Abstract
A poly-beta-hydroxybutyrate complex extracted from the plasma membranes of genetically competent Escherichia coli contained polyhydroxybutyrate:polyphosphate:calcium in molar ratios approximating 1:1:0.5. The chain length of the polyhydroxybutyrate was estimated as 120-200 subunits, and that of the polyphosphate was estimated as 130-170 subunits. The extracted complex, when incorporated into liposomes, exhibited a lipid phase transition in the same temperature range as that of the membrane complex in whole cells as well as the same properties of irreversibility, lability, and sensitivity to chelating buffers. Space-filling molecular models and molecular energy minimization methods (Charmm) were used to develop and evaluate a plausible structure for the complex. It is proposed that the polyhydroxybutyrate forms an exolipophilic-endopolarophilic helix around an inner framework helix of calcium polyphosphate. The calcium ions link the two polymers by forming ionic bonds with phosphoryl oxygens of the polyphosphate and ion-dipole bonds with the ester carbonyl oxygens of the polyhydroxybutyrate. This symmetrical structure forms a channel through the membrane and may play a role in the transport of calcium, phosphate, and DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brass J. M., Ehmann U., Bukau B. Reconstitution of maltose transport in Escherichia coli: conditions affecting import of maltose-binding protein into the periplasm of calcium-treated cells. J Bacteriol. 1983 Jul;155(1):97–106. doi: 10.1128/jb.155.1.97-106.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Brass J. M., Boos W. Ca2+-induced permeabilization of the Escherichia coli outer membrane: comparison of transformation and reconstitution of binding-protein-dependent transport. J Bacteriol. 1985 Jul;163(1):61–68. doi: 10.1128/jb.163.1.61-68.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornibert J., Marchessault R. H. Physical properties of poly- -hydroxybutyrate. IV. Conformational analysis and crystalline structure. J Mol Biol. 1972 Nov 28;71(3):735–756. doi: 10.1016/s0022-2836(72)80035-4. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., KORNBERG S. R., SIMMS E. S. Metaphosphate synthesis by an enzyme from Escherichia coli. Biochim Biophys Acta. 1956 Apr;20(1):215–227. doi: 10.1016/0006-3002(56)90280-3. [DOI] [PubMed] [Google Scholar]
- Kulaev I. S., Vagabov V. M. Polyphosphate metabolism in micro-organisms. Adv Microb Physiol. 1983;24:83–171. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDGREN D. G., ALPER R., SCHNAITMAN C., MARCHESSAULT R. H. CHARACTERIZATION OF POLY-BETA-HYDROXYBUTYRATE EXTRACTED FROM DIFFERENT BACTERIA. J Bacteriol. 1965 Jan;89:245–251. doi: 10.1128/jb.89.1.245-251.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N., Hiske T. W., Sadoff H. L. Poly-beta-hydroxybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986 Nov;168(2):553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R. N., Sadoff H. L. D-(-)-poly-beta-hydroxybutyrate in membranes of genetically competent bacteria. J Bacteriol. 1983 Nov;156(2):778–788. doi: 10.1128/jb.156.2.778-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch R., Hiske T., Sadoff H., Harris R., Beveridge T. Cellular incorporation of poly-beta-hydroxybutyrate into plasma membranes of Escherichia coli and Azotobacter vinelandii alters native membrane structure. Can J Microbiol. 1987 May;33(5):435–444. doi: 10.1139/m87-073. [DOI] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
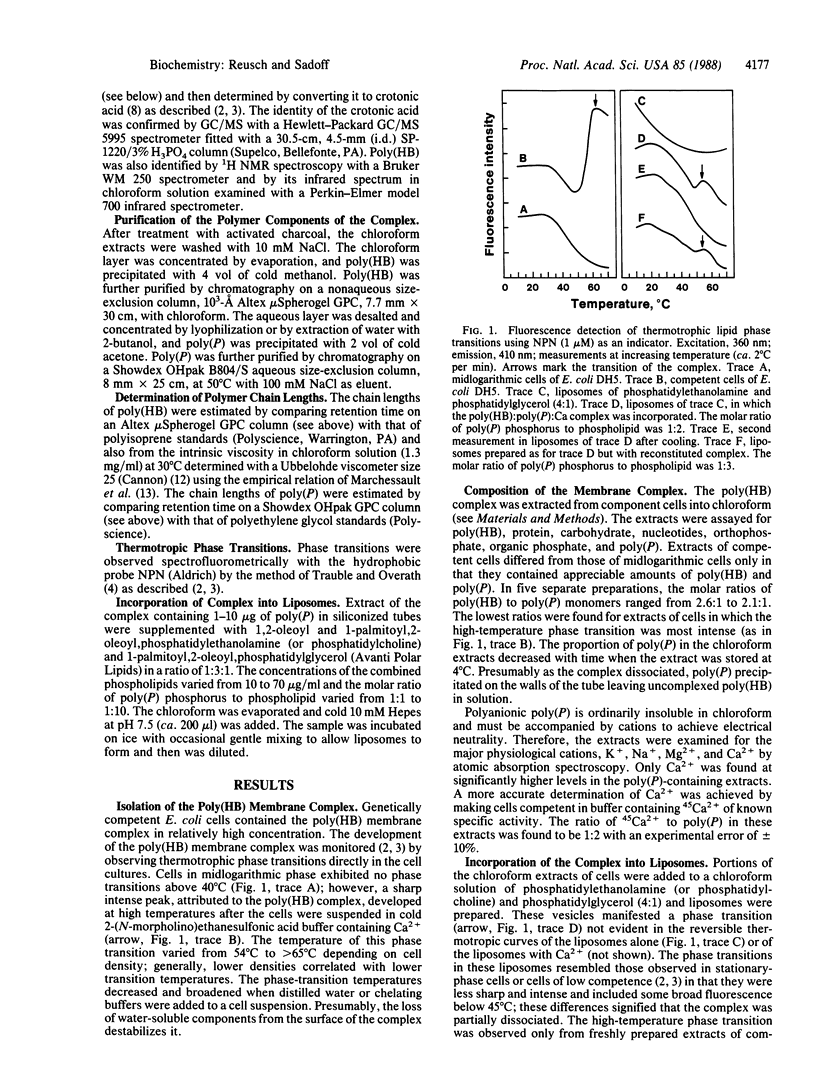
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Basic aspects of calcium chemistry and membrane interaction: on the messenger role of calcium. Ann N Y Acad Sci. 1978 Apr 28;307:3–27. doi: 10.1111/j.1749-6632.1978.tb41933.x. [DOI] [PubMed] [Google Scholar]