SUMMARY
Glutamate receptors play major roles in excitatory transmission in the vertebrate brain. Among ionotropic glutamate receptors (AMPA, kainate, NMDA), AMPA receptors mediate fast synaptic transmission and require TARP auxiliary subunits. NMDA receptors and kainate receptors play roles in synaptic transmission, but it remains uncertain whether these ionotropic glutamate receptors also have essential subunits. Using a proteomic screen, we have identified NETO2, a brain-specific protein of unknown function, as an interactor with kainate-type glutamate receptors. NETO2 modulates the channel properties of recombinant and native kainate receptors without affecting trafficking of the receptors and also modulates kainate-receptor-mediated mEPSCs. Furthermore, we found that kainate receptors regulate the surface expression of NETO2 and that NETO2 protein levels and surface expression are decreased in mice lacking the kainate receptor GluR6. The results show that NETO2 is a kainate receptor subunit with significant effects on glutamate signaling mechanisms in brain.
INTRODUCTION
Excitatory synaptic transmission in brain is primarily mediated by the neurotransmitter glutamate. Glutamate released from presynaptic terminals binds to three classes of ionotropic glutamate receptors, which are pharmacologically classified as AMPA-(amino-3-hydroxy-5-methylisoxazole-4-propionic acid), NMDA-(N-methyl-D-aspartic acid), and kainate-sensitive glutamate receptors (Dingledine et al., 1999; Hollmann and Heinemann, 1994; Seeburg, 1993). AMPA receptors mediate fast synaptic transmission, whereas NMDA receptors are involved in synaptic plasticity. Kainate receptors play multiple roles in synaptic transmission. Postsynaptic kainate receptors mediate slow EPSCs (Castillo et al., 1997; Kidd and Isaac, 1999; Vignes and Collingridge, 1997), and presynaptic kainate receptors modulate the release of the excitatory and inhibitory neurotransmitters glutamate and GABA (Chittajallu et al., 1996; Clarke et al., 1997; Kamiya and Ozawa, 1998; Rodriguez-Moreno et al., 1997). Furthermore, presynaptic kainate receptors are involved in long-term potentiation at mossy fiber-CA3 pyramidal cells in hippocampus (Bortolotto et al., 1999; Contractor et al., 2001; Schmitz et al., 2001).
Although mice in which kainate receptor expression is genetically disrupted show no kainate receptor activity, overexpression of kainate receptors does not enhance kainate-receptor-mediated excitatory postsynaptic current (EPSC), suggesting that native kainate receptors may contain additional modulatory proteins. For example, faithful reconstitution of native AMPA receptor properties in heterologous cells requires coexpression of transmembrane AMPA receptor auxiliary subunits (TARPs) (Nicoll et al., 2006). Several cytoplasmic proteins (SAP90/PSD95, the cadherin/catenin complex, KRIP6, Actinfillin, PICK1, Syntenin, GRIP) have been identified as kainate receptor interactors (Coussen et al., 2002; Garcia et al., 1998; Hirbec et al., 2003; Laezza et al., 2007; Salinas et al., 2006). The primary effect of these proteins is to modulate receptor localization. Garcia et al. (1998) showed with whole-cell recording that SAP90/PSD95 causes incomplete receptor desensitization in heterologous cells. However, subsequent studies at greater time resolutions using outside-out patch membranes showed that SAP90/PSD95 does not modulate the rate at which receptors desensitize, but rather accelerates recovery from desensitization (Bowie et al., 2003). Recently, KRIP6 was also shown to modulate receptor kinetics. KRIP6 enhanced the ratio of steady-state to peak currents, but did not significantly alter decay kinetics in heterologous cells (Laezza et al., 2007). Importantly, enhancement of kainate-receptor-mediated EPSCs by overexpression of these interactors has not yet been shown.
In this study, we identified a brain-specific transmembrane protein of unknown function, NETO2, using a proteomic screen. NETO2 slows the decay kinetics of kainate receptors in heterologous cells without affecting receptor expression at the cell surface. Single-channel analysis showed that NETO2 also increases the open probability (Popen) of kainate-receptor channels, resulting in significantly larger peak glutamate-evoked currents. NETO2 modulated the agonist sensitivity of kainate receptors in heterologous cells and neurons. Importantly, NETO2 slowed the decay of kainate-receptor-mediated EPSCs, demonstrating that it directly influences synaptic transmission. The total amount of NETO2 is decreased in mice lacking the kainate receptor subunit GluR6, and kainate receptors increase the surface expression of NETO2 in both heterologous cells and neurons. The results indicate that NETO2 is an accessory subunit of neuronal kainate receptors that has important effects on receptor function.
RESULTS
Identification of NETO2 as a Kainate Receptor Binding Protein
The kainate receptor subunit GluR6 plays major roles in kainate receptor function (Mulle et al., 1998) and is highly expressed in cerebellum (Bahn et al., 1994; Smith et al., 1999). To identify proteins that interact with kainate receptors, we used rat cerebella for coimmunoprecipitation experiments with anti GluR6/7 antibody, followed by silver staining. Mass spectrometry analysis of bands immunoprecipitated with anti GluR6/7 antibody identified a rat ortholog of NETO2/Btcl2 (99% shared identity of amino acids), a brain-specific mouse protein of unknown function (Michishita et al., 2004; Stohr et al., 2002), as well as AKAP8, the kainate receptor subunits GluR6 and GluR7, and a major contaminant, keratin (Figure 1A and Table S1 available online). NETO2 and GluR6/7 were also coimmunoprecipitated with an anti GluR5 antibody that also recognizes GluR6 directly (Figure S1A available online), whereas AKAP8 was not detected (Figure S1B), suggesting that the anti GluR6/7 antibody may directly recognize AKAP8. To test this possibility, we used Cos-7 cells transfected with FLAG-AKAP8 for immunoprecipitation experiments with anti GluR6/7 antibody. We found that AKAP8 was weakly immunoprecipitated with anti GluR6/7 antibody in the absence of GluR6/7 expression (Figure S1C). We therefore concluded that NETO2, but not AKAP8, is a kainate receptor interactor.
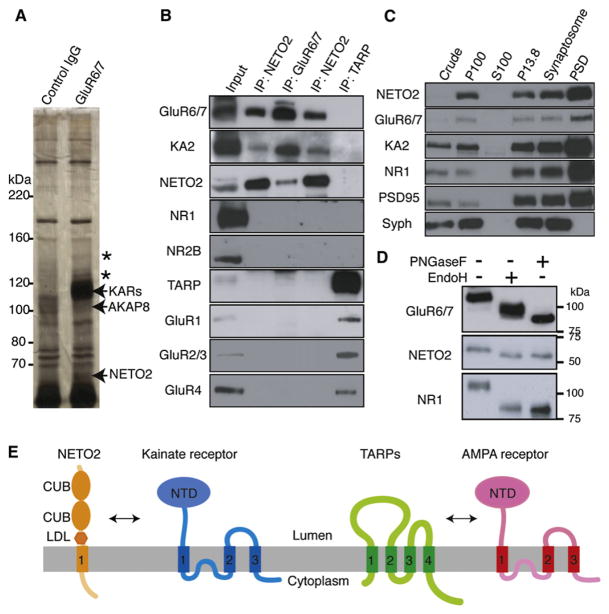
Figure 1. Native Kainate Receptor Complexes in Brain Contain NETO2.
(A) Immunoprecipitation of GluR6/7 from rat cerebella membranes showed five specific bands (asterisks and arrows). Mass spectrometry demonstrated that these bands contained NETO2, AKAP8, and the kainate receptor subunits GluR6 and GluR7 (KARs). No protein was identified from two bands (asterisks: 130, 150 kDa). Keratin was identified from all five bands.
(B) Coimmunoprecipitation from rat cerebella membranes showed that NETO2 interacts with kainate, but not NMDA and AMPA, receptors.
(C) NETO2, GluR6/7, and KA2 are cofractionated and highly enriched in the postsynaptic density (PSD) fraction together with NMDA receptor subunit NR1 and PSD95, whereas synaptophysin (Syph) is not.
(D) In rat cerebellum, NETO2 is sensitive to both PNGaseF and EndoH (similar to NR1).
(E) Domain organization of kainate receptor with NETO2 and AMPA receptor with transmembrane AMPA receptor regulatory proteins (TARPs).
To determine if NETO2 interacted specifically with kainate receptors, we generated antibodies against the cytoplasmic domain of NETO2. NETO2 is a 525 amino acid protein that contains a signal peptide, and the expected molecular weight of NETO2 protein after cleavage of the signal peptide is 56 kDa. Anti NETO2 antibody recognized 58–60 kDa bands in NETO2 transfected CHO cells, but not untransfected cells, and similar bands were recognized in lysates from rat brain and mouse cerebellar granule cell cultures (Figure S2). Thus, we concluded that the anti NETO2 antibody recognized NETO2 in brain and transfected cells. We used anti NETO2 antibody for coimmunoprecipitation experiments on rat cerebellar lysates. We found that NETO2 interacts specifically with kainate receptors, but not with AMPA and NMDA receptors (Figure 1B). In contrast, TARPs interact specifically with AMPA receptors, but not with kainate and NMDA receptors (Figure 1B). NETO2 codistributed with the kainate receptor subunits GluR6/7 and KA2, and was highly enriched in the postsynaptic density (PSD) fraction, along with PSD95 and the NMDA receptor subunit NR1 (Figure 1C).
Sequence analysis indicates that NETO2 contains one transmembrane domain, as well as two CUB domains and one LDLa domain that are extracellular. Like the NMDA receptor subunit NR1, NETO2 contains EndoH sensitive sugars (2–3 kDa), consistent with the idea that NETO2 is a transmembrane protein (Figure 1D). The predicted structure of NETO2 is substantially different from that of the TARP family of AMPA receptor auxiliary subunits (Figure 1E). Here NETO2 is identified as a CUB-domain-containing protein that binds to ion channels in the vertebrate. In Caenorhabditis elegans, CUB-domain-containing proteins LEV-10 and SOL-1 were identified as modulators of acetylcholine receptors and GLR-1 AMPA receptors (Gally et al., 2004; Zheng et al., 2004, 2006). Interestingly, the domain structure of NETO2 is more similar to the domain structure of invertebrate proteins that modulate acetylcholine receptors (LEV-10) than SOL-1, which modulates GLR-1 AMPA receptors (Figure S3). In addition, NETO2 is similar to the hypothetical proteins Q9XUU2 in C. elegans and Q9VYC7 in Drosophila melanogaster (Figure S3 and Discussion).
NETO2 Modulates the Functional Properties, but Not Surface Trafficking, of Kainate Receptors
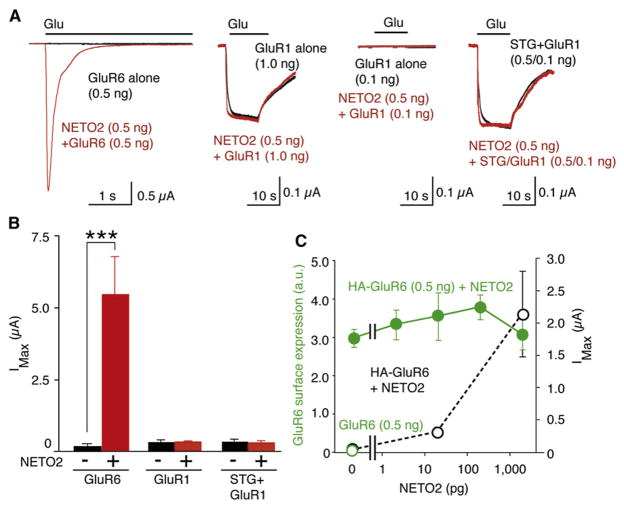
To begin to explore the functional role of NETO2 interactions with kainate receptors, we coexpressed these proteins in Xenopus laevis oocytes, in which protein expression could be more tightly regulated by complementary RNA (cRNA) injection (in comparison with transfection of cDNAs in mammalian cells). The detection of glutamate-evoked currents in oocytes injected with 0.5 ng GluR6 cRNA by two-electrode voltage-clamp recording (TEVC) required reduction of desensitization with concanavalin A (Wong and Mayer, 1993). In contrast, large glutamate-evoked currents were routinely recorded without concanavalin A treatment in oocytes coinjected with 0.5 ng NETO2 cRNA and 0.5 ng GluR6 cRNA, whereas coexpression of NETO2 had no effect on the activity of AMPA receptors or stargazin-like TARP/AMPA receptor complexes expressed at the minimum levels to detect further enhancement (Figures 2A, 2B, and S4). To determine whether the NETO2-associated increase in GluR6 currents was due to an increase in receptor expression at the cell surface, we measured in parallel glutamate-evoked currents by TEVC and the surface expression of kainate receptors by chemiluminescence. Oocytes were injected with extracellular HA-epitope-tagged GluR6 at nonsaturating amounts (0.5 ng) of HA-GluR6 cRNA (Figure S5) with varying amounts of NETO2 cRNA (Tomita et al., 2005). We found that NETO2 enhanced maximal glutamate-evoked currents in a dose-dependent manner, but had no effect on surface expression (Figure 2C and the raw data in Figure S6), suggesting that NETO2 enhances GluR6 currents by modulating the functional properties of the receptors, perhaps by slowing desensitization.
Figure 2. NETO2 Modulates Channel Properties, but Not Surface Trafficking, of Kainate Receptors.
Oocytes were injected with cRNAs encoding GluR6 (0.5 ng), GluR1 (1 ng, 0.1 ng) or GluR1 (0.1 ng) and stargazin (STG; 0.5 ng) with or without NETO2 (0.5 ng), and responses to 10 mM glutamate were measured with TEVC. (A and B) NETO2 enhances maximal glutamate-evoked currents (IMax) for GluR6, but not GluR1, channels (n = 8–10) (B). (C) Addition of NETO2 cRNA enhances currents in oocytes coinjected with HA-GluR6 cRNA (0.5 ng), but not surface expression of HA-GluR6 (0.5 ng). Data are shown as mean ± SEM. n = 10–11. ***p < 0.005.
NETO2 Modulates Decay Kinetics and Peak Open Probability of Kainate Receptors
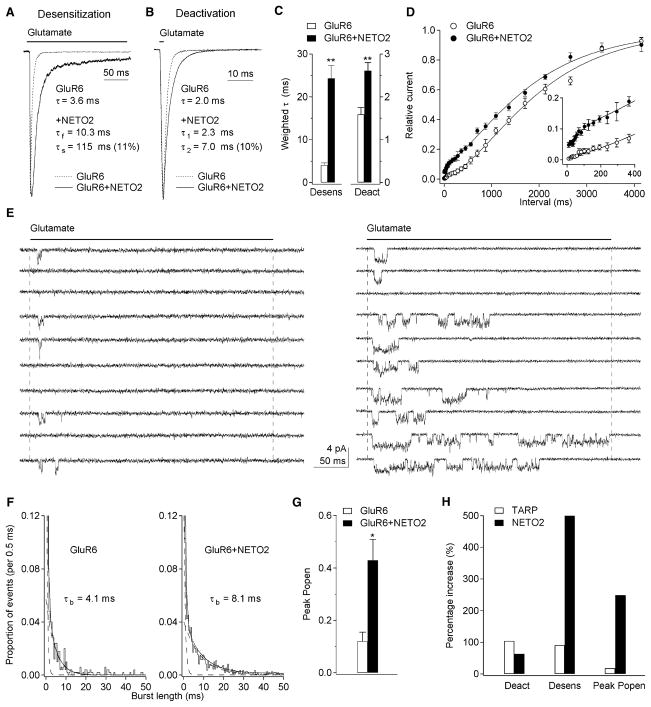
To examine NETO2 modulation of receptor properties at a better time resolution, we expressed NETO2 and GluR6 in tsA201 cells and applied glutamate to outside-out patches from the transfected cells with a fast piezoelectric system. Coexpression of GluR6 and NETO2 at a cDNA ratio of 1:10 resulted in significant slowing of both desensitization and deactivation (Figures 3A–3C). Relative to the corresponding values for GluR6 alone, the NETO2-associated increase in mean weighted tau values was 500% for desensitization and 64% for deactivation. The results obtained with cDNA ratios of 1:10 and 1:30 were similar, indicating that most GluR6 receptors in the patches studied contained NETO2. In addition, NETO2 did not modulate desensitization of GluR1 AMPA receptors (time constant of desensitization: GluR1 alone, 2.4 ± 0.2 ms, n = 5; GluR1 + NETO2, 2.2 ± 0.5 ms, n = 4). Two-pulse protocols showed that NETO2 also causes GluR6 receptors to recover faster from desensitization, especially at short interpulse intervals (Figure 3D). Together, the slower entry into desensitization and faster recovery seen with NETO2 coexpression resulted in a 9-fold enhancement of steady-state currents (0.11 ± 0.02% versus 1.01% ± 0.24% of the peak current, n = 6 and 9, respectively). Notably, the difference in kinetics suggests that there is no endogenous NETO2 in tsA201 cells, because human and rat NETO2 should have similar effects on receptor kinetics given the high degree of identity (97%) the two proteins share.
Figure 3. NETO2 Modulates Kainate Receptor Kinetics and Peak Open Probability.
(A and B) Responses to 10 mM glutamate in outside-out patches from tsA201 cells transfected with GluR6 alone or GluR6 and NETO2. Glutamate was applied for 200 ms (A) or 1 ms (B). The time constants (relative amplitude) are from monoexponential or biexponential fits to the decays of the currents.
(C) The fits were used to calculate weighted time constants of desensitization and deactivation. NETO2 coexpression slowed both desensitization and deactivation significantly (n = 6–9).
(D) Recovery from desensitization for GluR6 alone (n = 5) and GluR6 and NETO2 (n = 6). NETO2 markedly sped recovery at short intervals, but did not alter the slow component of recovery.
(E) Examples of single-channel currents evoked by 10 mM glutamate in patches containing four GluR6 (left) or three GluR6+NETO2 channels (right). NETO2 increased the probability of channel opening and the duration of channel activity.
(F) Burst-length distributions from the patches in (E). The similar fast component present in all such distributions is off-scale. NETO2 coexpression produced approximately a 2-fold increase in the time constant of the slow component.
(G) Mean values for peak Popen obtained from patches containing GluR6 and GluR6+NETO2 channels (n = 6).
(H) Mean percentage increases in the weighted time constants of deactivation and desensitization, as well as peak Popen, produced by stargazin coexpression with GluR1 (9) and NETO2 coexpression with GluR6. Data are given as mean ± SEM. *p < 0.05, **p < 0.01.
To determine the effect of NETO2 on unitary receptor properties, we made concentration jumps on patches containing only a few channels (Figure 3E). As reported before (Swanson et al., 1996), GluR6-Q channels displayed three open levels with conductances of approximately 7, 17, and 26 pS. NETO2 coexpression had no effect on unitary conductance and did not alter the relative frequency or the mean duration of openings to each conductance level (Table 1). However, the duration of bursts of openings (tcrit = 4 ms) was clearly longer with NETO2 (Figures 3E and 3F) and the mean duration of these bursts was increased significantly (Table 1).
Table 1.
Effect of NETO2 on the Unitary Properties of GluR6-Q Channels
| GluR6-Q | GluR6-Q + NETO2 | ||
|---|---|---|---|
| Conductance (pS) | O1 | 7.4 ± 0.3 (24.7%) | 7.4 ± 0.2 (18.2%) |
| O2 | 16.9 ± 0.4 (43.2%) | 16.4 ± 0.5 (51.3%) | |
| O3 | 27.0 ± 0.8 (32.1%) | 25.5 ± 0.6 (30.5%) | |
| Open time (ms) | O1 | 0.87 ± 0.13 | 0.70 ± 0.05 |
| O2 | 0.60 ± 0.09 | 0.80 ± 0.07 | |
| O3 | 0.81 ± 0.14 | 0.89 ± 0.07 | |
| Burst length (ms) | 0.64 ± 0.08 | 0.66 ± 0.10 | |
| 4.60 ± 0.38 | 7.45 ± 0.82* |
Mean ± SEM values from four or five patches are listed. Conductance levels and open times for each level were estimated with QuB software. Bursts were defined as a series of openings (to any level) that were separated by shuttings briefer than 4 ms. The distributions of burst durations were fitted with two exponential components. The fast component was similar in all distributions examined.
p < 0.05.
It was evident from inspection of the records that NETO2 coexpression dramatically increased channel activity. In patches in which the maximum number of receptors that open simultaneously was estimated to be three or four, the number of jumps that produced no detectable openings (“failures”) was much higher in the absence of NETO2 coexpression (Figure 3E). Our measurements of the relative frequency of openings to each conductance level with and without NETO2 (Table 1) were used to calculate the mean single-channel current, which together with the number of active receptors in the patch allowed us to estimate the Popen at the peak of the ensemble current (peak Popen). NETO2 coexpression increased peak Popen significantly (Figure 3G). In total, the results show that the inclusion of NETO2 in kainate receptor assemblies modulates both the amplitude and kinetics of ensemble currents evoked by rapid pulses of glutamate, leading to a marked increase in charge transfer. Our results suggest that the mechanism underlying NETO2-mediated kainate receptor modulation differs from TARP modulation of AMPA receptors. TARPs slow both the deactivation and desensitization of AMPA receptors to similar extents, suggesting that they decrease the activation energy for channel opening, whereas NETO2 has larger effects on desensitization and may primarily alter the rate constants governing transits in and out of desensitized states (Figure 3H).
NETO2 Modulates Kainate-Receptor-Mediated mEPSCs
We next examined whether NETO2 modulates the properties of the synaptic receptors that mediate kainate-receptor miniature EPSCs (mEPSCs). We used cerebellar granule cells for these studies because their small size allowed us to measure EPSC kinetics accurately. To minimize the contribution of AMPA receptors, we used neurons from stargazer mice, which lack AMPA-receptor-mediated EPSCs (Chen et al., 2000; Hashimoto et al., 1999). As published previously (Chen et al., 2000; Cho et al., 2007; Milstein et al., 2007), we observed AMPA-receptor-mediated mEPSCs in cerebellar granule cells from wild-type mice, but not in cells from stargazer mice (data not shown). NMDA receptors, GABA receptors, and voltage-gated sodium channels were blocked by the inclusion of AP-5, picrotoxin, and tetrodotoxin (respectively) in the external solution.
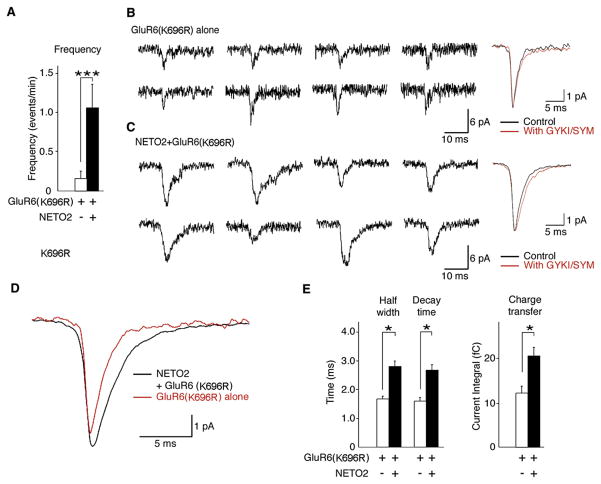
We did not detect mEPSCs in stargazer granule cells, even after transfecting the neurons with GluR6, although we routinely detected AMPA receptor mEPSCs in neurons in parallel cultures transfected with stargazin. We hypothesized that the low peak Popen of GluR6 channels limited our detection of kainate receptors at synapses. We therefore transfected granule cells with a GluR6 mutant in which substitution of lysine for arginine at position 696 reduces receptor desensitization (Priel et al., 2006). In oocytes injected with cRNAs, NETO2 enhanced glutamate-evoked currents of GluR6 (K696R) receptors without altering surface expression of the receptors (Figure S7). We did observe mEPSCs in some neurons transfected with GluR6 (K696R) alone, although the frequency of the events was very low (Figure 4A). In contrast, mEPSCs were observed in most neurons cotransfected with NETO2 and GluR6 (K696R), and the frequency of these events was significantly greater (Figure 4A). Examples of representative mEPSCs are shown in Figures 4B and 4C. The mEPSCs detected in each type of transfected neuron were mediated by kainate receptors, as confirmed by their resistance to block by selective AMPA receptor antagonists (50 μM GYKI 53655 and 100 μM SYM 2206) (Figures 4B and 4C). The decay kinetics of the kainate-receptor-mediated mEPSCs with and without NETO2 coexpression were different and were slower in neurons cotransfected with NETO2 (Figure 4D), and NETO2 significantly increased the half-width of individual mEPSCs, as well as the decay time and charge transfer of ensemble averages from individual neurons (Figure 4E). Additionally, in cerebellar granule cells from stargazer mice, NETO2 slowed the decay of spontaneous kainate-receptor EPSCs in neurons transfected with GluR5. The time constants obtained from fitting average EPSCs were 0.87 ± 0.10 ms for neurons transfected with GluR5 alone and 2.6 ± 0.36 ms for neurons cotransfected with GluR5 and NETO2 (p < 0.05). The results indicate that NETO2 can modulate kainate-receptor-mediated synaptic transmission.
Figure 4. NETO2 Modulates Kainate-Receptor-Mediated mEPSCs.
mEPSCs were recorded from stargazer cerebellar granule cells transfected with GluR6 (K696R) alone (69 events from 14 neurons) or GluR6 (K696R) and NETO2 (233 events from 17 neurons).
(A) NETO2 coexpression significantly increased the frequency of kainate receptor mEPSCs. ***p = 0.01.
(B and C) Representative mEPSCs (eight traces on left) and ensemble averages (right) from neurons transfected with GluR6 (K696R) alone (B) or with GluR6 (K696R) and NETO2 (C). Addition of the AMPA receptor antagonists GYKI 53655 (50 μM) and SYM 2206 (100 μM) did not alter mEPSC kinetics.
(D) Ensemble mEPSCs from neurons transfected with GluR6 (K696R) with or without NETO2 in the presence of the AMPA receptor antagonists GYKI 53655 (50 μM) and SYM 2206 (100 μM). NETO2 slows the decay kinetics of mEPSCs.
(E) Kinetic parameters (half-width, decay time, and charge transfer) calculated from mEPSCs from neurons transfected with GluR6 (K696R) alone or with GluR6 (K696R) and NETO2.
Data are given as mean ± SEM. *p < 0.05.
NETO2 Modulates the Agonist Sensitivity of Kainate Receptors
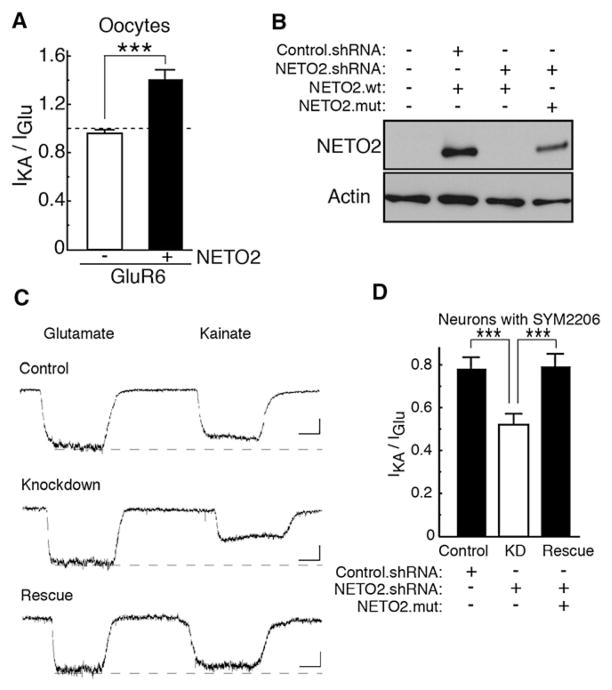
It is well known that kainate-receptor-mediated EPSCs are less than 10% of the amplitude of AMPA-receptor-mediated EPSCs (Castillo et al., 1997; Kidd and Isaac, 1999; Vignes and Collingridge, 1997), and in cerebellar granule cells whole-cell kainate receptor currents are on average smaller than 20 pA even when desensitization is reduced with concanavalin A (Pemberton et al., 1998; Smith et al., 1999). Because we were unable to routinely detect native kainate receptor mEPSCs in granule cells (or hippocampal neurons), we used a pharmacological approach to determine whether endogenous kainate receptors contain NETO2. For AMPA receptors, modulation of receptor kinetics by TARP auxiliary subunits is associated with changes in the relative efficacy of glutamate and kainate (Tomita et al., 2005). To determine whether NETO2 changed the pharmacology of kainate receptors, we compared glutamate- and kainate-evoked currents in oocytes injected with GluR6 or GluR6 coexpressed with NETO2. To reduce desensitization, the oocytes were pre-exposed to concanavalin A (Wong and Mayer, 1993). We found that the ratio of kainate- and glutamate-evoked currents is increased by coexpression of NETO2 (Figure 5A) without marked changes in agonist potency (Figure S8).
Figure 5. NETO2 Modulates the Agonist Sensitivity of Kainate Receptors.
(A) Oocytes injected with NETO2 (0.5 ng) and GluR6 (0.1 ng) cRNAs were pre-exposed to concanavalin A and currents evoked by glutamate (500 μM; IGlu) or kainate (20 μM; IKA) were measured with TEVC (n = 7–8). Coinjection of NETO2 increases the relative efficacy of kainate.
(B) CHO cells were cotransfected with expression vectors for wild-type (wt) or mutant (mut) NETO2 and NETO2 shRNA vector. NETO2 shRNAs eliminated detectable protein for NETO2 wild-type (wt) without affecting actin expression. The knockdown was prevented by silent point mutations in the NETO2 cDNA (NETO2.mut). shRNA parent vector (Control.shRNA) had no effect.
(C) Inward whole-cell currents evoked in cultured hippocampal neurons (−80 mV) by the successive application of 500 μM glutamate and 20 μM kainate. The neurons (DIV 10–11) were transfected at DIV 7. Agonists were applied by local superfusion with a large-bore glass pipette. Solution exchange times were about 1 s. Neurons were pre-exposed to concanavalin A to reduce kainate receptor desensitization and all solutions contained SYM 2206 (100 μM), AP-5 (50 μM), and tetrodotoxin (2 μM) to block AMPA receptors, NMDA receptors, and sodium channels, respectively.
(D) Currents evoked by glutamate (500 μM; IGlu) or kainate (20 μM; IKA) in hippocampal cultured neurons transfected as indicated. NETO2.shRNA decreased the ratio of kainate- and glutamate-evoked currents (n = 8). Coexpression of NETO2.mut rescued this suppression (n = 6). Data are given as mean ± SEM. ***p < 0.005.
To test whether NETO2 is incorporated in functional neuronal receptors, we therefore used whole-cell current measurements and the NETO2-mediated pharmacologic difference in kainate/glutamate efficacy. We first generated several NETO2 shRNA constructs and transfected NETO2 shRNA plasmids with NETO2 cDNA in CHO cells to detect NETO2 expression. One NETO2 shRNA construct effectively suppressed NETO2 expression, and this suppression could be rescued by coexpression of a NETO2 mutant carrying silent mutations (Rescue) in the shRNA target sequence (Figure 5B). Because we could verify the specificity of the shRNA with the Rescue construct, we transfected the NETO2 shRNA plasmid alone or with the NETO2 silent mutant in primary hippocampal cultures and measured kainate and glutamate-evoked steady-state currents by whole-cell recording after pre-exposing the cultures to concanavalin A. The vector used (pLLox3.7) directs the expression of both shRNA and GFP, allowing identification of neurons expressing the shRNA. The parent vector alone served as a control. Expression of NETO2 shRNA selectively decreased the ratio of kainate-and glutamate-evoked steady-state currents in hippocampal neurons (Figures 5C and 5D), strongly indicating that NETO2 interacts with native kainate receptors.
Kainate Receptors Modulate Cell Surface Expression of NETO2 in Heterologous Cells and Neurons
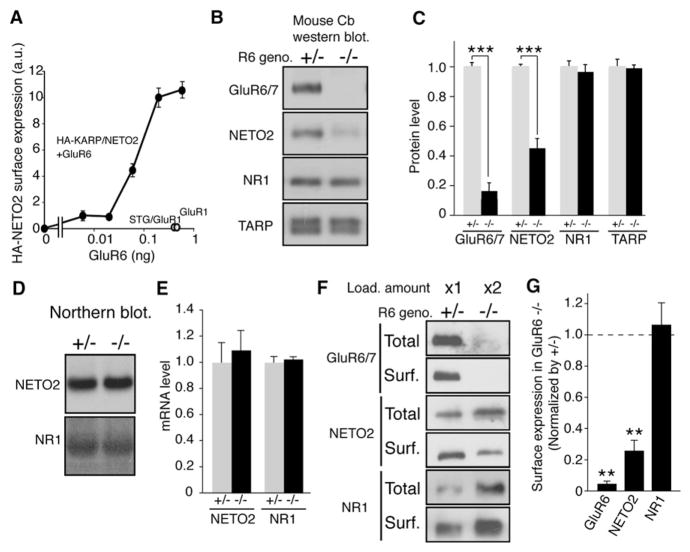
Although NETO2 did not modulate the surface expression of kainate receptors (Figure 2C), both kainate receptors and NETO2 are transmembrane proteins and likely interact in the endoplasmic reticulum (ER). We therefore wondered whether kainate receptors modulate NETO2 trafficking. To examine this possibility, we used chemiluminescence (quantified with a Luminometer) to measure surface expression of NETO2 in oocytes injected with extracellular HA-epitope-tagged NETO2 and varying amounts of GluR6 (Tomita et al., 2005). We found that GluR6 enhances the surface expression of NETO2 in a GluR6-dose-dependent manner (Figure 6A and raw data in Figure S9).
Figure 6. Kainate Receptors Modulate Cell Surface Expression of NETO2.
(A) Oocytes were injected with HA-NETO2 (20 pg) and various amounts of GluR6 cRNA as indicated, and surface expression of NETO2 was quantified by chemiluminescence (n = 8). GluR6 specifically and dose-dependently increased the surface expression of NETO2, whereas GluR1 or GluR1 with stargazin (STG) did not.
(B–E) Quantitative analysis of protein expression (B and C) and mRNA levels (D and E) in GluR6 heterozygous (+/−) and homozygous (−/−) littermate mice. (B and C) NETO2 expression was reduced in GluR6−/− mice, whereas expression of the NMDA receptor subunit NR1 and TARP was unaffected (n = 4). Arrow and asterisk indicate NETO2 and nonspecific band, respectively. Data are given as mean ± SEM. ***p < 0.005. (D and E) mRNA level of NETO2 and NR1 was unaffected in GluR6−/− mice (n = 3).
(F and G) Cerebellar cultures from GluR6 littermates were biotinylated, solubilized, and precipitated with neutravidin-beads. Twice as much total protein was loaded for the sample from GluR6 homozygous mice to account for the reduced NETO2 expression in these cultures relative to cultures from heterozygous littermates. The surface expression of NETO2 is dramatically decreased in GluR6 homozygous cultures, whereas NMDA receptor subunit NR1 is unaltered (n = 3). Data are given as mean ± SEM. **p < 0.01.
Mice lacking two different TARPs known to modulate AMPA receptor trafficking, stargazin and γ-8, have been reported to show a decreased total amount of AMPA receptors (Fukaya et al., 2006; Rouach et al., 2005; Tomita et al., 2003). Because GluR6 modulates NETO2 trafficking (Figure 6A), we asked whether the total amount of NETO2 is decreased in mice lacking GluR6. To test this, we measured NETO2 expression in heterozygous and homozygous littermates of GluR6 knockout mice (Mulle et al., 1998). We found that the total amount of NETO2 in the cerebella of GluR6 homozygous mice was reduced by 60% relative to a GluR6 heterozygous littermate, whereas the total amounts of TARP isoforms and the NMDA receptor subunit NR1 were unaltered (Figures 6B and 6C). The reduced NETO2 expression was not due to a decrease in transcription, since northern blot analysis of cerebella of GluR6 littermates demonstrated no alteration in NETO2 mRNA levels (Figures 6D and 6E).
To determine the extent to which GluR6 expression influences NETO2 trafficking in neurons, we used cell-impermeable NHS-SS-biotin to measure the surface expression of NETO2 in primary cerebellar granule cells from GluR6 heterozygous and homozygous littermates (Tomita et al., 2004). Because the total amount of NETO2 is decreased in GluR6 knockout mice (Figures 6B and 6C), we adjusted the protein loaded (2-fold) so that the total amount of NETO2 was similar in the two groups (Figure 6F). We found that surface expression of NETO2 in cultures from GluR6 homozygous mice was reduced 70% relative to cultures from GluR6 heterozygous littermates, whereas the surface expression of NR1 was unaltered (Figures 6F and 6G).
DISCUSSION
Here we identified a transmembrane protein, NETO2, which modulates kainate receptor function in brain. In summary, three main findings support the conclusion that NETO2 is a transmembrane accessory subunit of neuronal kainate receptors. First, NETO2 copurifies with kainate receptors from mammalian brain. Second, NETO2 modulates the kinetics, as well as the agonist sensitivity, of kainate receptors in both heterologous cells and neurons. Third, NETO2 trafficking in heterologous cells and neurons is modulated by kainate receptors.
Sequence alignment shows that rat NETO2 shares high sequence identity (56%) with NETO1 (Figure S3). Therefore, we also examined whether NETO1 modulated kainate receptor function. We found that NETO1 enhanced glutamate-evoked current from GluR6 (Figure S10); thus, we defined NETO1 and NETO2 as a family of kainate receptor regulatory proteins (KARPs). Although NETO1 and NETO2 are CUB-domain-containing transmembrane proteins that interact with ion channels in vertebrates, two other CUB-domain-containing proteins in C. elegans, SOL-1 and LEV-10, were previously identified as ion channel modulators of GLR-1 AMPA receptors and acetylcholine receptors, respectively (Gally et al., 2004; Zheng et al., 2004, 2006). Interestingly, the acetylcholine receptor modulator LEV-10 shares a similar domain structure with NETO2, as well as with the AMPA receptor subunit SOL-1. In addition, the domain structure of NETO2 is similar to the hypothetical proteins Q9XUU2 in C. elegans and Q9VYC7 in D. melanogaster.
Our identification of a CUB-domain-containing protein as an ion channel accessory subunit in vertebrates supports a conserved role for CUB-domain-containing proteins as ion channel subunits through evolution, and some of the 47 CUB-domain-containing proteins encoded in the human genome may play roles in ion channel modulation (Venter et al., 2001). In the invertebrate, three proteins, SOL-1, GLR-1, and the auxiliary subunit STG-1, form functional AMPA receptor complexes (Walker et al., 2006a). However, we find that NETO2 does not form protein complexes with AMPA receptors and their auxiliary subunit, stargazin-like TARP (STG-1 vertebrate homolog) (Figure 1B). SOL-1 slows desensitization of GLR-1 AMPA receptors without effects on receptor trafficking (Walker et al., 2006b), which is similar to the effects of NETO2 on kainate receptors; however, there is a distinct difference in the domain structure of NETO2 and SOL-1. NETO2 contains one LDLa domain in its extracellular domain, whereas SOL-1 does not. Therefore, we examined the role of the LDLa domain of NETO2 in modulating kainate receptor activity. Since cysteine residues in the LDLa domain play critical roles in its function, we generated a NETO2 mutant in which we substituted serines for two cysteine residues in the LDLa domain and coexpressed the mutant NETO2 with GluR6 in oocytes. The mutant NETO2 successfully expressed at the cell surface (Figure S11). Comparison of glutamate-evoked currents with and without expression of the mutant NETO2 indicated that the LDLa domain is necessary for modulation of kainate receptor activity (Figure S11). This result indicates that NETO2 and SOL-1 function distinctly and that there might be NETO2 homologs in the invertebrate kainate receptor complex and SOL-1 homologs in the vertebrate AMPA receptor complex.
Coexpression of NETO2 with GluR6 produced marked effects on both the peak amplitude and the kinetics of glutamate-evoked currents. Although other kainate-receptor interactors have been shown to increase steady-state currents and speed recovery from desensitization (Garcia et al., 1998; Laezza et al., 2007), we show that NETO2 is a unique accessory protein that alters the decay kinetics of currents evoked by rapid pulses of glutamate. Our single-channel data also indicate that, in the absence of NETO2, the vast majority of GluR6 receptors desensitize without opening when they are rapidly exposed to glutamate, a conclusion which differs from previous estimates of peak Popen values for GluR6 from nonstationary noise analysis (Traynelis and Wahl, 1997). The association of NETO2 with native kainate receptors would therefore be expected to modulate both the amplitude and the decay of kainate-receptor-mediated currents.
Indeed, coexpression of NETO2 increased significantly both the frequency and decay time of kainate-receptor-mediated mEPSCs. The increased frequency of mEPSCs in NETO2-transfected neurons likely reflects the ability of NETO2 to increase peak Popen and therefore the peak amplitude of kainate-receptor mEPSCs. Even with NETO2 coexpression the amplitude of the mEPSCs was near the limit of detection, and it is probable that many events were too small to detect in the absence of NETO2. The effect of NETO2 in slowing the decay of mEPSCs was also significant, and the extent of the slowing was consistent with the effect of NETO2 on the deactivation time course of recombinant GluR6 receptors.
NETO2/Btcl2 mRNA is abundant in the cerebellar granule cell layer and hippocampus CA3 region (Michishita et al., 2004) (also see websites of Allen Brain Atlas, http://www.brain-map.org/, and Brain Gene Expression Map, http://www.stjudebgem.org/web/view/probe/viewProbeDetails.php?id=10). Interestingly, these neurons are strongly labeled with [3H]-kainate in the rat brain (Foster et al., 1981; Monaghan and Cotman, 1982). In most neurons, kainate receptor EPSCs decay with time constants of 50–200 ms (Bannister et al., 2005; Castillo et al., 1997; Kidd and Isaac, 1999; Vignes and Collingridge, 1997), whereas CA1 interneurons in hippocampus show briefer time constants of 10 to 15 ms (Cossart et al., 1998; Frerking et al., 1998), which are closer to the time constants of deactivation (2–5 ms) and desensitization (5–10 ms) for recombinant kainate receptors (Dingledine et al., 1999; Erreger et al., 2004). Interestingly, in situ hybridization showed that NETO2/Btcl2 is not expressed in CA1 interneurons (Michishita et al., 2004), although CA1 interneurons do express NETO1. Our results showed that NETO1 modulates kainate receptor activity, but not as much as NETO2 does, which might in part explain the faster decay kinetics of kainate-receptor-mediated EPSCs in CA1 interneurons than that in CA3 pyramidal cells. While the effect of NETO2 on the kinetics of native kainate receptors is at this point unknown, the inclusion of NETO2 in native kainate receptors may contribute to the often noted discrepancy between the decay time course of kainate receptor synaptic currents and the decay kinetics of recombinant receptors (Castillo et al., 1997; Dingledine et al., 1999; Erreger et al., 2004; Huettner, 2003; Lerma, 2006; Vignes and Collingridge, 1997).
Our results indicate that NETO2, and the structurally distinct TARPs, modulate glutamate receptor properties through distinct mechanisms, with TARPs primarily influencing AMPA receptor activation and NETO2 primarily altering the rates at which kainate receptors enter and recover from desensitization. Crystal structures of the ligand binding domain of AMPA receptors and kainate receptors have been solved (Armstrong and Gouaux, 2000; Mayer, 2005; Nanao et al., 2005). Upon agonist binding, the clamshell-like structure of the ligand binding domain closes and triggers conformational changes that open the channel pore, which ultimately causes the dimer interface to rearrange and receptor desensitization (Sun et al., 2002). One explanation for our findings that NETO2 slows desensitization is that NETO2 stabilizes the dimer interface.
The AMPA receptor auxiliary subunit, TARP/stargazin, is required for the surface expression of AMPA receptors in cerebellar granule cells (Chen et al., 2000) and modulates receptor properties (Tomita et al., 2005), suggesting that most AMPA receptors contain TARP/stargazin (at least in cerebellar granule cells). However, kainate receptor trafficking does not require NETO2, but rather, kainate receptors regulate the surface expression of NETO2. The reduced expression of NETO2 in cerebella from GluR6 knockout mice may indicate that GluR6 enhances the surface trafficking of NETO2 and that, in the absence of GluR6, NETO2 accumulates intracellularly and is degraded. Alternatively, NETO2 may traffic to the cell surface in neurons lacking GluR6, but it is unstable when not coassembled with GluR6. Similar to NETO2, total protein and surface expression of KA2 were reduced in hippocampus of GluR6 and GluR5 knockout mice (Christensen et al., 2004; Nasu-Nishimura et al., 2006; Ruiz et al., 2005). Experiments with EndoH showed that the ER retention of KA2 is increased in GluR6 knockout mice, indicating that KA2 does not traffic by itself (Nasu-Nishimura et al., 2006). Because NETO2 is sensitive to EndoH treatment like NR1 (Figure 1D), it remains unclear whether NETO2 degrades in GluR6 knockout mice at the cell surface or in the ER.
In GluR6 knockout mice, about 30% of total and surface expression of NETO2 was still detected in cerebellum and cerebellar granule cells, respectively. The residual NETO2 might be trafficked or stabilized by other kainate receptor subunits in cerebellum, or a small portion of NETO2 could be trafficked without GluR6. Because GluR6 could traffic to the cell surface without NETO2, there is also the possibility that two types of kainate receptors may exist in brain, one with and one without NETO2. Since these two types of kainate receptors display different channel properties, kainate receptors with and without NETO2 may play roles in distinct functions or may be targeted to different subcellular compartments. Further studies on the mechanisms of kainate receptor trafficking and localization are required to examine this possibility. Related to this question, the stoichiometry of NETO2 and kainate receptors is unknown. Both NETO2 and kainate receptors are transmembrane proteins (like TARPs and AMPA receptors). It is therefore likely that the molecular structure of NETO2/kainate receptor complexes at high resolution will be required to ascertain the stoichiometry of the two proteins.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were used: rabbit polyclonal antibodies to GluR1, GluR2/3, GluR4, GluR5, GluR6/7, KA2, and TARP (Millipore); goat polyclonal antibody to AKAP8 (abcam); and mouse monoclonal antibodies to NR1 and NR2B (BD Biosciences), PSD95 (ABR), synaptophysin (Sigma), and actin (Chemicon). Polyclonal antisera to NETO2 proteins were raised by injecting rabbits with a GST-NETO2 fusion protein encoding the last 192 amino acids of NETO2. Antisera were affinity purified on agarose columns containing the His-NETO2 fusion proteins.
Plasmid Construction
Rat NETO2 cDNA was cloned by RT-PCR and subcloned into pGEMHE and pCDNA3.1 (Invitrogen). NETO2 shRNA oligonucleotide (AGTGTTGCTAATAAC GGTA TTCAAGAGA TACCGTTATTAGCAACACT) was inserted into pLLox3.7. The NETO2 silent mutant carries five mutations (GGTACTACTGATAACGGTA), which do not alter amino acids encoded.
Immunoprecipitation
Rat cerebella membranes were suspended in lysis buffer (20 mM Tris-Cl [pH 8.0], 2 mM EDTA, and 1 mM DTT), 1% Triton X-100, and protease inhibitors (1 mM PMSF and 10 μg/ml leupeptin), and were centrifuged at 100,000 × g for 1 hr. Precleared supernatants were then incubated with 3 μg of affinity-purified antibodies and 20 μl of protein A sepharose beads. Bound proteins were eluted by heating the resin in 20 μl of 1X SDS-PAGE sample buffer and were separated by SDS-PAGE, which was followed by silver staining.
Mass Spectrometry Analysis
Protein bands were excised from a silver-stained gel and diced. The gel pieces were reduced with 2.1 mM dithiothreitol, alkylated with 4.2 mM iodoacetamide, and digested for 12 hr with trypsin (12 ng/L) at 37°C. Peptides were separated using a 75 μm × 15 cm reverse phase C18 column (LC Packings, Sunnyvale, CA). The LC eluent was coupled to a microionspray source attached to a QSTAR Pulsar mass spectrometer (Applied Biosystems, Foster City, CA).
PSD Fraction and Glycosylation Assay
PSD fractionation and glycosylation assay were performed as described (Tomita et al., 2003).
Electrophysiology Using X. laevis Oocytes
TEVC was performed as described (Tomita et al., 2004). Briefly, GluR6, NETO2, GluR1, and stargazin constructs were subcloned into pGEMHE vector and cRNAs were transcribed in vitro using T7 mMessage mMachine (Ambion). TEVC analysis was performed 4–5 days after injection at room temperature in recording solution containing (in mM) 100 NaCl, 1.0 KCl, 1.0 MgCl2, 0.5 BaCl2, and 5 HEPES (pH 7.4). The membrane potential was held at −70 mV. Maximal glutamate-evoked currents were taken as responses to 10 mM glutamate. In some experiments, steady-state responses to glutamate (Glu: 500 μM) or kainate (KA: 20 μM) were recorded after 5 min incubation with concanavalin A (10 mg/ml).
Surface Labeling of Oocytes
Surface labeling was performed as described (Tomita et al., 2005; Zerangue et al., 1999). Briefly, 4–5 days after injection oocytes were incubated for 1 hr with 0.25 μg ml−1 rat anti-HA antibody (3F10, Roche), followed by 30 min with horseradish-peroxidase-conjugated anti-rat immunoglobulin. Individual oocytes were then placed into 50 μl of SuperSignal ELISA Femto maximum sensitivity substrate (Pierce) and quantified by chemiluminescence using a Veritas Microplate Luminometer (Turner Biosystems).
Primary Neuronal Cultures and Biotinylation of Cell Surface Proteins
Cerebellar granule cell cultures were maintained as described (Cho et al., 2007). Surface expression of glutamate receptors and NETO2 was quantitated as described (Tomita et al., 2004). Briefly, cerebellar granule cell cultures at DIV 10 were labeled for 12 min at 4°C with 1.5 mg/ml sulfo-NHS-SS-biotin. Membranes were prepared and the biotinylated proteins were precipitated with Neutravidin-agarose and detected by western blotting.
Outside-Out Patch Recordings
tsA201 cells were maintained and transfected as previously described (Robert and Howe, 2003). Individual GluR6 and NETO2 cDNAs were cotransfected at a 1:3, 1:10, or 1:30 ratio. Individual GluR1 and NETO2 cDNAs were cotransfected at a 1:10 ratio. Recordings from outside-out patches were performed 1 to 3 days posttransfection at room temperature with an EPC-9 amplifier (HEKA) at a holding potential of −100 mV (Robert and Howe, 2003). The external solution was (in mM) 150 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 5 glucose, and 10 HEPES (pH 7.4). Patch pipettes (open tip resistance, 3–5 MΩ) were filled with a solution containing (in mM) 135 CsF, 33 CsOH, 2 MgCl2, 1 CaCl2, 11 EGTA, and 10 HEPES (pH 7.4). The size of the peak currents was 0.2 to 2 nA (60%–70% series resistance compensation). Glutamate was added to the external solution and applied with theta glass pipettes mounted on a piezoelectric bimorph. Agonist-evoked currents were analog low-pass filtered at 3 kHz, sampled at 20–30 kHz, and analyzed with Igor software (Tomita et al., 2005). Recovery from desensitization was studied by making two consecutive 200 ms applications of 1 mM glutamate at different intervals. The results were fitted with an equation consisting of two Hodgkin-Huxley components (Zhang et al., 2006).
Single-Channel Data Analysis
Single-channel currents evoked by 10 mM glutamate were analog low-pass filtered at 5 kHz, sampled at 25 kHz, and written directly to the hard drive of two computers. One computer stored the records in PULSE format (the software used to run the EPC-9) and the other stored the data in QuB (http://www.qub.buffalo.edu) format (the software used for single-channel analysis). The patches analyzed gave maximum peak currents of 5 to 15 pA at −100 mV and appeared to contain 3 to 10 active channels. Responses to 200 to 500 concentration jumps (400 ms applications) were obtained from each patch. After filtering the digitized records at 4 kHz and manually setting the closed-channel current (r.m.s., 240 to 270 pA) to 0, the entire filtered data set from a given patch was idealized with the segmental k-means (SKM) algorithm of QuB to identify single-channel transitions and estimate conductance levels and event durations (Qin, 2004; Qin et al., 1996). The time resolution was set to four sample intervals (160 μs, 1.5 filter rise times). Open and shut times and burst durations were exported to Channelab (Synaptosoft Inc) and histograms (10–12 bins per decade) of the dwell times were displayed and fitted with log-likelihood log-binned subroutines. All burst distributions contained a fast component (primarily brief single openings) that was unaltered by NETO2. Peak Popen was estimated in patches containing three to five channels by dividing the peak ensemble current by the weighted mean single-channel current determined from measurements of the amplitude and frequency of subconductance levels (Table 1).
Whole-Cell Recordings from Rat Hippocampal Neurons
Dissociated hippocampal cells were prepared from E20 rats as described (Tomita et al., 2003). Neurons were transfected by calcium phosphate transfection at DIV 7 and whole-cell recordings were made from DIV 12–14 neurons at −100 mV and room temperature with an EPC-9 amplifier (HEKA). The solutions and recording conditions were the same as those used for the outside-out patch recordings. Cultures were incubated in concanavalin A (50 μM) for 20 min prior to recording and control and agonist-containing solutions were applied from thin glass pipettes positioned near the cells.
Synaptic Currents in Transfected Cerebellar Granule Cells
Stargazer mice were obtained from Jackson Laboratory and maintained at the Yale animal facility under the guidelines of the Institutional Animal Care and Use Committee. Heterozygous male and female mice were mated to obtain homozygous stargazer mice. Cerebellar granule cell cultures were prepared from postnatal day 7–9 (P7–9) homozygous stargazer mice and were transfected at DIV 5 as described (Cho et al., 2007). Patch-clamp recordings from cerebellar granule cells (DIV 7–10) were made in external solution containing (in mM) 10 HEPES, 140 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 2.7 MgCl2, and 10 glucose. The frequency of mEPSCs was increased by making local applications of barium (5 mM) or sucrose (200 mM). Barium was applied for 250 ms three to six times at 10 s intervals. Sucrose was applied for 2.5 s, also at 10 s intervals. After five cycles of this sucrose protocol, the cell was locally superfused with normal external solution for 5 min. Release occurred both during the sucrose application and the wash. Patch pipettes were filled with recording solution (pH 7.2, 320 mOsm) that contained (in mM) 130 cesium methanesulfonate, 5 HEPES, 5 Mg-ATP, 0.2 Na-GTP, 20 TEA, and 5 EGTA. All recordings were performed at room temperature. To isolate and record kainate-receptor-mediated mEPSCs, tetrodotoxin (1 μM), AP-5 (100 μM), and picrotoxin (50 μM) were added to the external solution. mEPSCs were recorded from cerebellar granule cells in whole-cell configuration at a holding potential of −70 mV. The current was analog low-pass filtered at 3 kHz and digitally sampled at 25 kHz.
Supplementary Material
Acknowledgments
The authors thank members of the Tomita lab and the CNNR program for helpful discussions, and Pietro De Camilli for critical reading of the manuscript. We thank Dr. H. Ariga (Hokkaido University) for providing FLAG-AKAP8/95 cDNA and Dr. S.F. Heinemann (Salk institute) for generating GluR6 knockout mice. We also thank Daniel DiMaio for his Veritas Microplate Luminometer. S.T. is supported by Yale start-up funds, Alfred P. Sloan research fellowship, NARSAD young investigator award, the Esther A. & Joseph Klingenstein Fund, and the Edward Mallinckrodt Jr. Foundation. J.R.H. and A.L.B. are supported by grants from the NIH.
Footnotes
The supplemental data for this article include 11 Supplemental Figures and 1 Supplemental Table and can be found at http://www.neuron.org/supplemental/S0896-6273(08)01085-4.
Author contributions: S.T. directed all the experiments except for the patch-clamp studies, which J.R.H. and C.P.G. directed. S.T., M.M-T., K.S.K., and A.S. performed biochemistry and molecular biology. F.S. performed experiments using X. laevis oocytes, W.Z. performed the patch-clamp recordings, and C.P.G performed synaptic physiology. J.C.T. and A.L.B performed mass spectrometry analysis. The manuscript was written by S.T. and J.R.H. All authors commented on the manuscript.
References
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister NJ, Benke TA, Mellor J, Scott H, Gurdal E, Crabtree JW, Isaac JT. Developmental changes in AMPA and kainate receptor-mediated quantal transmission at thalamocortical synapses in the barrel cortex. J Neurosci. 2005;25:5259–5271. doi: 10.1523/JNEUROSCI.0827-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, et al. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J Physiol. 2003;547:373–385. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Went-hold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two Families of TARP Isoforms that Have Distinct Effects on the Kinetic Properties of AMPA Receptors and Synaptic Currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Christensen JK, Paternain AV, Selak S, Ahring PK, Lerma J. A mosaic of functional kainate receptors in hippocampal interneurons. J Neurosci. 2004;24:8986–8993. doi: 10.1523/JNEUROSCI.2156-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, et al. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson G, Heinemann SF. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Coussen F, Normand E, Marchal C, Costet P, Choquet D, Lambert M, Mege RM, Mulle C. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J Neurosci. 2002;22:6426–6436. doi: 10.1523/JNEUROSCI.22-15-06426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Foster AC, Mena EE, Monaghan DT, Cotman CW. Synaptic localization of kainic acid binding sites. Nature. 1981;289:73–75. doi: 10.1038/289073a0. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe K, Natsume R, Kano M, Kamiya H, Watanabe M, Sakimura K. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- Gally C, Eimer S, Richmond JE, Bessereau JL. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol. 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Laezza F, Wilding TJ, Sequeira S, Coussen F, Zhang XZ, Hill-Robinson R, Mulle C, Huettner JE, Craig AM. KRIP6: a novel BTB/kelch protein regulating function of kainate receptors. Mol Cell Neurosci. 2007;34:539–550. doi: 10.1016/j.mcn.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Michishita M, Ikeda T, Nakashiba T, Ogawa M, Tashiro K, Honjo T, Doi K, Itohara S, Endo S. Expression of Btcl2, a novel member of Btcl gene family, during development of the central nervous system. Brain Res Dev Brain Res. 2004;153:135–142. doi: 10.1016/j.devbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP Subtypes Differentially and Dose-Dependently Control Synaptic AMPA Receptor Gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 1982;252:91–100. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci USA. 2005;102:1708–1713. doi: 10.1073/pnas.0409573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu-Nishimura Y, Hurtado D, Braud S, Tang TT, Isaac JT, Roche KW. Identification of an endoplasmic reticulum-retention motif in an intracellular loop of the kainate receptor subunit KA2. J Neurosci. 2006;26:7014–7021. doi: 10.1523/JNEUROSCI.0573-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Pemberton KE, Belcher SM, Ripellino JA, Howe JR. High-affinity kainate-type ion channels in rat cerebellar granule cells. J Physiol. 1998;510:401–420. doi: 10.1111/j.1469-7793.1998.401bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Selak S, Lerma J, Stern-Bach Y. Block of kainate receptor desensitization uncovers a key trafficking checkpoint. Neuron. 2006;52:1037–1046. doi: 10.1016/j.neuron.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rouach N, Byrd RK, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP g-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Sachidhanandam S, Utvik JK, Coussen F, Mulle C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J Neurosci. 2005;25:11710–11718. doi: 10.1523/JNEUROSCI.4041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas GD, Blair LA, Needleman LA, Gonzales JD, Chen Y, Li M, Singer JD, Marshall J. Actinfilin is a Cul3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J Biol Chem. 2006;281:40164–40173. doi: 10.1074/jbc.M608194200. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Nicoll RA. Presynaptic kainate receptor mediation of frequency facilitation at hippocampal mossy fiber synapses. Science. 2001;291:1972–1976. doi: 10.1126/science.1057105. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The Trends Neurosci/TiPS Lecture The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Smith TC, Wang LY, Howe JR. Distinct kainate receptor phenotypes in immature and mature mouse cerebellar granule cells. J Physiol. 1999;517:51–58. doi: 10.1111/j.1469-7793.1999.0051z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr H, Berger C, Frohlich S, Weber BH. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene. 2002;286:223–231. doi: 10.1016/s0378-1119(02)00438-9. [DOI] [PubMed] [Google Scholar]
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly single-channel properties of recombinant kainate receptors. J Physiol. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci USA. 2006a;103:10781–10786. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Francis MM, Brockie PJ, Madsen DM, Zheng Y, Maricq AV. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc Natl Acad Sci USA. 2006b;103:10787–10792. doi: 10.1073/pnas.0604520103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91:1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature. 2004;427:451–457. doi: 10.1038/nature02244. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Walker CS, Francis MM, Maricq AV. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2006;103:1100–1105. doi: 10.1073/pnas.0504612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.