Abstract
Gas-phase ion/ion reactions are emerging as flexible means for probing and manipulating analyte ions with particular utility in bioanalysis.
What are the elements of an analytically useful chemical reaction? This question is implicit in much of analytical chemistry but is rarely addressed explicitly. Nevertheless, sometimes it is useful to articulate the obvious in order to place into context the relative strengths and weaknesses of the “chemical” part of an analytical method. Any list of desirable reaction characteristics would include high reaction efficiency—ideally, 100% conversion of a reactant of interest (usually the analyte) to product(s). This trait underlies titrimetry, for example. Another obvious characteristic on the list is formation of products that are readily distinguishable from the reactants by available methods. For example, the naked eye can detect the color change associated with an acid–base or redox indicator. Instrumental techniques are a much more sophisticated means for “seeing” differences between reactants and products via, for example, spectroscopic, electrochemical, chromatographic, or mass spectral properties. A third criterion is that the reaction must perform a desirable function, such as precipitating an analyte in solution or making a transformation that facilitates a subsequent step in the process. These three characteristics are either necessary or desirable for any chemical reaction used in analysis. A fourth characteristic of selectivity might also be included, although no generalizations can be made in this regard. In some cases, for example, assays based on selective catalysts, such as enzymes, benefit from a high degree of selectivity. In other cases, “universal” reactions are desired, at least within a class of compounds (e.g., in the conversion of organic nitrogen compounds to ammonia in a Kjeldahl determination).
This report describes the emergence of a new class of analytically useful chemical reactions—viz., gas-phase ion/ion reactions—implemented within the context of an MS experiment. The advent of ionization methods that can generate multiply-charged ions, principally ESI and its variants (1), has made the exploration of this reaction type possible. Chemical reactions have always played key roles in organic and biological MS experiments when both mass and structural information are of interest. The formation of gaseous ions, a sine qua non for an MS experiment, generally involves both chemical and physical processes, the former often causing the removal or attachment of an electron or ion in the gas phase. Mass and structural information for a molecule of interest is obtained via measurement of the mass-to-charge ratios (m/z) of the ions derived from the molecule. In this sense, the gaseous ion serves as a surrogate for the molecule of interest. Structural information, which has largely been restricted to information about bond connectivity, has generally been derived from unimolecular dissociation of the ion. This fragmentation may result from energy deposited into the ion during the ionization step or as a result of a separate activation step, as is usually the case in MS/MS. Hence, most of the chemistry that takes place within a mass spectrometer is part of the ion formation step or the fragmentation step. Therefore, ionization and ion activation/dissociation constitute much of organic and biological MS research..
The nature of the ion (e.g., a radical cation, a protonated molecule, a deprotonated molecule, a multiply-protonated molecule, etc.), the internal energy distribution of the ion, and the observation timescale determine the structural information that can be derived from fragmentation of a gaseous ion. For this reason, exploration of various combinations of ion types and activation methods for every important analyte class generates the most informative chemistry. From a practical standpoint, however, this exploration is often highly constrained by the ionization method. The most useful structural information may not come from the ion type most readily formed by the available ionization method.
Gas-phase ion/ion reactions involving multiply-charged ions are emerging as very useful means for converting one ion type into another for subsequent analysis via MS or MS/MS. Most major commercial mass spectrometer vendors have adapted tandem mass spectrometers for ion/ion reaction experiments in prototyping studies, and several vendors currently offer ion/ion reaction capabilities as options in commercial products. The reactions are efficient (e.g., generation of conditions in which all analyte ions react is generally straightforward), MS can easily distinguish reactants from products, and a wide variety of useful transformations can be made to support chemical analysis objectives. Hence, all the elements of an analytically useful chemical reaction are present. This report does not focus on instrumentation; a description of the evolution of instrumentation for ion/ion reactions has recently appeared (2). Rather, the emphasis is placed on reaction efficiency and ion transformation capabilities to illustrate the unique characteristics of ion/ion reactions and the new types of experiments that they enable.
EFFICIENCIES AND RATES
Placing ion/ion reactions into context with other types of ion chemistry, such as ion/molecule chemistry, requires consideration of both the thermodynamics and kinetics associated with these reactions. Because of the strong mutual attraction of oppositely-charged ions in the absence of a dielectric medium (such as liquid water), processes that lead to charge reduction are highly exothermic. Several mechanisms, such as electron transfer, proton transfer, other cation or anion transfer, or complex formation, can lead to neutralization. The key point is that virtually any cation/anion combination in the gas phase, regardless of the chemical natures of the reactants, will undergo a charge reduction reaction if the cations and anions collide. The mechanism for charge reduction, however, is highly dependent upon the chemical nature of the reactants. Hence, in contrast with ion/molecule collisions, which may or may not lead to a reaction, ion/ion collisions lead to a reaction of some kind. In this sense, ion/ion collisions are unit efficient. With ion/ion chemistry, the question is not if a reaction will take place. Rather, the key question is which of the various charge reduction mechanisms will dominate because this will determine the nature of the products.
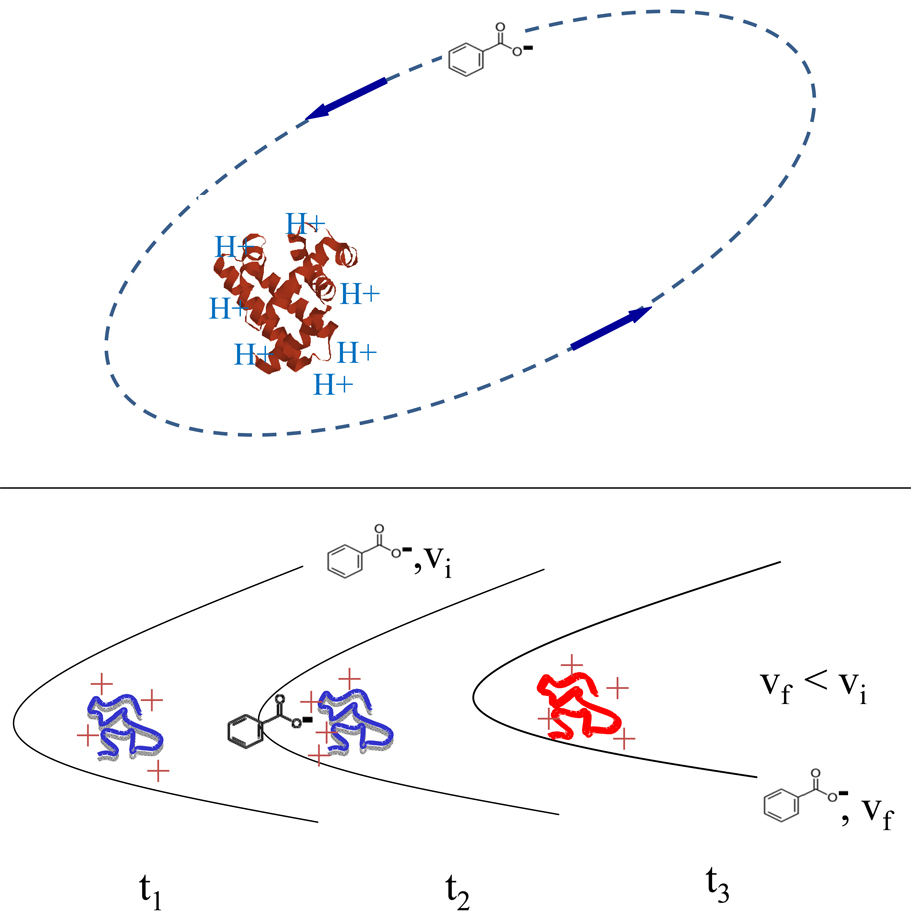
The kinetics of ion/ion reactions is also quite distinct from that of ion/molecule reactions. The attractive potential for ions of opposite polarity follows an r−1 dependence, whereas the attractive ion/dipole and ion/induced-dipole potentials relevant to ion/molecule reactions follow r−2 and r−4 dependencies, respectively. The Coulombic attraction associated with oppositely-charged ions falls off slowly with distance and is therefore a much longer range interaction than those associated with ions and neutrals. The practical result is that the cross-sections for ion/ion reactions can be orders of magnitude larger than for unit efficient ion/molecule reactions. In fact, the rates of ion/ion reactions directly relate to the squares of the charges of the reactant ions (3), which is consistent with the formation of a stable orbit for the two reactants as the rate-determining step (Figure 1, top). The cross-section for this process is approximated by the classical Thomson model for a three-body interaction (Equation 1) (4):
| (1) |
where Z1 and Z2 are the unit charges of the ions, e is the electron charge, ε0 is the vacuum permittivity, v is the relative velocity, and μ is the reduced mass. The distances associated with the stable orbit can be too large for chemistry to occur. However, mechanisms for reducing the relative velocities of the reactants in the orbits, such as the excitation of internal modes of the reactants from the relative motions of the oppositely-charged ions (a so-called “tidal” mechanism; Figure 1, bottom), can bring the reactants into sufficiently close proximity for a reaction to occur. To summarize, the rate-determining step of an ion/ion reaction is largely independent of the chemical identities of the reactants, the kinetics shows a Z2 dependence, and the cross-sections for the reactions can be orders of magnitude larger than for ion/molecule reactions because of the long-range mutual attraction of opposite charges.
Figure 1.
(Top) A depiction of a stable ion/ion orbit (most are elliptical). (Bottom) A schematic diagram of the conversion of relative translation into internal motion of the reactants, which serves as a mechanism for reducing the size of the orbit to the point at which chemistry can occur.
Once the two ionic reactants are captured in their mutually attractive electric field, a charge reduction reaction can take place either via the formation of a relatively long-lived chemical complex (i.e., the reactants undergo an intimate collision) or via a small charged particle (an electron or a proton) transfer without the formation of a long-lived complex. The latter processes are most likely to be most important when the distance at which a proton or electron can transfer is larger than the distance for a physical collision. In the case of a proton transfer, for example, the maximum distance over which a proton can hop, rPT, can be estimated by Equation 2 (5):
| (2) |
where Z1 and Z2 represent the unit charges of the reactants and ΔHPT represents the enthalpy of the proton transfer reaction. The analytical significance of the competition between complex formation and proton or electron transfer without complex formation is that the relative contributions of these processes can have a significant impact on the partitioning of products into the desired reaction channel. For example, as discussed below, some reaction types must proceed through a long-lived complex. The experimentalist can influence the likelihood for complex formation via the judicious choice of reagent ion. To maximize the formation of a long-lived complex, for example, the use of reagents with large physical cross-sections and reactant ions of relatively low charge increases the fraction of ion/ion encounters that result in intimate collisions.
Important experimental considerations also impact both the efficiencies and rates of gas-phase ion/ion reactions. A particularly important consideration is the spatial and temporal overlap of the oppositely-charged ions. For ion/molecule reactions, achieving overlap of the reactants is generally a trivial undertaking. Leaking the neutral reagent into a reaction chamber is straightforward, and this leads to a roughly constant number density of the neutral reactant throughout the reaction region. In the case of ion/ion reactions, on the other hand, care must be taken to ensure spatial and temporal overlap of the reactant ion populations both because generating high ion number densities presents a challenge and because the presence of external electric fields can prevent good spatial overlap. For this reason, ion/ion reaction studies have generally been restricted either to near atmospheric pressure ionization regions that lack strong external fields (6,7) or to electrodynamic ion traps that confine both ion polarities to relatively well-defined overlapping regions of space (8). In the former case, the reactions take place prior to sampling the products into the mass spectrometer, and in the latter case, the reactions can take place between stages of mass analysis (i.e., within the context of an MSn experiment). Ion/ion reactions have been implemented using electrodynamic ion trapping in a variety of instrument platforms that include stand-alone 3D or Paul traps (9,10), stand-alone linear ion traps (LITs) (11), and in a variety of hybrid instruments, such as a hybrid triple quadrupole/LIT (12), a quadrupole/TOF instrument (13), a LIT/Orbitrap instrument (14,15), and a hybrid quadrupole/ion cyclotron resonance instrument (16). The stand-alone ion traps serve both as the reactor and mass analyzer, whereas the hybrid instruments physically separate the reaction region from the mass analyzer. The overall ion/ion reaction rates that are observed are first order in each reactant with the rate constant determined by the cross-section for the formation of the bound ion/ion orbit and the relative velocity of the reactants (i.e., v•σorbit). Therefore, to maximize the rate/efficiency for the reaction of an analyte ion, supplying the oppositely-charged reagent ion in great excess is desirable to ensure that the reaction rate is pseudo-first order in analyte ion and to maximize both the spatial overlap of the reactants and the number density of the reagent. Under such conditions, ion/ion reaction rates in the range of 10–1000 s−1 have been achieved with the ion-trap-based instruments. These rates translate to reaction times of 1–100 ms for efficient (>90%) conversion of reactant analyte ions to product ions.
ION TRANSFORMATIONS AND APPLICATIONS
Whereas the kinetics of ion/ion reactions is largely independent of the chemical identities of the reactant ions, the outcome of the reaction is determined by the chemical characteristics of the reactant ions. Given today’s array of ionization methods, which generate gaseous ions from almost every form of matter, a potentially enormous range of possible reactions exist. For the purpose of illustration, this Feature focuses on analyte ions derived from peptides, proteins, and oligonucleotides, in part because analytical strategies involving ion/ion reactions of such species are the most extensively developed (17,18). However, this is not intended to imply that analytically useful ion/ion reactions are restricted to these biopolymers.
Mechanisms for ion/ion reactions can be placed into three categories: single charged particle transfer, multiple charged particle transfers in a single collision, and covalent bond formation. The first category includes proton transfer, electron transfer, and metal ion transfer. The second category includes ion/ion reactions that result in the inversion of charge of one of the reactants. The third category is an emerging area that represents a very wide array of possible reactions. The remainder of this article illustrates examples of analytically useful ion/ion reactions involving proton transfer, electron transfer, metal ion transfer, charge inversion, and covalent bond formation.
Proton transfer
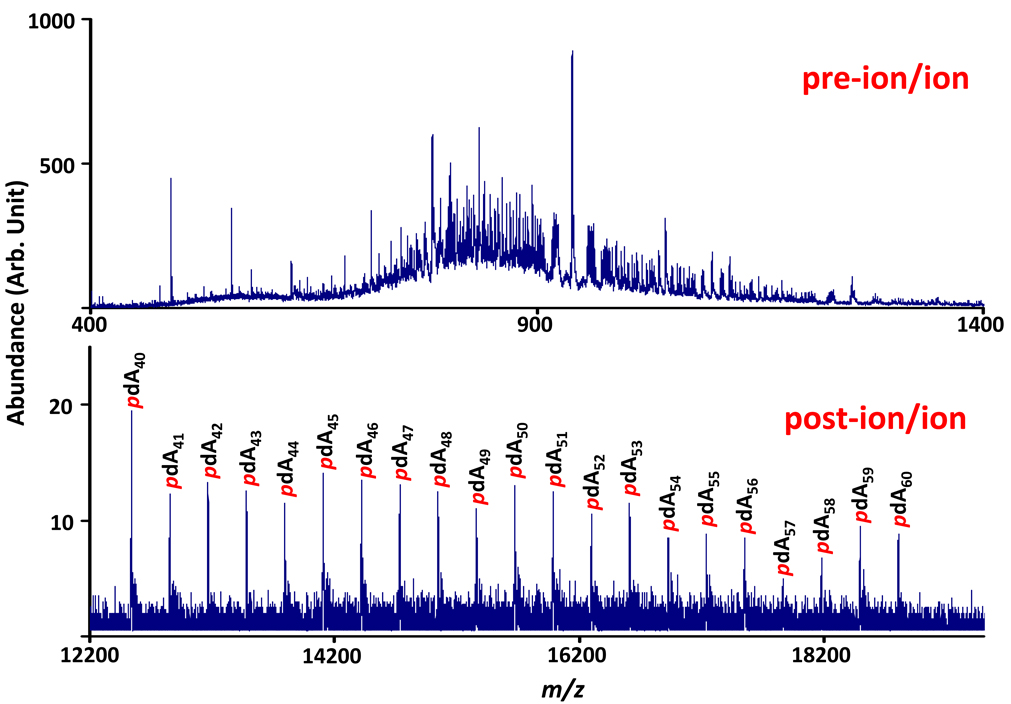
Since the introduction of ESI, which tends to yield a distribution of charge states for biopolymers, means for altering the charge state distribution have been explored. A common objective is to reduce charge states to simplify the interpretation of electrospray mass spectra of complex mixtures. Ion/ion reactions have been the most robust means for removing excess protons from multiply protonated analytes, as well as for protonating multiply deprotonated analytes. Ion/ion proton transfer reactions have been applied to the simplification of electrospray mass spectra of protein mixtures (positive ion mode) and oligonucleotide mixtures (negative ion mode), both prior to sampling ions into the mass spectrometer (19) and in electrodynamic ion traps (Figure 2) (20,21). The Z2 rate dependence facilitates this application because it is straightforward to establish conditions that make neutralization of the Z = 1 charge state of the analyte ion sufficiently slow such that only a small fraction of analyte ions are completely neutralized.
Figure 2.
Proton transfer to multiply-charged pdA40–60 anions. (Top) Negative ion electrospray mass spectrum of an oligonuncleotide mixture. (Bottom) Post-ion/ion reaction mass spectrum after protonated benzoquinoline was allowed to react with the mixture of ions reflected in the top spectrum.
Another mixture analysis problem is generated when multiply-charged precursor ions are subjected to fragmentation. The product ion distribution consists of a mixture of masses and charges, which can lead to ambiguity in spectral interpretation. This problem can be solved either by collecting spectra at high resolution and measuring the spacings between isotope peaks (22) or by manipulating the charge state. Charge state manipulation of the multiply-charged fragment ions from whole protein dissociation has been conducted with bench-top ion trap mass spectrometers using proton transfer ion/ion reactions (23) (Figure 3 (24)). All product ion charge states can be reduced to mostly singly-charged ions with subsequent mass analysis over a wide m/z range (if possible with the mass analyzer). When coupled with TOF mass analysis, which has good resolution and mass measurement accuracy and a wide m/z range, this approach can provide high degrees of confidence in unknown protein identification/characterization (25).
Figure 3.
(Top) Ion trap CID product ion spectrum of the (M+19H)19+ ion of porcine elastase. (Bottom) Product ion spectrum collected after the mixture of ions shown in the top trace was subjected to proton transfer reactions with perfluorocarbon anions Adapted with permission from ref. 24.
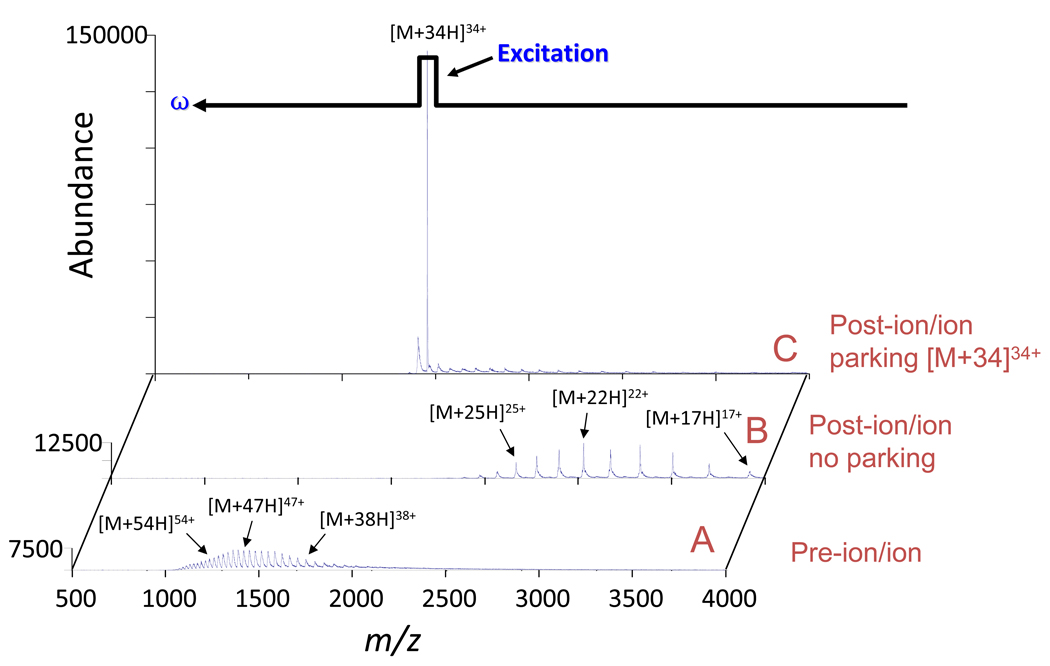
Alternatively, the low mass region of the spectrum can be collected without complications from products of high mass and high charge, thereby providing structural information from the two ends of the molecule (26). The Z2 dependence of the kinetics is particularly significant in these applications because dissociation of a highly-charged precursor ion often generates a wide range of masses and charges. Ion/ion reactions can transform the ions of the highest initial charge states largely to singly-charged ions without neutralizing most of the product ions that start with a single charge. Hence, a single ion/ion reaction period is sufficient for generating all of the mass information inherent in the pre-ion/ion reaction population. When ion/ion reactions are conducted in quadrupole ion traps, it is possible to concentrate most of the analyte ions into a single charge state. This capability is particularly useful in so-called “top-down” MS/MS applications (27) in which precursor ions are normally generated with a range of charge states. The technique is referred to as “ion parking” (28) because the ion/ion reaction rate of a selected ion can be greatly inhibited while those of other ions are unaffected. Hence, the selected ion is “parked” while the others continue to react. Ion parking relies on the fact that ions stored in quadrupole ion traps undergo m/z dependent frequencies of motion. This characteristic underlies mass analysis with ion traps, as well as most forms of ion trap collisional activation. In the ion parking experiment, a supplemental frequency is applied in dipolar fashion to a pair of opposing electrodes that matches the fundamental frequency of motion of the ion of interest. Any ions of the selected m/z are accelerated, which reduces both the overlap with the oppositely-charged reagent ions and the cross-section for formation of the ion/ion orbit because of the inverse fourth power dependence on the relative velocity of the reactants (Equation 1). The net result is that an analyst can choose to reduce all high charge states into a single charge state (Figure 4 (29)). The ion parking experiment is particularly useful in top-down applications because it focuses the analyte signal, which electrospray tends to disperse over many charge states, into a single charge state (30). The ability to access charge states that are lower than those often formed directly via electrospray is desirable because the greatest extents of sequence information tend to come from moderate to low protein charge states (31). Inhibiting the reaction rates of ions over broad ranges of m/z is also possible, which can simplify mixture analyses by combining ion/ion reactions with selective ion trap excitation techniques. This is referred to as “parallel ion parking” (32) because multiple analyte species can be parked simultaneously.
Figure 4.
(A) Pre-ion/ion reaction ESI mass spectrum of BSA. (B) Post-ion/ion reaction spectrum after 30 ms of ion/ion proton transfer reactions with a perfluorocarbon anion (no ion parking). (C) Post-ion/ion reaction spectrum with acceleration of the [M+34H]34+ ion to effect ion parking. Adapted with permission from ref. 29.
Electron transfer
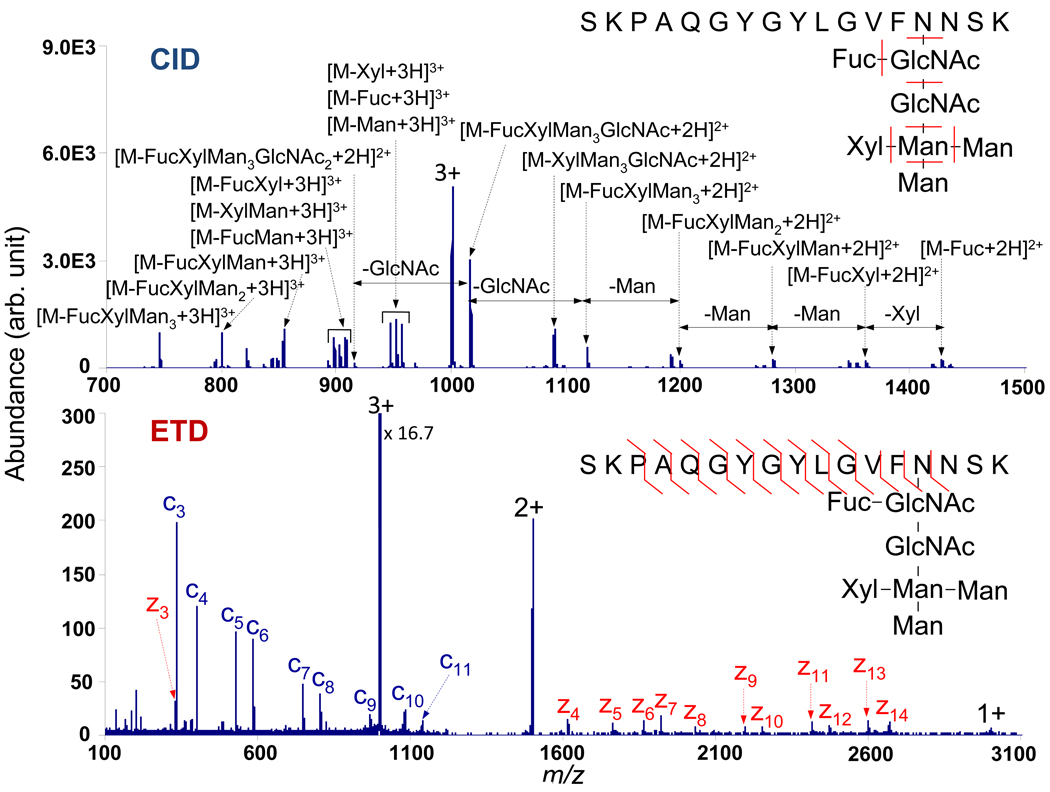
Research in the latter part of the 1990s showed that capture of a thermal electron by a multiply protonated protein results in a hypervalent species that fragments quite distinctly from fragmentation by conventional activation methods (33). This process, referred to as electron capture dissociation (ECD), often provides more sequence information than conventional collision-induced dissociation (CID) and also preserves labile post-translational modifications (PTMs). The discovery of anions that transfer electrons to polypeptide cations was needed for the development of an ion/ion reaction analog to ECD. The first such reagents were reported in 2004 (11), giving rise to the technique referred to as electron transfer dissociation (ETD) (34). Since that time, ECD and ETD of polypeptides and proteins have produced similar, though not always identical, results. Research into the underlying dynamics of ECD and ETD remains very active, and both methods have become very useful additions to the array of MS-based techniques for protein identification and characterization. This very important application has driven the implementation of ion/ion reactions on commercial instruments. Perhaps the most important feature of ECD/ETD is the extent to which they complement the widely available CID methods. Glycopeptides illustrate this particularly well (Figure 5): CID fragments the sugar portion of the molecule, whereas ECD (35) and ETD (36) fragment the peptide portion of the molecule.
Figure 5.
(Top) CID product ion spectrum of a triply protonated glycopeptides. (Bottom) ETD product ion spectrum of the same glycopeptide ion. Adapted from ref. 36.
Given the roles that ECD and ETD play in MS/MS, it is appropriate to refer to them as “dissociation” techniques, but they should not be classified as “activation” methods in the same sense as CID or photodissociation. The latter techniques add energy to a precursor ion to induce dissociation. ETD and ECD, on the other hand, transform one ion type to a less stable ion type (37). Separating the role of the energy transferred in the electron capture/transfer process from the formation of a new ion type is difficult, but the energy transferred alone cannot account for the extent of dissociation observed.
Metal transfer
Many ion/ion reactions require the formation of a relatively long-lived complex; an example of this is metal cation transfer. Metal ions, including alkali, alkaline earth, and transition metals, can replace protons in polypeptide cations by reacting multiply-protonated polypeptides with metal-containing anion complexes (38). For a singly-charged metal, for example, the process is summarized by:
| (3) |
in which M represents the analyte, Me represents a metal cation, and L represents a singly-charged anionic ligand. The reaction involves the transfer of the metal ion to the peptide, as well as the transfer of protons to the ligands. For a singly-charged cation, two protons are transferred, as shown in Equation 3. An example is the transfer of Au(I) to a polypeptide via the ion/ion reaction with AuCl2− (39). The transfer of multivalent cations is also straightforward, as with the transfer of Au(III) to a polypeptide via reaction with AuCl4−. The process represented by Equation 3 can be conducted in an ion trap, which affords the ability to select each of the reactants and to activate the intermediate, if necessary. This method provides a higher degree of control in forming metal cationized species than the conventional approach of dissolving a salt of the metal of interest in the analyte solution prior to electrospray. Whereas the insertion of metal ions into polypeptide cations is emphasized here, the insertion of metal cations into multiply-deprotonated oligonucleotides (40) and peptides (41) by ion/ion reactions has also been demonstrated.
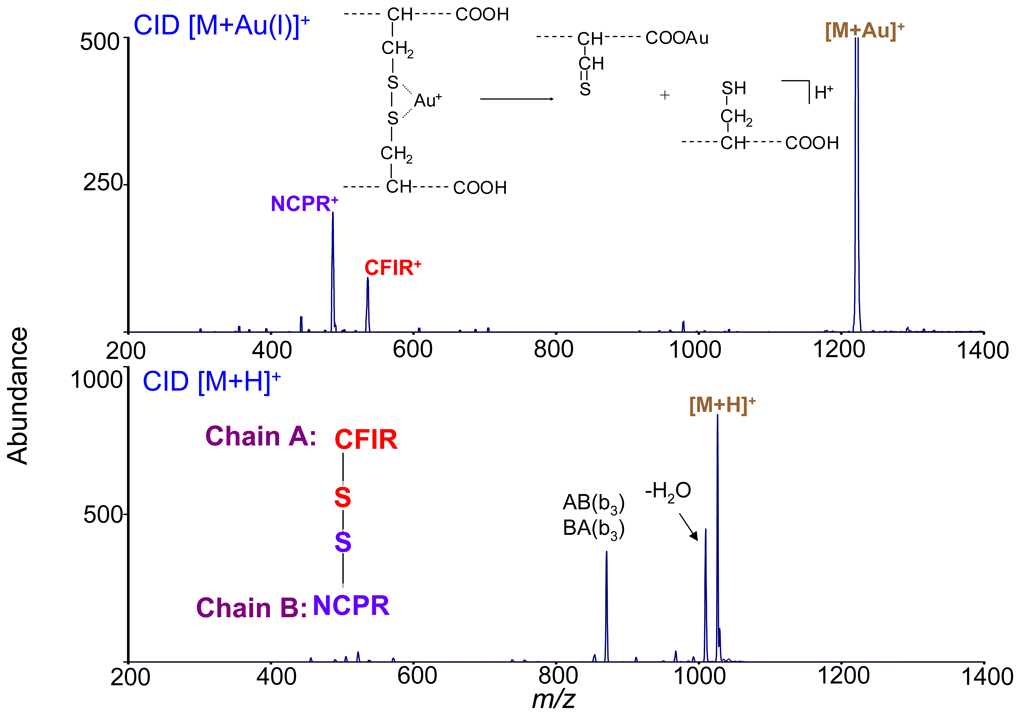
The behavior of metal-containing bio-ions is a very active area of research, spurred by two desires: to understand the role of metal ions in biology and to use metal ions as cationizing agents for MS/MS. The latter motivation follows from the role of ion type in MS/MS. Metal cationized species often show fragmentation that differs from the analogous protonated species. Disulfide-linked Au(I)-cationized peptides are cases in point: whereas protonated species tend not to show much disulfide bond cleavage, dissociation of the S–S bond is a dominant pathway for Au(I)-cationized species (39) (Figure 6).
Figure 6.
CID spectra of two disulfide linked peptides. (Top) [M+Au(I)]+. (Bottom) [M+H]+. Adapted from ref. 39.
Charge inversion
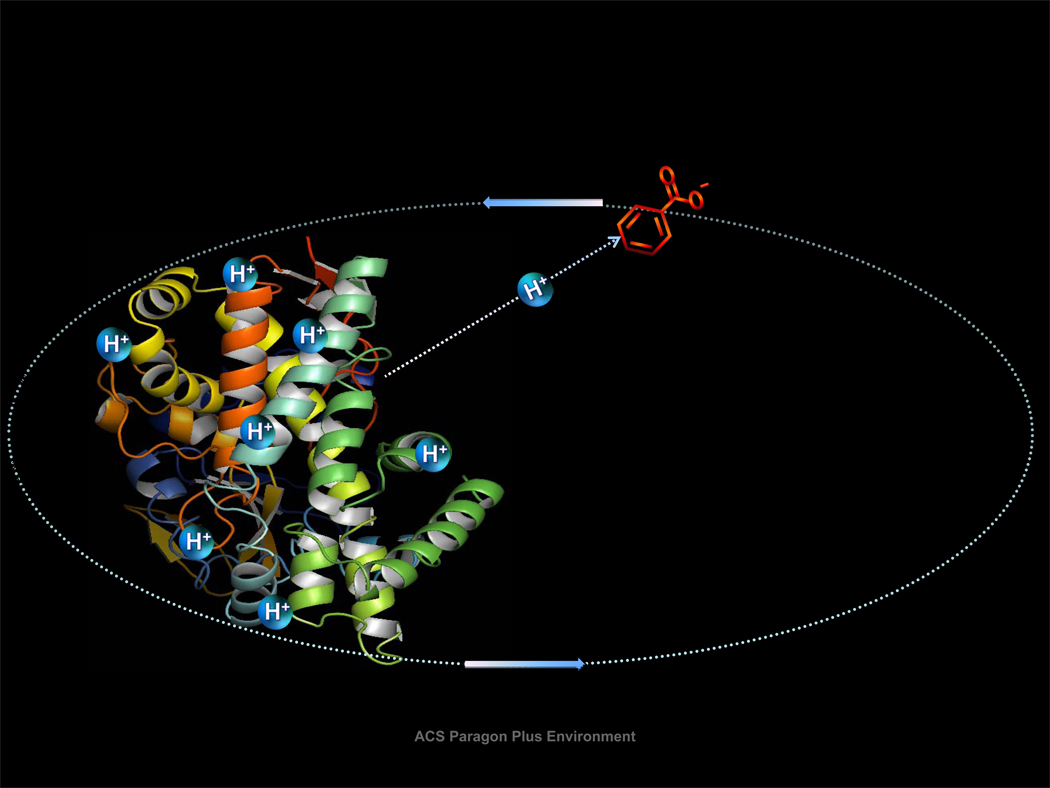
While most applications have been developed for multiply-charged analyte species, ion/ion reactions involving singly-charged analyte ions can also be useful, provided that the reagent ions are multiply charged and that the reaction leads to a reversal of the analyte charge. This so-called “charge inversion” process must form a relatively long-lived complex because it requires the transfer of at least two charges in a single ion/ion encounter. The transfer of a single charge via a single proton or electron transfer without formation of a long-lived complex neutralizes the analyte. Hence, charge inversion reagents with good efficiency tend to have large physical cross-sections.
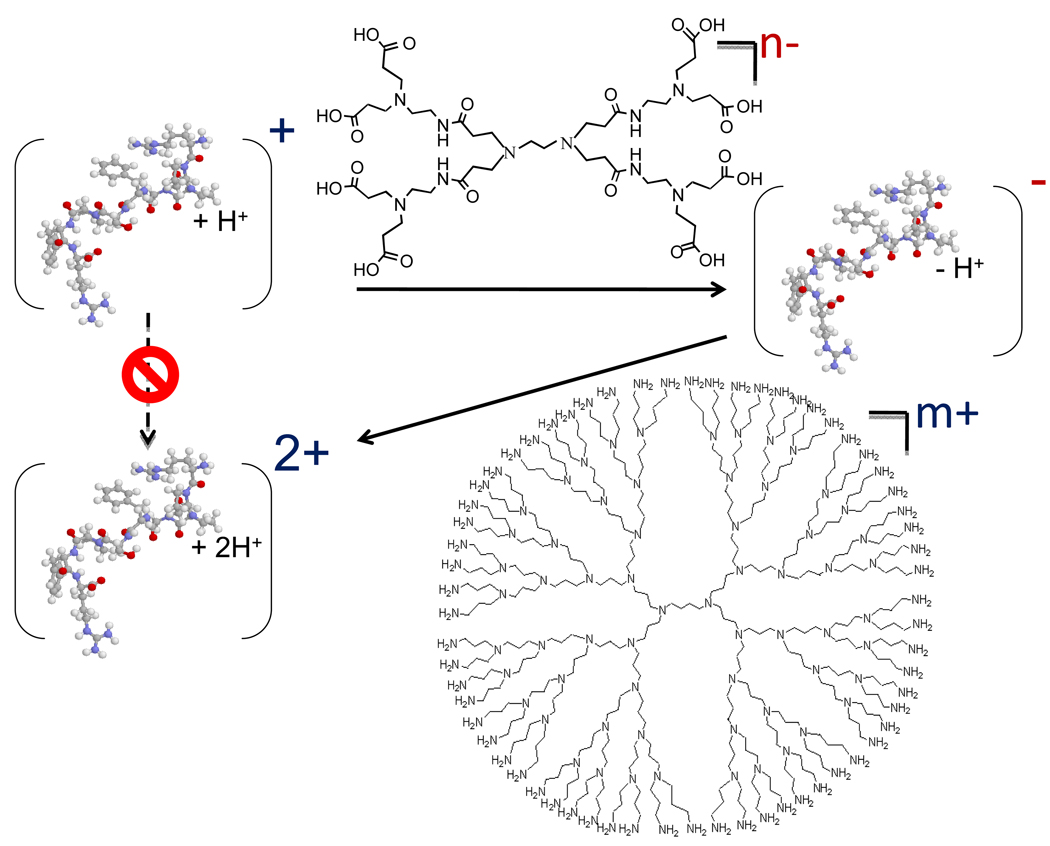
An example of an application of charge inversion is an increase in the charge of a singlycharged analyte ion (42). This strategy employs two charge inversion steps, as illustrated in Figure 7 for a singly-protonated peptide. The first step inverts the polarity of the ion by transforming the ion type from (M+H)+ to (M−H)− using a multiply-deprotonated reagent, such as a carboxylate-terminated dendrimer. The second step transforms the (M−H)− ion to (M+2H)2+ via reaction with a multiply-protonated reagent, such as an amine-terminated dendrimer. The net result is the transformation of (M+H)+ to (M+2H)2+. Overall efficiencies [i.e., the percentage of the initial (M+H)+ ion population converted to (M+2H)2+] of a few tens of percent have been measured (40). An analogous set of reactions has been shown to be effective for transforming (M−H)− to (M−2H)2− ions (43).
Figure 7.
Ion/ion reaction strategy for increasing the net charge state of an ion in the gas phase.
In another charge inversion application, a single step charge inversion reaction has been employed to transform the (M−H)− ion derived from a phosphopeptide present in a tryptic digest mixture to the (M+2H)2+ species for subsequent ETD (44). This process was enabling because no analyte ions derived from the phosphopeptide were observable in the positive ion mass spectrum of the mixture, thereby excluding the use of ETD for structural characterization. By transforming the anionic phosphopeptide to a doubly-protonated ion type, the ETD experiment became feasible and was able to locate the phosphorylation site. In yet another application, charge inversion reactions are being examined for use in screening for compound classes by exploiting the different tendencies for ions to undergo multiple charge transfers in a single collision. Species with both acidic and basic sites, for example, are significantly more likely to undergo efficient charge inversion than are species with only one such functionality.
Covalent bond formation
When the ionic reactants of an ion/ion reaction undergo a condensation reaction (i.e., they form a long-lived complex), a very wide range of chemical phenomena can take place, in principle. The transfer of a transition metal cation into a peptide, for example, almost surely leads to complexation of the metal by the peptide. Investigating the possibility for selective chemical reactions that lead to covalent bond formation is also of interest. This is a little-explored area in ion/ion chemistry, but the principle has been demonstrated recently. For example, the use of singly-deprotonated 4-formyl-1,3-benzenedisulfonic acid as a reagent anion leads to the formation of Schiff base adducts with primary amine groups (e.g., the N-terminus and the amine group of lysine) of multiply-protonated peptides (45). This observation opens up the possibility for other functional-group-specific reagents that can form bioconjugates in the gas phase and within the context of an MSn experiment. This possibility holds particular promise for enhancing structural characterization capabilities of MS/MS by providing the analyst with flexible means for modifying analyte ions without resorting to solution-based experiments. A few examples of gas-phase modification using ion/molecule chemistry have been described (46,47). However, the greater control over the reagent species in ion/ion reactions, along with the much wider range of accessible reagents (the need to vaporize the neutral reagent in ion/molecule chemistry severely limits the range of possible reagents), makes ion/ion chemistry particularly attractive as a means for gas-phase bioconjugate formation.
CONCLUSIONS AND PROGNOSIS
Gas-phase ion/ion reactions involving either multiply-charged analyte ions, multiply-charged reagent ions, or both exhibit all the characteristics of an analytically useful reaction. They can be highly efficient, fast, and informative and can be readily implemented in MS. They are particularly promising as means for converting an ion formed directly by an ionization technique into a wide array of other ion types that can generate additional information regarding an analyte of interest. Experiments conducted in electrodynamic ion traps capable of executing MSn procedures can employ multiple ion/ion reaction steps, possibly involving distinct reaction mechanisms (e.g., electron transfer followed by proton transfer). Given the vast array of ions that can be formed via today’s panoply of ionization methods, as well as our ability to manipulate ions, the potential dimensionality of ion/ion chemistry is enormous. The main barrier to use of ion/ion reactions for most chemists has been lack of access to appropriate instrumentation. This situation is changing quickly with the growing commercial availability of instruments capable of executing ion/ion reaction experiments along with software to support them. The main impetus for implementation on commercial instrument platforms has been the important ETD experiment. Proton transfer reactions are also accessible with these instruments. Many other applications for ion/ion reactions, a few of which were briefly illustrated here, will likely be developed in the coming years as this class of reactions becomes widely accepted in the analytical MS community.
Figure 8.
ACKNOWLEDGEMENTS
The authors acknowledge support in their laboratory from the Office of Basic Energy Sciences, Office of Sciences, U.S. Department of Energy under Award No. DE-FG02-00ER15105 for the study of fundamental aspects of ion/ion chemistry, the National Institutes of Health under Grant GM 45372 for supporting the development of instrumentation and applications related to the ion/ion chemistry of peptides and proteins, and the National Science Foundation under CHE-0808380 for our recent ion/ion reaction work with nucleic acids.
Biographies
Scott A. McLuckey is the John A. Leighty Distinguished Professor of Chemistry at Purdue University. His research career has been devoted to advancing the state-of-the-art in organic and biological MS. His laboratory currently emphasizes the exploration and development of ion/ion reactions in analytical MS.
Teng-Yi Huang is a senior graduate student pursuing the Ph.D. degree at Purdue University. His research is focused on the characterization of biopolymers, with particular emphasis on nucleic acids, via MS/MS using ion/ion reactions and a range of dissociation methods.
References
- 1.Cole RB, editor. Electrospray Ionization Mass Spectrometry, Fundamentals, Instrumentation, and Applications. New York: Wiley; 1997. [Google Scholar]
- 2.Xia Y, McLuckey SA. J. Am. Soc. Mass Spectrom. 2008;19:173–189. doi: 10.1016/j.jasms.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson JL, Jr., McLuckey SA. J. Am. Chem. Soc. 1996;118:7390–7397. [Google Scholar]
- 4.Thomson JJ. Philos. Mag. 1924;47:337–378. [Google Scholar]
- 5.McLuckey SA. In: Principles of Mass Spectrometry Applied to Biomolecules. Laskin J, Lifshitz C, editors. Hoboken, NJ: Wiley-Interscience; 2006. pp. 519–564. Chapter 14. [Google Scholar]
- 6.Ogorzalek Loo RR, Udseth HR, Smith RD. J. Am. Soc. Mass Spectrom. 1992;3:695–705. doi: 10.1016/1044-0305(92)87082-A. [DOI] [PubMed] [Google Scholar]
- 7.Scalf M, Westphall MS, Krause J, Kaufman SL, Smith LM. Science. 1999;283:194–197. doi: 10.1126/science.283.5399.194. [DOI] [PubMed] [Google Scholar]
- 8.Mather RE, Todd JF. J. Int. J. Mass Spectrom. Ion Processes. 1980;33:159–165. [Google Scholar]
- 9.Herron WJ, Goeringer DE, McLuckey SA. J. Am. Soc. Mass Spectrom. 1995;6:529–532. doi: 10.1016/1044-0305(95)00199-N. [DOI] [PubMed] [Google Scholar]
- 10.Hartmer R, Kaplan DA, Gebhardt CR, Lederheil T, Brekenfeld A. Int. J. Mass Spectrom. 2008;276:82–90. [Google Scholar]
- 11.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Chrisman PA, Erickson DE, Liu J, Liang X, Londry FA, Yang MJ, McLuckey SA. Anal. Chem. 2006;78:4146–4154. doi: 10.1021/ac0606296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAlister GC, Berggren WT, Griep-Raming J, Horning S, Makarov A, Phanstiel D, Stafford G, Swaney DL, Syka JEP, Zabrouska V, Coon JJ. J. Proteome Res. 2008;7:3127–3136. doi: 10.1021/pr800264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAlister GC, Phanstiel D, Good DM, Berggren WT, Coon JJ. Anal. Chem. 2007;79:3525–3543. doi: 10.1021/ac070020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DA, Hartmer R, Speir JP, Stoermer C, Gumerov D, Easterling ML, Brekenfeld A, Kim T, Laukien F, Park MA. Rapid Commun. Mass Spectrom. 2008;22:271–278. doi: 10.1002/rcm.3356. [DOI] [PubMed] [Google Scholar]
- 17.McLuckey SA, Stephenson JL., Jr. Mass Spectrom. Rev. 1998;17:369–407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Pitteri SJ, McLuckey SA. Mass Spectrom. Rev. 2005;24:931–958. doi: 10.1002/mas.20048. [DOI] [PubMed] [Google Scholar]
- 19.Scalf M, Westphall MS, Smith LM. Anal. Chem. 2000;72:52–60. doi: 10.1021/ac990878c. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson JL, Jr., McLuckey SA. J. Am. Soc. Mass Spectrom. 1998;9:585–596. doi: 10.1016/S1044-0305(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 21.McLuckey SA, Wu J, Bundy JL, Stephenson JL, Jr., Hurst GB. Anal. Chem. 2002;74:976–984. doi: 10.1021/ac011015y. [DOI] [PubMed] [Google Scholar]
- 22.Henry KD, McLafferty FW. Org. Mass Spectrom. 1990;25:490–492. [Google Scholar]
- 23.Stephenson JL, Jr., McLuckey SA. Anal. Chem. 1998;70:3533–3544. doi: 10.1021/ac9802832. [DOI] [PubMed] [Google Scholar]
- 24.Hogan JM, McLuckey SA. J. Mass Spectrom. 2003;38:245–256. doi: 10.1002/jms.458. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Huang T-Y, McLuckey SA. Anal. Chem. 2009;81:1433–1441. doi: 10.1021/ac802204j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coon JJ, Ueberheide B, Syka JEP, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. USA. 2005;102:9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelleher NL. Anal. Chem. 2004;76:196A–203A. [PubMed] [Google Scholar]
- 28.McLuckey SA, Reid GE, Wells JM. Anal. Chem. 2002;74:336–346. doi: 10.1021/ac0109671. [DOI] [PubMed] [Google Scholar]
- 29.Reid GE, Wells JM, Badman ER, McLuckey SA. Int. J. Mass Spectrom. 2003;222:243–258. [Google Scholar]
- 30.Reid GE, Shang H, Hogan JM, Lee GU, McLuckey SA. J. Am. Chem. Soc. 2002;124:7353–7362. doi: 10.1021/ja025966k. [DOI] [PubMed] [Google Scholar]
- 31.Chanthamontri C, Liu J, McLuckey SA. Int. J. Mass Spectrom. 2009;283:9–16. doi: 10.1016/j.ijms.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrisman PA, Pitteri SJ, McLuckey SA. Anal. Chem. 2005;77:3411–3414. doi: 10.1021/ac0503613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]
- 34.Coon JJ. Anal. Chem. 2009;81:3208–3215. doi: 10.1021/ac802330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Anal. Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- 36.Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. J. Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turecek F. J. Am. Chem. Soc. 2003;125:5954–5963. doi: 10.1021/ja021323t. [DOI] [PubMed] [Google Scholar]
- 38.Newton KA, Amunugama R, McLuckey SA. J. Phys. Chem. A. 2005;109:3608–3616. doi: 10.1021/jp04416i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gunawardena HP, O’Hair RAJ, McLuckey SA. J. Proteome Res. 2006;5:2087–2092. doi: 10.1021/pr0602794. [DOI] [PubMed] [Google Scholar]
- 40.Barlow CK, Hodges BDM, Xia Y, O'Hair RAJ, McLuckey SA. J. Am. Soc. Mass Spectrom. 2008;19:281–293. doi: 10.1016/j.jasms.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Crizer DM, Xia Y, McLuckey SA. J. Am. Soc. Mass Spectrom. doi: 10.1016/j.jasms.2009.05.008. DOI 10.1016/j.jasms.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 42.He M, McLuckey SA. J. Am. Chem. Soc. 2003;125:7756–7757. doi: 10.1021/ja0354521. [DOI] [PubMed] [Google Scholar]
- 43.He M, McLuckey SA. Anal. Chem. 2004;76:4189–4192. doi: 10.1021/ac496087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunawardena HP, Emory JF, McLuckey SA. Anal. Chem. 2006;78:3788–3793. doi: 10.1021/ac060164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han H, McLuckey SA. J. Am. Chem. Soc. DOI 10.1021/ja904812d. [Google Scholar]
- 46.Gur EH, de Koning LJ, Nibbering NMM. Int. J. Mass Spectrom. Ion Processes. 1997;167/168:135–147. [Google Scholar]
- 47.O'Hair RAJ, Reid GE. J. Am. Soc. Mass Spectrom. 2000;11:244–256. doi: 10.1016/S1044-0305(99)00142-7. [DOI] [PubMed] [Google Scholar]