Abstract
2,3,7,8,-Tetrachlorodibenzo-p-dioxin (TCDD) administered in vivo causes drastic reduction in the weight of the mouse thymus at low doses (e.g., 30 micrograms/kg single i.p. injection), the reduction becoming statistically significant after 2 days. To understand the cause for such thymic involution TCDD-evoked changes in various biochemical parameters in this tissue were examined. The most noticeable change was observed in the increased activity of specific protein-tyrosine kinases and protein kinase C and an increased level of p21ras-associated binding of [3H]GTP. Since no significant change was observed with cAMP-stimulated protein kinases and cAMP levels, the above changes appear to be a selective effect on these special classes of proteins. As a result of a time sequence study it has become apparent that the rise in protein-tyrosine kinase activities becomes significant within 24 hr, whereas the rise in protein kinase C does not become significant until 48 hr. Among protein-tyrosine kinases, pp60c-src and probably pp561skT were found to be significantly elevated by TCDD treatment. In view of similarities between TCDD and thyroid hormones in causing thymic involution, the levels of c-erb-A expression were assessed in the liver by using avian 32P-labeled v-erb-A probe and RNA transfer blot hybridization technique. The results clearly indicate that TCDD has the property to elevate levels of mRNA bearing homology to v-erb-A. Such changes in c-erb-A expression and protein-tyrosine kinase occurred only in TCDD-susceptible (responsive) strains but not in tolerant (nonresponsive) strains of mice at the dose tested. Based on such observations a hypothesis has been proposed that TCDD owes its potency to its ability to stimulate the expression of one of a family of DNAs bearing homology to v-erb-A and that one of the major consequences of such an action is stimulation of various tyrosine kinases.
Full text
PDF
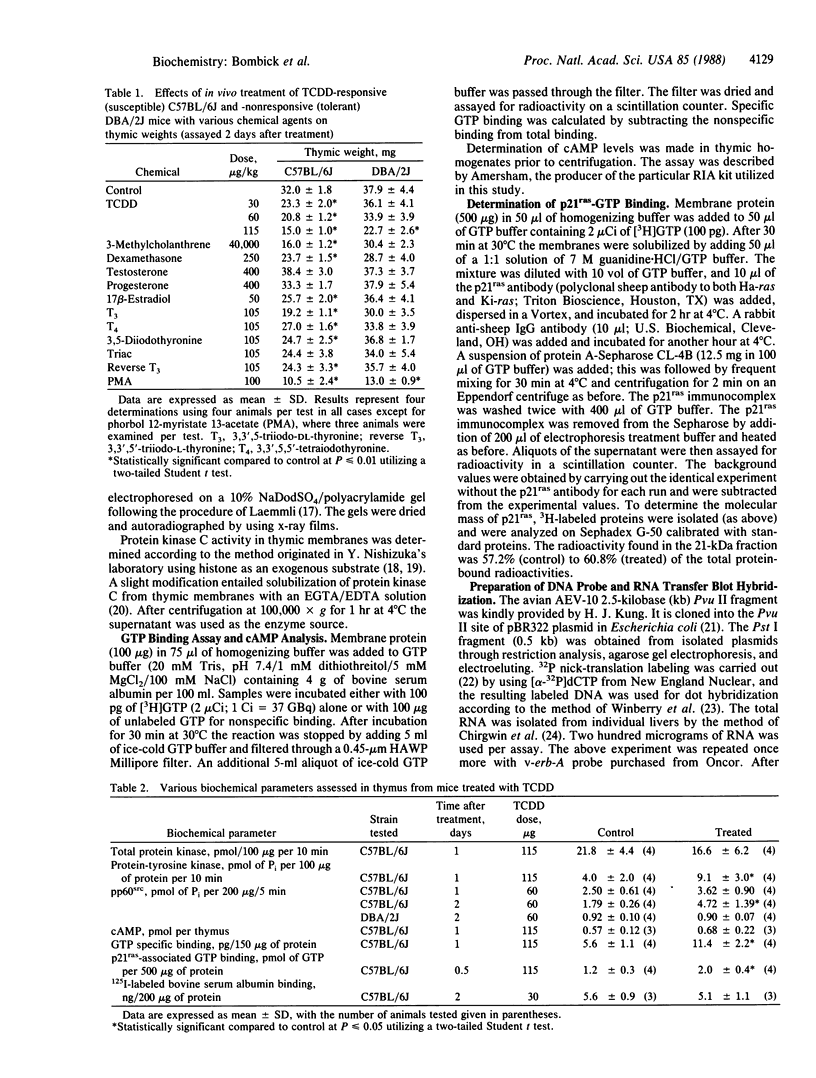
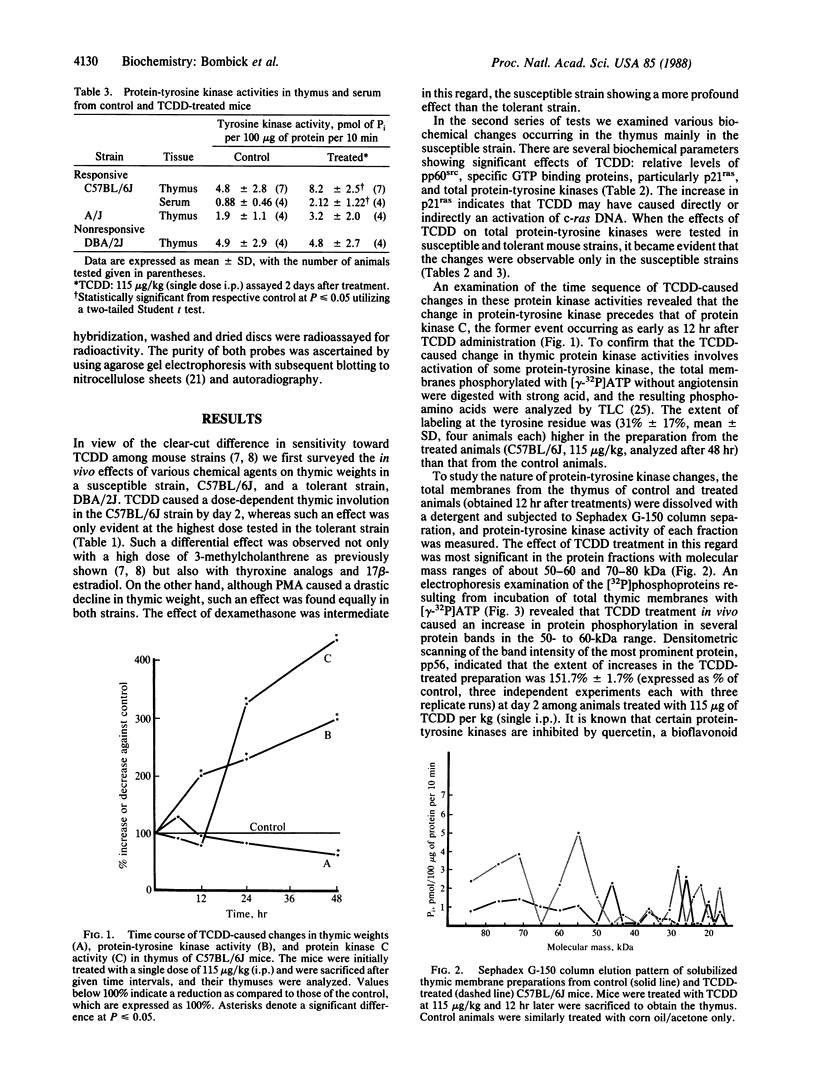
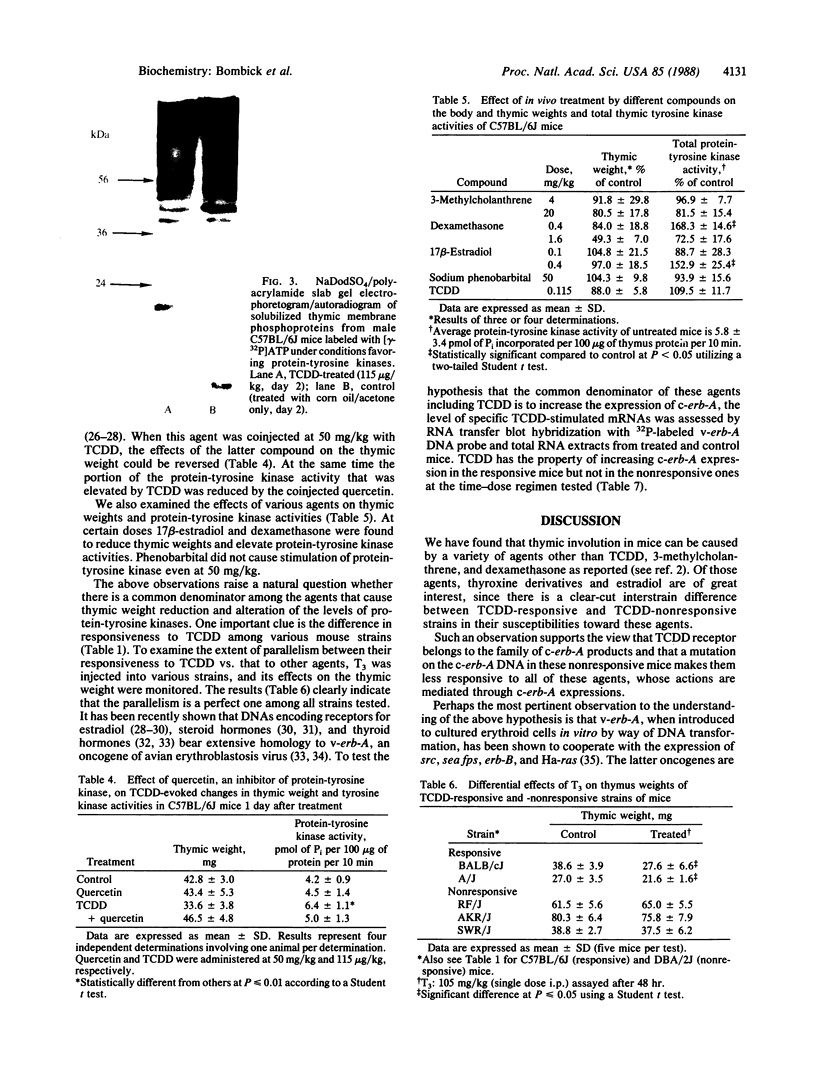

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Oncogenes. Tricks with tyrosine kinases. 1986 Feb 27-Mar 5Nature. 319(6056):722–723. doi: 10.1038/319722a0. [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Madhukar B. V., Brewster D. W., Matsumura F. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes increases in protein kinases particularly protein kinase C in the hepatic plasma membrane of the rat and the guinea pig. Biochem Biophys Res Commun. 1985 Feb 28;127(1):296–302. doi: 10.1016/s0006-291x(85)80158-3. [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes elevation of the levels of the protein tyrosine kinase pp60c-src. J Biochem Toxicol. 1987 Summer;2:141–154. doi: 10.1002/jbt.2570020207. [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Matsumura F., Madhukar B. V. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in the low density lipoprotein (LDL) receptor activities in the hepatic plasma membrane of the guinea pig and rat. Biochem Biophys Res Commun. 1984 Jan 30;118(2):548–554. doi: 10.1016/0006-291x(84)91337-8. [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Matsumura F. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes an increase in protein tyrosine kinase activities at an early stage of poisoning in vivo in rat hepatocyte membranes. Life Sci. 1987 Jul 27;41(4):429–436. doi: 10.1016/0024-3205(87)90218-9. [DOI] [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma cell line expressing elevated levels of tyrosine protein kinase activity. J Biol Chem. 1983 Sep 10;258(17):10738–10742. [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Hellstrom K. E., Krebs E. G. A lymphoma protein with an in vitro site of tyrosine phosphorylation homologous to that in pp60src. J Biol Chem. 1982 Dec 10;257(23):13877–13879. [PubMed] [Google Scholar]
- Casnellie J. E., Harrison M. L., Pike L. J., Hellström K. E., Krebs E. G. Phosphorylation of synthetic peptides by a tyrosine protein kinase from the particulate fraction of a lymphoma cell line. Proc Natl Acad Sci U S A. 1982 Jan;79(2):282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely O. M., Sullivan W. P., Toft D. O., Birnbaumer M., Cook R. G., Maxwell B. L., Zarucki-Schulz T., Greene G. L., Schrader W. T., O'Malley B. W. Molecular cloning of the chicken progesterone receptor. Science. 1986 Aug 15;233(4765):767–770. doi: 10.1126/science.2426779. [DOI] [PubMed] [Google Scholar]
- Earp H. S., Austin K. S., Buessow S. C., Dy R., Gillespie G. Y. Membranes from T and B lymphocytes have different patterns of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2347–2351. doi: 10.1073/pnas.81.8.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Graziani Y., Erikson E., Erikson R. L. The effect of quercetin on the phosphorylation activity of the Rous sarcoma virus transforming gene product in vitro and in vivo. Eur J Biochem. 1983 Oct 3;135(3):583–589. doi: 10.1111/j.1432-1033.1983.tb07692.x. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Jackson T. The ras gene. Transformer and transducer. Nature. 1987 Aug 20;328(6132):668–669. doi: 10.1038/328668a0. [DOI] [PubMed] [Google Scholar]
- Hayden L. J., Severson D. L. Correlation of membrane phosphorylation and epidermal growth factor binding to hepatic membranes isolated from triiodothyronine-treated rats. Biochim Biophys Acta. 1983 May 5;730(2):226–230. doi: 10.1016/0005-2736(83)90337-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy J., Teuerstein I., Marbach M., Radian S., Sharoni Y. Tyrosine protein kinase activity in the DMBA-induced rat mammary tumor: inhibition by quercetin. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1227–1233. doi: 10.1016/s0006-291x(84)80264-8. [DOI] [PubMed] [Google Scholar]
- Madhukar B. V., Brewster D. W., Matsumura F. Effects of in vivo-administered 2,3,7,8-tetrachlorodibenzo-p-dioxin on receptor binding of epidermal growth factor in the hepatic plasma membrane of rat, guinea pig, mouse, and hamster. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7407–7411. doi: 10.1073/pnas.81.23.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Brewster D. W., Madhukar B. V., Bombick D. W. Alteration of rat hepatic plasma membrane functions by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Arch Environ Contam Toxicol. 1984 Sep;13(5):509–515. doi: 10.1007/BF01056330. [DOI] [PubMed] [Google Scholar]
- Mukku V. R. Regulation of epidermal growth factor receptor levels by thyroid hormone. J Biol Chem. 1984 May 25;259(10):6543–6547. [PubMed] [Google Scholar]
- Neal R. A., Beatty P. W., Gasiewicz T. A. Studies of the mechanisms of toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Ann N Y Acad Sci. 1979 May 31;320:204–213. doi: 10.1111/j.1749-6632.1979.tb56602.x. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin: segregation of toxocity with the Ah locus. Mol Pharmacol. 1980 Jan;17(1):86–94. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Dasgupta J. D., Garbers D. L. Tyrosine protein kinase activity of rat spleen and other tissues. J Biol Chem. 1983 Sep 10;258(17):10341–10347. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Voronova A. F., Buss J. E., Patschinsky T., Hunter T., Sefton B. M. Characterization of the protein apparently responsible for the elevated tyrosine protein kinase activity in LSTRA cells. Mol Cell Biol. 1984 Dec;4(12):2705–2713. doi: 10.1128/mcb.4.12.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A. Suppression of cellular immunity in rats and mice by maternal treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int Arch Allergy Appl Immunol. 1974;47(5):777–794. doi: 10.1159/000231268. [DOI] [PubMed] [Google Scholar]
- Vos J. G., Moore J. A., Zinkl J. G. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the immune system of laboratory animals. Environ Health Perspect. 1973 Sep;5:149–162. doi: 10.1289/ehp.7305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Domain structure of human glucocorticoid receptor and its relationship to the v-erb-A oncogene product. Nature. 1985 Dec 19;318(6047):670–672. doi: 10.1038/318670a0. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Winberry L., Priehs C., Friderici K., Thompson M., Fluck M. Expression of proto-oncogenes in normal and papovavirus-transformed or -infected rat fibroblasts. Virology. 1985 Nov;147(1):154–168. doi: 10.1016/0042-6822(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. In vitro phosphorylation of angiotensin analogs by tyrosyl protein kinases. J Biol Chem. 1983 Jan 25;258(2):1022–1025. [PubMed] [Google Scholar]