Abstract
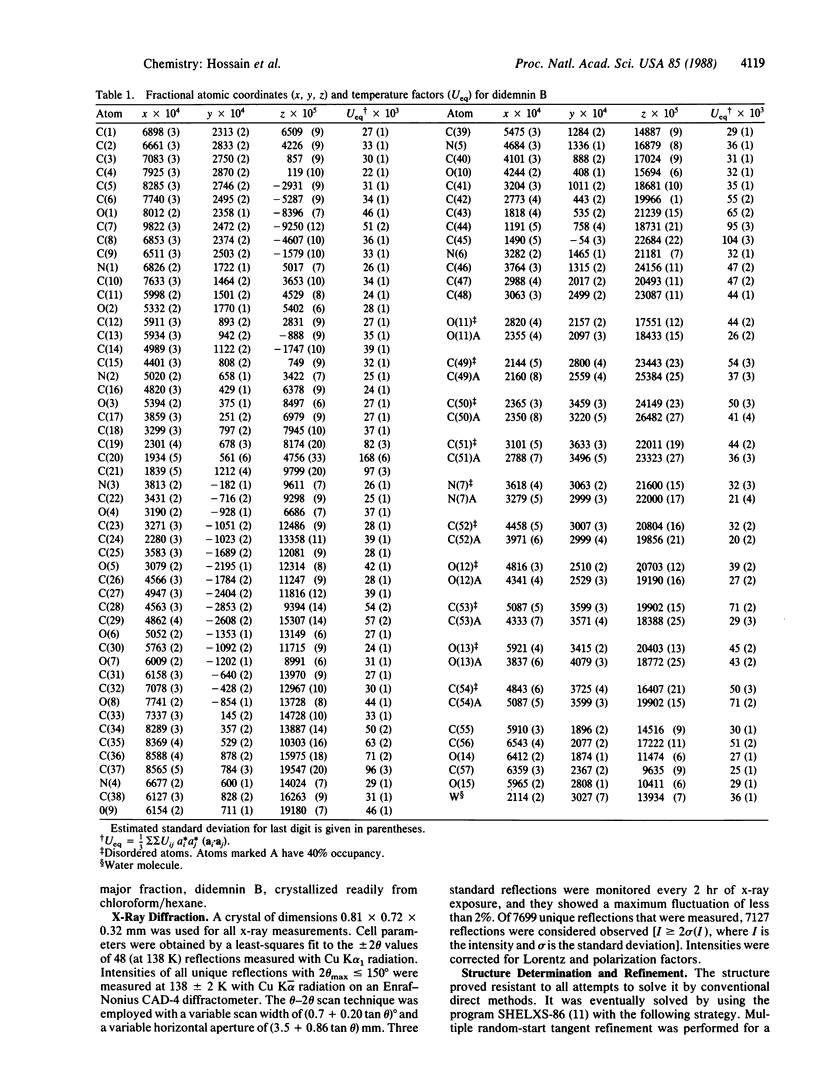
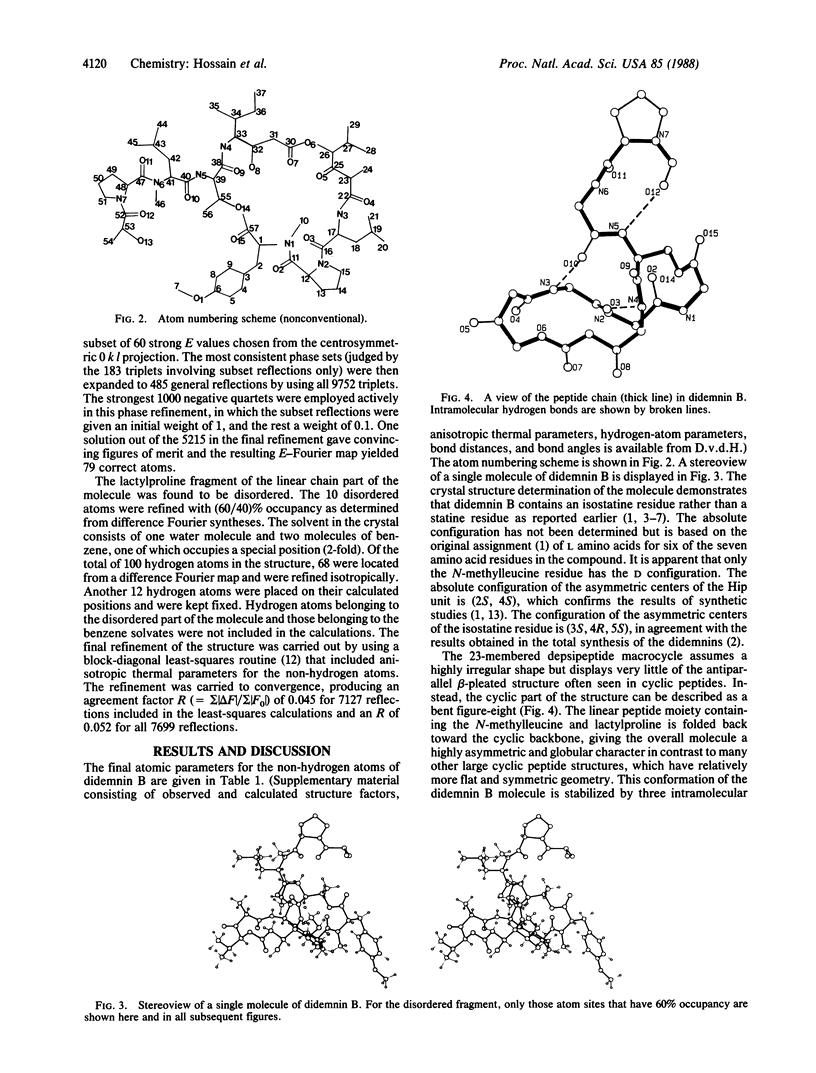
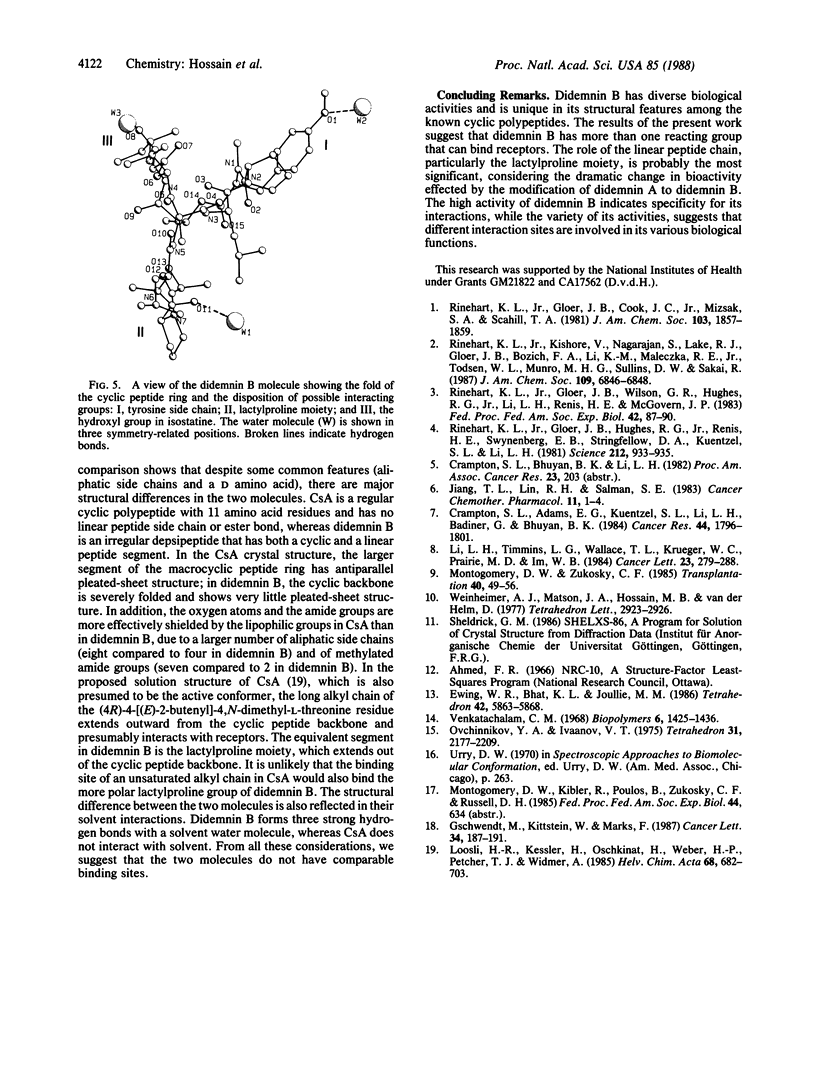
Didemnin B, a highly active depsipeptide isolated from a Caribbean tunicate, crystallizes from chloroform/benzene in the orthorhombic space group C2221, with cell parameters a = 14.990 +/- 0.003 A, b = 22.574 +/- 0.004 A, c = 41.112 +/- 0.009 A, V = 13911.7 A3 at 138 K and a calculated density of 1.143 g/cm3 based on C57H89N7O15, 1.5C6H6.H2O and eight formula units per cell. The overall agreement factor R = 0.052 for 7699 reflections, 20 theta max = 150 degrees, Cu K-alpha radiation. The structure determination revealed that didemnin B contains an isostatine residue instead of a statine residue. The conformation of the 23-membered depsipeptide ring is stabilized by one transannular hydrogen bond. The ring does not show the antiparallel beta-pleated-sheet structure but, instead, has a fold in the shape of a bent figure-eight. The linear peptide moiety, containing N-methylleucine and lactylproline, forms a beta (II)-bend and is folded back toward the cyclic backbone, giving the overall molecule a globular character. Comparison with the structure of cyclosporin A shows distinct stereochemical differences between the two molecules. It is suggested that didemnin B and cyclosporin A are unlikely to have a common receptor binding site.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crampton S. L., Adams E. G., Kuentzel S. L., Li L. H., Badiner G., Bhuyan B. K. Biochemical and cellular effects of didemnins A and B. Cancer Res. 1984 May;44(5):1796–1801. [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W., Marks F. Didemnin B inhibits biological effects of tumor promoting phorbol esters on mouse skin, as well as phosphorylation of a 100 kD protein in mouse epidermis cytosol. Cancer Lett. 1987 Feb;34(2):187–191. doi: 10.1016/0304-3835(87)90009-7. [DOI] [PubMed] [Google Scholar]
- Jiang T. L., Liu R. H., Salmon S. E. Antitumor activity of didemnin B in the human tumor stem cell assay. Cancer Chemother Pharmacol. 1983;11(1):1–4. doi: 10.1007/BF00257406. [DOI] [PubMed] [Google Scholar]
- Li L. H., Timmins L. G., Wallace T. L., Krueger W. C., Prairie M. D., Im W. B. Mechanism of action of didemnin B, a depsipeptide from the sea. Cancer Lett. 1984 Jul;23(3):279–288. doi: 10.1016/0304-3835(84)90095-8. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F. Didemnin B: a new immunosuppressive cyclic peptide with potent activity in vitro and in vivo. Transplantation. 1985 Jul;40(1):49–56. [PubMed] [Google Scholar]
- Rinehart K. L., Jr, Gloer J. B., Hughes R. G., Jr, Renis H. E., McGovren J. P., Swynenberg E. B., Stringfellow D. A., Kuentzel S. L., Li L. H. Didemnins: antiviral and antitumor depsipeptides from a caribbean tunicate. Science. 1981 May 22;212(4497):933–935. doi: 10.1126/science.7233187. [DOI] [PubMed] [Google Scholar]
- Rinehart K. L., Jr, Gloer J. B., Wilson G. R., Hughes R. G., Jr, Li L. H., Renis H. E., McGovren J. P. Antiviral and antitumor compounds from tunicates. Fed Proc. 1983 Jan;42(1):87–90. [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]



