidiabetic medications in women when adjusted for a 10-km performance.
Conclusions
Although these results do not prove causality, they show that exercise intensity is inversely associated with the prevalence of hypertension, hypercholesterolemia, and diabetes independent of exercise volume and cardiorespiratory fitness (10-km performance), suggesting that the more vigorous the exercise, the healthier the health benefits.
INTRODUCTION
Physical activities may be characterized by their energy expenditure into light intensity, requiring less than threefold the energy expenditure of sitting at rest (3 METs, 1 MET = 3.5 mL/min/kg), moderate intensity (3 to 6 METs), and vigorous intensity (> 6 METs) [1]. Most of what is known about the relationships of exercise intensity to health outcomes pertain to differences between moderate and vigorous exercise [31]. When total energy is held constant, vigorous exercise is reported to produce greater reductions in diastolic blood pressure and to produce greater improvements in glucose control than moderate exercise, whereas vigorous and moderate activities are reported to have equivalent effects on systolic blood pressure, lipoproteins, and body fat [31]. There are also reports on the health effects of varying intensities of light and moderate exercise [31], including those showing that the prevalence or incidence of coronary heart disease [22], diabetes [15,16,36], hypertension [36], and hypercholesterolemia [36] decline with faster walking speed.
The focus on moderate versus vigorous exercise arises from the public health issue of whether it is necessary to break a sweat to obtain the health benefits of physical activity [12]. Little has been reported on the health effects of increasing intensities of vigorous exercise. Running typically spans a range from 8 [12 min/mile] to 15 METs (6.5 min/mile) [1]. This range exceeds the entire intensity range of all light-to-moderate exercise. We have reported that faster 10-km performance times were associated with lower BMI [33,35] and lower mean blood pressure levels when adjusted for BMI [33]. In addition, greater cardiorespiratory fitness is associated with lower risks for hypercholesterolemia, diabetes, hypertension, and coronary heart disease [5,37,38].
This report examines the prevalence of hypertension, hypercholesterolemia, and diabetes in relation to usual running speed when adjusted for age, body mass, and total exercise dose. Because increases in cardiorespiratory fitness are greater for vigorous than moderate exercise [30] and are greater for more vigorous than less vigorous exercise [32], we hypothesized that more intense vigorous exercise may produce greater health benefits than less intense vigorous exercise. Although related, cardiorespiratory fitness and usual training intensity are distinct characteristics. Cardiorespiratory fitness is inherited in addition to being affected by training [6,9]. Exercise intensity is not simply determined by cardiorespiratory fitness but rather is a behavior based on other factors in addition to fitness (e.g., motivation), subject to individual control and amenable to change. Although cross-sectional associations cannot prove that running intensity causally affects disease, the results are consistent with the hypothesis that the more vigorous the vigorous exercise, the healthier the health benefits.
SUBJECTS AND METHODS
Although the design and methods of the National Runners’ Health Study are described elsewhere [33–39], information on usual running pace was obtained only for those runners recruited between 1998 and 2001. Briefly, a two-page questionnaire was distributed nationally to runners identified among running magazine subscribers and participants of foot race events. The questionnaire solicited information on demographics, running history, weight history, smoking habits, history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. From our initial cohort, we estimate that approximately 15% returned the questionnaires among the total number contacted. The University of California Berkeley Committee for the Protection of Human Subjects approved the study protocol, and all participants signed committee-approved informed consents.
Participants reported whether they took medications for diabetes, high blood pressure, and high cholesterol at the time of the survey. Usual running pace was reported as minutes per mile, which was then converted by us into meters per second. Running distances were reported in usual miles run per week for the year in which the baseline survey was taken, which has been shown to correlate strongly between duplicate questionnaires (r = 0.89) [34]. Running represented (mean ± SD) 91.5 ± 19.1% of all vigorously intense activity and 73.5 ± 23.7% of total leisure time physical activity in these runners.
BMI was calculated as weight in kilograms divided by the square of height in meters. Self-reported waist circumferences were elicited by the statement, “Please provide, to the best of your ability, your waist circumference in inches,” without further instruction. Elsewhere, we have reported the strong correlations between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [34]. Self-reported waist circumferences are somewhat less precise, as indicated by their correlations with reported circumferences on a second questionnaire (r = 0.84) and with their clinical measurements (r = 0.68) [34]. Others report that clinically measured waist circumferences correlate significantly with visceral (r = 0.68), abdominal subcutaneous (r = 0.74), and total abdominal adipose tissue masses (r = 0.82) as measured by computed tomography [40].
Intakes of meat and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat?”, and “…pieces of fruit do you eat?”. Alcohol intake was estimated from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs and computed as 14.2 mL per 4-oz glass of wine, 14.2 mL per 12-oz bottle of beer, and 17.7 mL per mixed drink. Correlations between these responses and values obtained from 4-day diet records in 110 men were r = 0.65 for alcohol intake, r = 0.46 for red meat, and r = 0.38 for fruit. For this report, cardiorespiratory fitness is defined as speed (m/s) of the participant’s best 10-km race during the previous 5 yr (reported as finish time in min). Published data support the use of running performance to estimate maximal oxygen consumption (VO2max) [3,8,13], and 10-km race performance times have been shown to be more strongly correlated with the incidence and prevalence of diabetes, hypertension, and hypercholesterolemia than distance run [37,38].
Statistical analyses
Logistic regression analyses (JMP Software Version 5.0; SAS Institute, Cary, NC) were used to estimate the dose response relationships of medication use to usual running pace. Reported weekly intakes of alcohol, meat, and fruit along with age, smoking history, and running distance (km/d) were used as covariates. Additional adjustment for BMI and 10-km performance (m/s) was included as indicated. Analyses were performed using usual running pace as both continuous and categorical measures although the statistical power to detect associations may be less for the categorical scale, reexpressing the exposure variables for categorical measures provides greater robustness to outliers.
RESULTS
From the original 59,506 surveys received on or after 1998 (when the question on usual running pace was introduced), we excluded 1 survey for missing sex, 76 for missing age, 967 for missing BMI, 1139 for missing running distance (many presumably nonrunners), and 1494 for missing usual running pace. We also excluded 1129 current smokers. Thus, complete data on distance run per day, usual running pace, and BMI were available for 25,552 male and 29,148 female nonsmoking runners. The sample was primarily white (men = 86.7%; women = 88.5%), with smaller percentages of Hispanic (men = 6.6%; women = 5.1%), Asian (men = 3.1%; women = 3.1%), African American (men = 1.9%; women = 1.5%), Native American (men = 0.6%; women = 0.5%), and mixed race (men = 0.9%; women, 1.1%). The men were significantly older than the women (mean ± SD: 44.4 ± 11.4 vs. 38.0 ± 10.1 yr), whereas their years of education were similar (16.5 ± 2.6 vs. 16.2 ± 2.6 yr, respectively).
The characteristics of the sample in relation to their self-reported usual pace are presented in Table 1. Faster runners of both sexes were younger, lighter, leaner, and smaller waisted but not necessarily distinguished by stature. Faster runners were also less likely to be ex-smokers. Younger age probably explains the shorter running history of the faster runners. Medication use was 8- to 14-fold greater in the slowest than fastest men and 5- to 7-fold greater in the slowest than fastest women. Intakes of meat and fruit were inversely related to running pace in women (P < 0.0001) but not men, whereas alcohol intake was inversely related to pace in men (P < 0.0001) but not women. On average, the men ran at 88.9% and the women at 92.7% of their 10-km performance speed. Usual running pace averaged 0.47 ± 0.44 m/s slower than the 10-km performance times in men and 0.30 ± 0.46 m/s slower in women; however, usual running pace and 10-km performance were strongly correlated (P < 0.0001) when unadjusted for other factors (men, r = 0.68; women, r = 0.58) and when adjusted for age (men, r = 0.61; women, r = 0.56) and kilometers per day (male, r = 0.55; females, r = 0.49) or age, kilometers per day, and BMI (men, r = 0.51; women, r = 0.46).
Table 1.
Characteristics of male and female runners by reported usual running speed
| Usual running speed | |||||
|---|---|---|---|---|---|
| Slowest | Slow | Intermediate | Faster | Fastest | |
| Males (min/mile) | >10 | 9 to 10 min | 8 to 9 min | 7 to 8 min | ≤7 min mile |
| Sample (N) | 1102 | 2774 | 6670 | 10233 | 4773 |
| Anti-hypertensive medications (%) | 15.8 | 11.4 | 6.5 | 3.5 | 1.4 |
| LDL-cholesterol lowering medications (%) | 14.2 | 8.4 | 5.9 | 3.0 | 1.0 |
| Anti-diabetic medications (%) | 2.5 | 1.2 | 0.7 | 0.4 | 0.3 |
| Ex-smokers (%) | 49.2 | 43.8 | 39.0 | 27.9 | 16.5 |
| Age (years) | 56.5±12.8 | 51.3±11.1 | 47.7±9.9 | 43.0±9.5 | 36.1±10.3 |
| Education (years) | 16.2±2.8 | 16.7±2.6 | 16.6±2.5 | 16.5±2.5 | 16.0±2.7 |
| Height (m) | 1.8±0.1 | 1.8±0.1 | 1.8±0.1 | 1.8±0.1 | 1.8±0.1 |
| Mass (kg) | 83.2±13.0 | 81.9±11.7 | 79.6±10.1 | 76.6±9.0 | 72.7±8.6 |
| BMI (kg/m2) | 26.5±3.7 | 25.9±3.3 | 25.1±2.8 | 24.1±2.5 | 23.0±2.5 |
| Waist circumference (cm) | 96.6±10.0 | 94.0±8.7 | 91.5±7.7 | 88.2±7.0 | 84.6±6.8 |
| Alcohol (ml/wk) | 80.3±127.1 | 81.7±119.3 | 83.9±111.1 | 78.4±105.3 | 67.0±97.7 |
| Meat (servings/wk) | 3.2±3.0 | 3.2±3.2 | 3.2±2.9 | 3.0±2.7 | 3.1±3.1 |
| Fruit (pieces/wk) | 10.6±9.4 | 10.2±9.1 | 9.8±8.4 | 10.1±8.2 | 10.3±8.4 |
| Years run | 14.3±10.9 | 12.6±9.7 | 12.2±9.1 | 12.3±8.6 | 11.9±8.6 |
| Running distance (km/wk) | 3.4±2.4 | 4.0±2.4 | 4.6±2.4 | 5.3±2.9 | 6.8±4.1 |
| Usual run speed (m/s) | 2.24±0.31 | 2.73±0.69 | 3.06±0.09 | 3.44±0.12 | 4.01±0.30 |
| 10 km performance (m/s) | 2.93±0.61 | 3.21±0.43 | 3.53±0.40 | 3.92±0.42 | 4.42±0.51 |
| Females (min/mile) | >11 | 10 to 11 min | 9 to 10 min | 8 to 9 min | ≤8 min mile |
| Sample (N) | 1948 | 1494 | 7156 | 10582 | 7968 |
| Anti-hypertensive medications (%) | 7.2 | 3.8 | 2.1 | 1.2 | 0.8 |
| LDL-cholesterol lowering medications (%) | 2.6 | 1.5 | 0.8 | 0.4 | 0.3 |
| Anti-diabetic medications (%) | 1.0 | 0.5 | 0.3 | 0.2 | 0.1 |
| Ex-smokers (%) | 34.2 | 35.5 | 32.4 | 27.7 | 19.0 |
| Age (years) | 46.2±12.3 | 44.1±10.5 | 40.2±9.9 | 37.4±8.8 | 33.6±8.7 |
| Education (years) | 15.8±4.6 | 16.2±2.4 | 16.3±2.6 | 16.3±2.3 | 16.1±2.5 |
| Height (m) | 1.6±0.1 | 1.6±0.1 | 1.6±0.1 | 1.7±0.1 | 1.7±0.1 |
| Mass (kg) | 64.8±11.3 | 62.6±8.6 | 60.2±7.7 | 58.4±6.7 | 56.6±6.3 |
| BMI (kg/m2) | 24.2±3.9 | 23.3±3.0 | 22.3±2.6 | 21.4±2.2 | 20.7±2.1 |
| Waist circumference (cm) | 80.5±11.4 | 78.5±9.4 | 76.2±8.8 | 74.4±7.7 | 73.0±7.6 |
| Alcohol (ml/wk) | 43.1±72.2 | 47.8±72.0 | 50.1±74.4 | 52.1±68.9 | 47.5±68.9 |
| Meat (servings/wk) | 2.4±2.6 | 2.1±2.3 | 2.0±2.2 | 1.7±2.2 | 1.6±2.1 |
| Fruit (pieces/wk) | 10.6±8.6 | 10.4±8.1 | 9.8±7.0 | 10.3±7.5 | 10.6±8.0 |
| Years run | 8.7±8.1 | 8.0±7.3 | 8.1±7.0 | 8.9±6.9 | 9.8±6.9 |
| Running distance (km/wk) | 2.7±2.3 | 3.4±2.2 | 3.8±2.3 | 4.8±2.5 | 6.0±3.4 |
| Usual run speed (m/s) | 2.02±0.32 | 2.47±0.05 | 2.73±0.07 | 3.05±0.09 | 3.50±0.25 |
| 10 km performance (m/s) | 2.72±0.74 | 2.82±0.58 | 3.03±0.46 | 3.33±0.39 | 3.76±0.47 |
ANOVA for differences between running speeds all significant at P<0.0001 except height (P=0.13), meat intake (P=0.05) and fruit intake (P=0.92) in men, and education (P=0.48), alcohol intake (P=0.10) and fruit intake (P=0.009) in women. 10-km performance times were reported by 75% of men and 64.5% of women, and waist circumferences were reported by 78% of men and 72% of women. Shown are means±SD except for percentages.
Table 2 presents the odds ratios per meters-per-second increment in usual running speed adjusted for age, ex-smoker status, and diet. The odds for taking medications for high blood pressure, high cholesterol, or diabetes decreased in association with the meters per second of usual running speed (P < 0.0001), and these decreases were little affected by additional adjustment for distance. Further adjustment for BMI somewhat diminished the significances of the odds reductions; however, all remained statistically significant. With the exception of antidiabetic use in men, the odds reductions also remained significant when adjusted for waist circumference. Adjustment for the separate effects of height and weight produced odds ratios and significance levels entirely consistent with the BMI-adjusted odds ratios of Table 2 (analyses not displayed).
Table 2.
Odds ratio (95% confidence intervals) for the prevalence of anti-hypertensive, LDL-cholesterol lowering, and anti-diabetic medication use per m/s of reported running speed during usual run (N=29,726 men and 12,222 women).
| Unadjusted for km/day & BMI (kg/m2) | Adjusted for km/day | Adjusted for km/day & BMI (kg/m2) | Adjusted for km/day & waist circumference (cm) | |
|---|---|---|---|---|
| Males | ||||
| Anti-hypertensive | 0.43§ (0.38, 0.49) | 0.46§ (0.40, 0.53) | 0.58§ (0.50, 0.67) | 0.55§ (0.46, 0.65) |
| LDL-cholesterol lowering | 0.41§ (0.36, 0.47) | 0.45§ (0.39, 0.52) | 0.51§ (0.44, 0.60) | 0.42§ (0.35, 0.51) |
| Anti-diabetic | 0.43§ (0.30, 0.60) | 0.50§ (0.35, 0.71) | 0.62† (0.43, 0.91) | 0.71 (0.45, 1.11) |
| Females | ||||
| Anti-hypertensive | 0.51§ (0.42, 0.62) | 0.54§ (0.44, 0.66) | 0.69‡ (0.56, 0.87) | 0.56§ (0.43, 0.73) |
| LDL-cholesterol lowering | 0.45§ (0.33, 0.61) | 0.52§ (0.38, 0.73) | 0.71* (0.50, 1.00) | 0.42§ (0.28, 0.63) |
| Anti-diabetic | 0.25§ (0.16, 0.38) | 0.25§ (0.16, 0.41) | 0.35§ (0.21, 0.59) | 0.30‡ (0.16, 0.57) |
Odds ratios adjusted for age (age and age2), ex-smoker status, and intakes of meat, fruit, and alcohol. Additional adjustment for km/day, BMI and waist circumference where indicated. Significance levels are coded
P<0.05,
P<0.01,
P<0.001, and
P<0.0001.
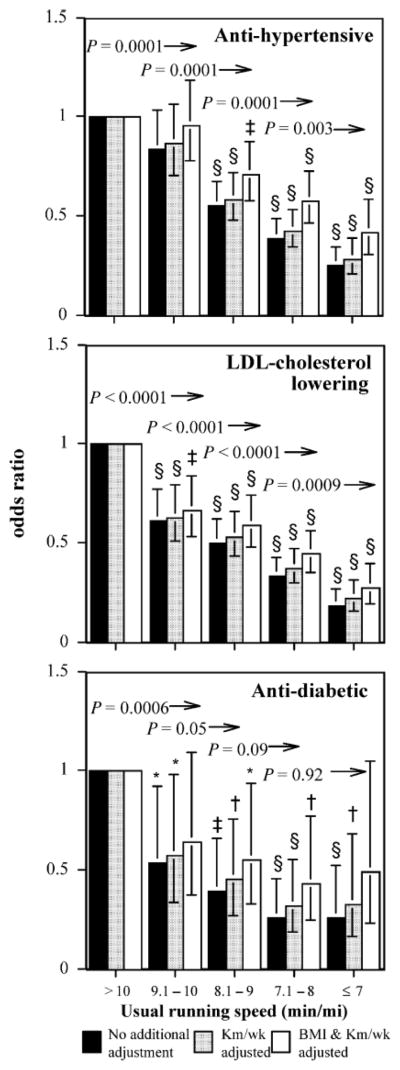
Figure 1 presents the men’s odds ratios for usual running speed adjusted for age, diet, ex-smoker status, and alcohol. The ratios are calculated relative to the odds for medication use in men who ran slower than a 10-min/mile (<2.68 m/s). The odds ratio for antihypertensive medication use declined linearly with usual speed and was significantly less than 1 in men who ran 9 min/mile or faster. Additional adjustment for running distance increased the odds ratios only slightly. Above the bars are the kilometers-per-day–adjusted significance levels for exceeding the running pace represented by the bar (i.e., the odds for using antihypertensive medications were significantly lower, P = 0.003, in men who ran faster than 7 min/mile than men who ran 7.1 to 8 min/mile). These significance levels show significant incremental reductions for antihypertensive medication usage throughout the range of running speeds when adjusted for distance run. Additional adjustment for BMI lessened the reductions in the odds ratio with usual running speed; however, medication usage remained significantly lower in men who ran 8.1 to 9, 7.1 to 8, or 7 min/mile or less compared to those running slower than 10 min/mile.
FIGURE 1.
Odds ratios for medication use by usual running pace relative to the slowest men (referent group >10 min/mile), adjusted for age and intakes of meat, fish, fruit, and alcohol. Additional adjustment for running distance (km/wk) and BMI where indicated. Brackets designate 95% confidence intervals. Significance levels relative to the slowest men are coded *P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001. Significance levels relative to all faster men are presented above the bars and to the left of the arrows (e.g., men who ran faster than 9 min/mile were significantly less likely to use antidiabetic medications than those who ran a 9- to 10-min/mile at P = 0.05 when adjusted for km/d run).
When adjusted for distance, reductions in men’s use of LDL-cholesterol–lowering medication were significant for each minute per mile increment in usual running speed. The results were similar with or without kilometers per day and BMI adjustment. Relative to the slowest men, the odds for medication use in those who ran 7 min/mile or faster were reduced 81% without adjustment for kilometers per day, 78% with adjustment for kilometers per day, and 72% adjusted for kilometers per day and BMI.
There were also substantial reductions in antidiabetic medication use with usual running speed, particularly with respect to slower than 10 min/mile versus faster paces. Adjustment for BMI did lessen the significance of the odds reduction; however, men whose speed was 8 min/mile or faster had less than one half the odds for using antidiabetic medications than those requiring over 10 minutes to run a mile.
Figure 2 presents the corresponding graphs for women. Because the women averaged about a mile per minute slower than the men (mean ± SD: 9.2 ± 1.8 vs. 8.3 ± 1.4 min/mile), their speed categories were shifted to be 1 min/mile slower. Running an 11-min/mile or faster was associated with significantly lower odds for medication use than slower women (P < 0.0001 for antihypertensive and antidiabetic medications, and P = 0.0006 for LDL-cholesterol–lowering medications). Running a 10-min/mile or faster was also associated with lower odds for antihypertensive (P = 0.03), LDL-cholesterol–lowering (P = 0.02), and antidiabetic medications (P = 0.06) than running 10.1 to 11 min/mile. Adjustment for running distance had little effect on the women’s odds ratios, and although adjustment for BMI had a somewhat greater effect, the significance of the odds ratios was generally not lost except for LDL-cholesterol–lowering.
FIGURE 2.
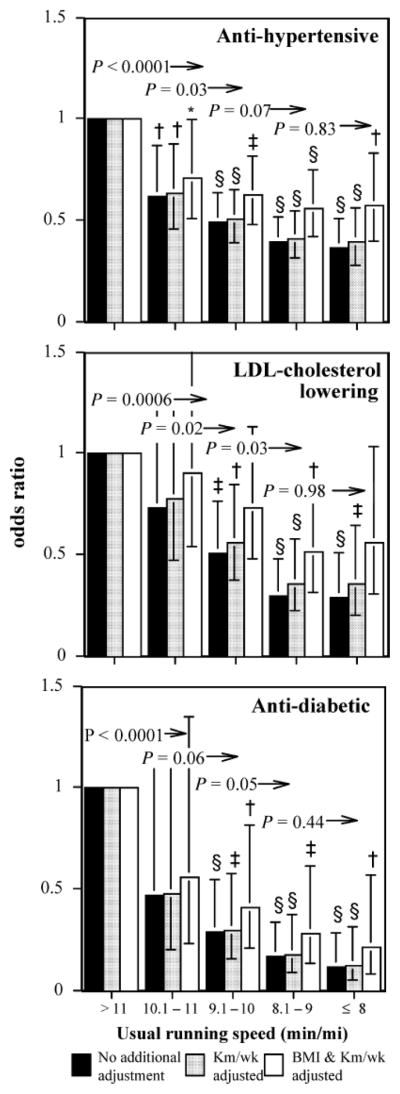
Odds ratios for medication use by usual running pace relative to the slowest women (referent group >11 min/mile), adjusted for age and intakes of meat, fish, fruit, and alcohol. Additional adjustment for running distance (km/wk) and BMI where indicated. Brackets indicate 95% confidence intervals. Significance levels relative to the slowest women are coded *P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001. Significance levels relative to all faster women are presented above the bars and to the left of the arrows (e.g., women who ran 9 min/mile or faster were significantly less likely to use antidiabetic medications than those who ran 9.1 to 10 min/mile at P = 0.05 when adjusted for km/d run).
Table 3 presents the odds ratios for antihypertensive, LDL-cholesterol–lowering, and antidiabetic medication use by running distance in this sample. In men, usual running distance was associated with lower medication use both before and after adjustment for usual running speed, albeit the odds reductions per kilometers-per-day run were reduced when adjusted. The women’s odds reductions for antihypertensive and LDL-cholesterol–lowering medications were similar to those of men but lacked statistical significance when adjusted for usual running speed.
Table 3.
Odds ratio (95% confidence intervals) for the prevalence of anti-hypertensive, LDL-cholesterol lowering, and anti-diabetic medication use by running distance (km/day) before and after adjustment usual running speed (N=29,726 men and 12,222 women).
| Unadjusted for usual running speed | Adjusted for usual running speed | |
|---|---|---|
| Males | ||
| Anti-hypertensive | 0.93§ (0.91, 0.95) | 0.96‡ (0.94, 0.98) |
| LDL-cholesterol lowering | 0.92§ (0.90, 0.94) | 0.95§ (0.92, 0.97) |
| Anti-diabetic | 0.89‡ (0.84, 0.95) | 0.92† (0.86, 0.98) |
| Females | ||
| Anti-hypertensive | 0.94‡ (0.91, 0.97) | 0.97 (0.94, 1.01) |
| LDL-cholesterol lowering | 0.90‡ (0.85, 0.96) | 0.94 (0.88, 1.01) |
| Anti-diabetic | 0.92 (0.84, 1.01) | 1.02 (0.93, 1.12) |
Odds ratios adjusted for age (age and age2), ex-smoker status, and intakes of meat, fruit, and alcohol. Additional adjustment for usual running speed where indicated. Significance levels are coded
P<0.05,
P<0.01,
P<0.001, and
P<0.0001.
Table 4 examines the independent contributions of running speed (m/s) during a usual run and maximum performance over a 10-km footrace by simultaneously including both in the logistic regression model. The analyses are restricted to 75% of men and 64.5% of women who provided their race times. Compared with those excluded for lacking these data, the runners included in these analyses were similar in age (mean ± SD for included vs. excluded males = 44.3 ± 10.6 vs. 44.9 ± 12.0 yr, females = 38.5 ± 9.6 vs. 37.7 ± 11.0 yr) but tended to be leaner (males = 24.0 ± 2.7 vs. 24.8 ± 3.1 kg/m2, females = 21.4 ± 2.5 vs. 22.1 ± 3.2 kg/m2), and run farther (males = 39.2 ± 22.5 vs. 28.8 ± 21.3 km/wk, females = 36.9 ± 21.0 vs. 26.6 ± 19.3 km/wk) and faster during their usual runs (males = 3.35 ± 0.45 vs. 3.22 ± 0.55 m/s, females = 3.05 ± 0.39 vs. 2.90 ± 0.51 m/s). The subset shows the same odds reduction in medication use with usual running speed in men as the complete sample but somewhat weaker odds reduction compared with the complete sample of women (cf. Table 2 and Table 4). There is also a general agreement between the odds ratio for medication use versus the 10-km performance for this subset and the entire cross-sectional sample [38]. The analyses show that when adjusted for 10-km performance times (a measure of cardiorespiratory fitness), the odds ratio per meters-per-second increment in usual running speed remains significantly less than 1 for antidiabetic, LDL-cholesterol–lowering, and antidiabetic medication use in men and for antidiabetic and antihypertensive medication use in women. After additional adjustment for BMI, the separate contributions of usual running speed and 10-km performance are less distinguishable for antidiabetic medication use in men and all three medications in women. However, men’s usual running speed remains significantly related to their odds ratio for antihypertensive and LDL-cholesterol–lowering medications when adjusted for 10-km performance and BMI in addition to age, diet, and ex-smoker status. Faster 10-km performance was significantly related to lower antihypertensive and LDL-cholesterol–lowering medication use in men and antihypertensive medication use in women when adjusted for usual running speed. However, adjusting for the 10-km performance diminishes the odds reduction for medication use versus running speed, and correspondingly, adjusting for running speed diminishes the odds reduction for medication use versus the 10-km performance.
Table 4.
Odds ratio (95% confidence intervals) for the prevalence of anti-hypertensive, LDL-cholesterol lowering, and anti-diabetic medication use per m/s of running speed during usual run and 10km foot race (N=19,228 men and 18,806 women).
| Unadjusted for BMI (kg/m2) | Adjusted for BMI (kg/m2) | ||||
|---|---|---|---|---|---|
| Model | Odds ratio for usual running speed (m/s) | Odds ratio for 10km performance (m/s) | Odds ratio for usual running speed (m/s) | Odds ratio for 10km performance (m/s) | |
| Males | |||||
| Anti-hypertensive | Usual speed only | 0.43§ (0.36, 0.52) | 0.55§ (0.45, 0.67) | ||
| 10 km performance only | 0.52§ (0.45, 0.60) | 0.62§ (0.53, 0.71) | |||
| Usual speed & 10 km | 0.59§ (0.47, 0.74) | 0.64§ (0.54, 0.76) | 0.70† (0.55, 0.87) | 0.70§ (0.60, 0.83) | |
| LDL-cholesterol lowering | Usual speed only | 0.42§ (0.34, 0.51) | 0.49§ (0.39, 0.60) | ||
| 10 km performance only | 0.56§ (0.49, 0.65) | 0.63§ (0.54, 0.74) | |||
| Usual speed & 10 km | 0.52§ (0.41, 0.66) | 0.74‡ (0.62, 0.88) | 0.57§ (0.45, 0.73) | 0.78† (0.65, 0.93) | |
| Anti-diabetic | Usual speed only | 0.39§ (0.24, 0.62) | 0.45† (0.27, 0.76) | ||
| 10 km performance only | 0.51‡ (0.35, 0.73) | 0.57† (0.39, 0.84) | |||
| Usual speed & 10 km | 0.51* (0.29, 0.92) | 0.66 (0.43, 1.02) | 0.57 (0.31, 1.04) | 0.70 (0.45, 1.09) | |
| Females | |||||
| Anti-hypertensive | Usual speed only | 0.55‡ (0.40, 0.76) | 0.69* (0.49, 0.96) | ||
| 10 km performance only | 0.64‡ (0.51, 0.81) | 0.73† (0.58, 0.92) | |||
| Usual speed & 10 km | 0.68* (0.47, 0.97) | 0.74* (0.57, 0.95) | 0.81 (0.56, 1.17) | 0.78* (0.61, 1.00) | |
| LDL-cholesterol lowering | Usual speed only | 0.58* (0.34, 0.98) | 0.84 (0.48, 1.46) | ||
| 10 km performance only | 0.80 (0.54, 1.16) | 0.96 (0.67, 1.39) | |||
| Usual speed & 10 km | 0.60 (0.33, 1.09) | 0.94 (0.62, 1.42) | 0.83 (0.45, 1.53) | 1.01 (0.68, 1.50) | |
| Anti-diabetic | Usual speed only | 0.28† (0.13, 0.63) | 0.40* (0.16, 1.00) | ||
| 10 km performance only | 0.43† (0.23, 0.81) | 0.56 (0.29, 1.07) | |||
| Usual speed & 10 km | 0.37* (0.14, 0.98) | 0.61 (0.30, 1.23) | 0.50 (0.18, 1.40) | 0.69 (0.34, 1.38) | |
Analyses restricted to the 75.3% of men and 64.5% of women who provided their 10-km performance times. Odds ratios adjusted for age (age and age2), weekly running distance (km/day and km2/day2), ex-smoker status, and intakes of meat, fruit, and alcohol. Additional adjustment for BMI (BMI and BMI2) where indicated. Significance levels are coded
P<0.05,
P<0.01,
P<0.001, and
P<0.0001.
Additional analyses to assess whether relative intensity predicted the odds for medication use showed that runners who typically ran at a higher percentage of their 10-km performance were not at significantly lower odds for using antihypertensive, antidiabetic, or LDL-lowering medications (analyses not displayed).
DISCUSSION
The health benefits of exercising at greater intensities have been previously demonstrated by comparing vigorous to moderate exercise [31], by comparing across intensities of primarily light to moderate exercise [31], and by comparing different walking speeds [15,16,22,36]. Running is vigorously intense [1]. There is a linear relationship between V̇O2 and running speed below the lactate threshold [4]. The estimated MET expenditures by running speed range from 8 to 18 METs for running paces of 12 to 5.5 min/mile [1]. These MET values translate into an exercise dose that is solely a function of distance (1.02 kcal/kg per kilometer). Thus, when statistically adjusted for average kilometers-per-week run, the effects of exercise intensity on health outcomes are obtained independent of the total activity dose. By this approach, we have shown that the prevalence of hypertension, hypercholesterolemia, and diabetes were all significantly and inversely correlated with usual running intensity.
Recent updates to the physical activity recommendations by the American College of Sports Medicine and the American Heart Association [12] largely adhere to the tenet that training response and adaptation are directly related to exercise volume [10]. A minimum of 30 min of moderate-intensity aerobic activity (such as brisk walking) for 5 d/wk, or 20 min of vigorous-intensity aerobic activity (such as jogging) 3 d/wk are specifically recommended [12]. The updates acknowledge that exceeding the recommended amounts may yield additional health benefits. Our analyses, and those by others [31], suggest that the benefits of more intense exercise go beyond providing flexibility in achieving the minimum exercise dose. When total volume is held constant, more vigorous exercise provides greater health benefits than moderate exercise [31], and more intense vigorous exercise is associated with greater health benefits than less-intense vigorous exercise (Table 2, Fig. 1 and Fig. 2).
The ACSM position statement on exercise and hypertension [24] endorses moderate-intensity physical activity. In part, this is because meta-analyses show no influence of exercise intensity on blood pressure lowering during training. Those meta-analyses considered different intensities from different clinical trials; however, when different intensities are compared within the same clinical trial, an intensity effect on resting blood pressure is suggested [23,31]. The inverse relationship we observed between running intensity and the prevalence of hypertension in both men and women runners (Figs. 1 and 2) may suggest a greater influence of exercise intensity than currently acknowledged by the position statement. This effect may be more evident in our data because the effect may be greater for different intensities of vigorous exercise than between moderate and vigorous exercise. Alternatively, self-selection on the basis of blood pressure or other factors that confound cross-sectional relationships but not training studies may account for the discrepancy.
Prior research on running intensities has focused primarily on the maximal efforts that determine 10-km or marathon performance times rather than the physiological and motivational determinants of self-selected usual running pace. In our study, the faster runners were younger, leaner, weighed less, and were smaller waisted (Table 1). Others have shown that increases in cardiorespiratory fitness are greater for more vigorous than for less vigorous exercise [32]. The correlations between usual running speed and 10-km performance in our men (r = 0.68) and women (r = 0.58) were strongly significant and were significant when adjusted for age (r = 0.61 and r = 0.56, respectively) or for age and BMI (r = 0.51 and r = 0.46, respectively). Greater cardiorespiratory fitness facilitates the ability to maintain a faster usual pace, and conversely, training harder improves cardiorespiratory fitness. However, our data suggest a significant association between disease prevalence and usual exercise intensity that is independent of maximum performance. For any given fitness, there is variation in the runners’ chosen training intensities, which appears to be associated with the prevalence of diabetes, hypertension, or hypercholesterolemia independent of the runner’s 10-km performance.
In addition to greater cardiorespiratory fitness facilitating the ability to run faster, improved substrate use may contribute to running speed. Although faster marathon speed is achieved by a combination of both fat and carbohydrate oxidation, the use of carbohydrate-derived energy increases with marathon running intensity [28]. Significant improvements in running performance do not necessarily require concomitant improvements in peak oxygen uptake [19]. Additional requirements for running a fast marathon include high cardiac output, transport of oxygen to exercising muscles, fractional V̇O2max use, running economy, and lactate response [11]. Exercise-induced hypoxemia, a condition characterized by reduced arterial oxygen content, significantly reduces exercise performance and maximum aerobic capacity in 50% of endurance athletes [17]. The ability to run faster may also be in part genetic. As of 2005, over 187 genetic variants have been associated with performance and health-related fitness phenotypes, including 17 mitochondrial sequence variants [25]. For example, the I/I genotype of the ACE I/D polymorphism is reported to be significantly more common in the fastest marathon runners compared to controls [2,14], although its significance at lower intensities is less clear.
The inverse relationship between usual running pace and these conditions remained significant when adjusted for 10-km performance (Table 4), suggesting its independent contribution to disease risk. More intense training may accentuate muscular changes beyond that produced by volume alone. For example, despite the 10-fold difference in exercise volume, comparable increases in skeletal muscle carbohydrate metabolism, lipid oxidation, and mitochondrial biogenesis have been reported for four to six 30-s sprints performed thrice weekly as for 200–300 min/wk of submaximal cycling [7]. Whether the principles involved apply in part to slow and fast running remains to be determined, given other differences between sprinting and endurance training.
Although some of the reduced medication usage may be attributable to lower adiposity of the faster runners (Table 2), the proportions are modest. The prevalence of hypertension, hypercholesterolemia, and diabetes all remained significantly related with usual running pace when adjusted for BMI, with very modest changes in the odds ratios. However, even among lean, vigorously active men and women who are ostensibly at a healthy weight and have desirable waist circumferences, those with greater BMI and waist circumference are at an increased risk for these conditions [39]. With the exception of diabetes in women, we were also unable to attribute the relationships between disease prevalence and running intensity to abdominal adiposity as measured by waist circumference, nor to the shorter stature or smaller body size of faster runners. These adjustments were pursued because abdominal adiposity increases metabolic risk [18] and is reduced by training [26] independent of BMI and because of the greater running economy in being smaller [27].
We have previously demonstrated that faster 10-km performance, a measure of cardiorespiratory fitness, is associated with decreased prevalence of hypertension, hypercholesterolemia, and diabetes cross-sectionally [38], and their decreased incidence prospectively [37], independent of total kilometers-per-week run. The current results suggest that the lower prevalence for hypertension, hypercholesterolemia, and diabetes is related to at least three characteristics of physically active individuals: their exercise dose, exercise intensity, and cardiorespiratory fitness. Tables 2–4 show that the odds reductions for each characteristic are diminished by adjustment for the other characteristics, and yet all three retain their significance, suggesting that exercise dose, intensity, and cardiorespiratory fitness may affect these conditions through both common and independent mechanisms.
CAVEATS AND LIMITATIONS
There are important limitations to these analyses. As with all cross-sectional associations, it is not possible to distinguish the causal direction of the relationship; that is, whether running intensity reduces the need for medications, or contrariwise, whether medications reduce usual running speed. Underlying hypertension, hypercholesterolemia, diabetes, or atherosclerosis might also affect exercise intensity. The analyses included 357 runners (0.65%) who reported having had a heart attack before their baseline survey; however, excluding these subjects from the analyses did not affect the results (analyses not displayed).
Self-reported antihypertension, LDL-cholesterol–lowering, and antidiabetic medication use and usual running speed have not been validated in this sample of runners. The proportion of patients with type 1 diabetes, whose medication use would not be affected by running intensity, is not known. The runners’ best 10-km performance time during the previous 5 yr may not reflect the participants’ cardiorespiratory fitness at the time of their baseline survey. We also note that our findings rely on the adequacy of the statistical procedures to correct for age, adiposity, and running distance because all three factors related to the prevalence of hypertension, diabetes, and hypercholesterolemia [38,39]. Elsewhere, we have shown that the relationships of running distance and BMI with each other and with other variables differ by the percentile of the dependent variable [34], which violates the principles of statistical adjustment. In addition, we caution that adjustment for BMI may not adequately correct for adiposity differences between faster and slower runners given that for any given BMI, men with high cardiorespiratory fitness have less total, visceral, and subcutaneous abdominal adipose tissue than those of low cardiorespiratory fitness [40]. Although the findings for BMI adjustment were corroborated by adjustment for waist circumference, imprecision in self-reported waist circumference, and its correlation with fat mass suggest that adjustment for waist circumference may not eliminate the effects of adiposity [40]. We do not believe that the declining incidence of hypertension, hypercholesterolemia, and diabetes with running speed is because of the avoidance of opportunities for diagnosis in the more athletic men and women. The Health Professionals Study reported that their more vigorously active participants had more routine medical checkups than less active men [20], and there was no difference in routine medical check-up by activity level in the Nurses’ Health Study [21].
Finally, the analyses were restricted to runners, and it is not known whether other vigorous activities performed at greater intensities lead to greater health benefits. However, restricting our analyses exclusively to running may provide a cleaner analysis than provided by earlier epidemiological studies that report the sum calorie expenditure over a diverse set of physical activities. In summary, the results are consistent with the hypothesis that physiological and metabolic characteristics associated with preferred running speed may confer important health benefits beyond those associated with usual distance or cardiorespiratory fitness as assessed by 10-km performance. The odds for medication use remained significantly related to the volume of vigorous exercise when adjusted for usual running speed (Table 3) and significantly related to the intensity of vigorous exercise when adjusted for volume (Table 2). If these findings are causal and apply more generally to vigorous physical activities in the population at large, then they suggest that the more vigorous the vigorous exercise, the healthier the health benefits.
Acknowledgments
This study was supported in part by grants HL45652, HL072110, and DK066738 from the National Heart Lung and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Amir O, Amir R, Yamin C, et al. The ACE deletion allele is associated with Israeli elite endurance athletes. Exp Physiol. 2007;92:881–6. doi: 10.1113/expphysiol.2007.038711. [DOI] [PubMed] [Google Scholar]
- 3.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–88. [PubMed] [Google Scholar]
- 4.Bickham DC, Gibbons C, Le Rossignol PF. V̇O2 is attenuated above the lactate threshold in endurance-trained runners. Med Sci Sports Exerc. 2004;36(2):297–301. doi: 10.1249/01.MSS.0000113667.64064.36. [DOI] [PubMed] [Google Scholar]
- 5.Blair SN, Kohl HW, 3rd, Pattenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for V̇O2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30(2):252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Burgomaster KA, Howarth KR, Phillips SM, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–60. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 9.Fagard R, Bielen RE, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise. J Appl Physiol. 1991;70:357–62. doi: 10.1152/jappl.1991.70.1.357. [DOI] [PubMed] [Google Scholar]
- 10.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol. 1975;228:1029–33. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- 11.Foster C, Lucia A. Running economy: the forgotten factor in elite performance. Sports Med. 2007;37:316–9. doi: 10.2165/00007256-200737040-00011. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 13.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–94. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 14.Hruskovicová H, Dzurenková D, Selingerová M, Bohus B, Timkanicová B, Kovács L. The angiotensin converting enzyme I/D polymorphism in long distance runners. J Sports Med Phys Fitness. 2006;46:509–13. [PubMed] [Google Scholar]
- 15.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Int Med. 2001;161:1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women. JAMA. 1999;282:1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 17.Judelson DA, Rundell KW, Beck KC, King TM, Laclair KL. Effect of high-intensity submaximal work, with or without rest, on subsequent V̇O2max. Med Sci Sports Exerc. 2004;36(2):292–6. doi: 10.1249/01.MSS.0000113480.21438.A8. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Cupples LA, Ramaswami R, Stokes J, III, Kreger E, Higgins M. Regional obesity and risk of cardiovascular disease: the Framingham Study. J Clin Epidemiol. 1991;44:183–90. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 19.Krahenbuhl GS, Morgan DW, Pangrazi RP. Longitudinal changes in distance-running performance of young males. Int J Sports Med. 1989;10:92–6. doi: 10.1055/s-2007-1024881. [DOI] [PubMed] [Google Scholar]
- 20.Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, Wing AL. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–8. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto K, Gen-no H, Masuki S, Okazaki K, Nose H. Effects of high-intensity interval walking training on physical fitness and blood pressure in middle-aged and older people. Mayo Clin Proc. 2007;82:803–11. doi: 10.4065/82.7.803. [DOI] [PubMed] [Google Scholar]
- 24.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. Position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 25.Rankinen T, Bray MS, Hagberg JM, et al. The human gene map for performance and health-related fitness phenotypes: the 2005 update. Med Sci Sports Exerc. 2006;38(11):1863–88. doi: 10.1249/01.mss.0000233789.01164.4f. [DOI] [PubMed] [Google Scholar]
- 26.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 27.Scott RA, Pitsiladis YP. Genotypes and distance running, clues from Africa. Sports Med. 2007;37:424–7. doi: 10.2165/00007256-200737040-00039. [DOI] [PubMed] [Google Scholar]
- 28.Spriet LL. Regulation of substrate use during the marathon. Sports Med. 2007;37:332–6. doi: 10.2165/00007256-200737040-00015. [DOI] [PubMed] [Google Scholar]
- 29.Sundet JM, Magnus P, Tambs K. The heritability of maximal aerobic power: a study of Norwegian twins. Scand J Med Sci Sports. 1994;4:181–5. [Google Scholar]
- 30.Swain DP. Moderate or vigorous intensity exercise: which is better for improving aerobic fitness? Prev Cardiol. 2005;8:55–8. doi: 10.1111/j.1520-037x.2005.02791.x. [DOI] [PubMed] [Google Scholar]
- 31.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–7. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 32.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3:346–56. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 33.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch Intern Med. 1998;158:237–45. doi: 10.1001/archinte.158.3.237. [DOI] [PubMed] [Google Scholar]
- 34.Williams PT. Exercise and the population distribution of body weight. Int J Obes. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 35.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obesity (Silver Spring) 2008;16:102–6. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 36.Williams PT. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med Sci Sports Exerc. 2008;40(3):433–43. doi: 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PT. Vigorous exercise, fitness, and incident hypertension, high cholesterol, and diabetes. Med Sci Sports Exerc. 2008;40(6):998–1006. doi: 10.1249/MSS.0b013e31816722a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT, Franklin B. Vigorous exercise and diabetic, hypertensive, and hypercholesterolemia medication use. Med Sci Sports Exerc. 2007;39(11):1933–41. doi: 10.1249/mss.0b013e318145b337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT, Hoffman K, La I. Weight-related increases in hypertension, hypercholesterolemia, and diabetes risk in normal weight male and female runners. Arterioscler Thromb Vasc Biol. 2007;27:1811–9. doi: 10.1161/ATVBAHA.107.141853. [DOI] [PubMed] [Google Scholar]
- 40.Wong SL, Katzmarzyk P, Nichaman MZ, Church TS, Blair SN, Ross R. Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc. 2004;36(2):286–91. doi: 10.1249/01.MSS.0000113665.40775.35. [DOI] [PubMed] [Google Scholar]