Abstract
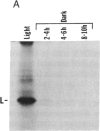
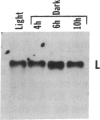
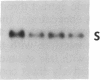
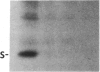
When light-grown seedlings of amaranth are transferred to total darkness, synthesis of the large subunit (LS) and small subunit (SS) of ribulose-1,5-bisphosphate carboxylase [RbuP2Case; 3-phospho-D-glycerate carboxylase (dimerizing), EC 4.1.1.39] is rapidly depressed. This reduction in RbuP2Case synthesis occurs in the absence of any corresponding changes in levels of functional mRNA for either subunit. Four hours after light-to-dark transition little, if any, changes in the distribution of LS and SS mRNAs on polysomes could be detected. The association of these mRNAs with polysomes was authenticated by treatment with RNase A or puromycin. Furthermore, polysomes were able to synthesize LS and SS precursor in cell-free translation systems supplemented with inhibitors of initiation. Therefore, during a light-to-dark transition LS and SS mRNAs remained bound to polysomes but were not translated in vivo, suggesting that control is exercised, in part, at the translational elongation step.
Keywords: light regulation, sucrose-gradient analysis, dot blot analysis, in vitro polysome run-off analysis, translational elongation arrest
Full text
PDF
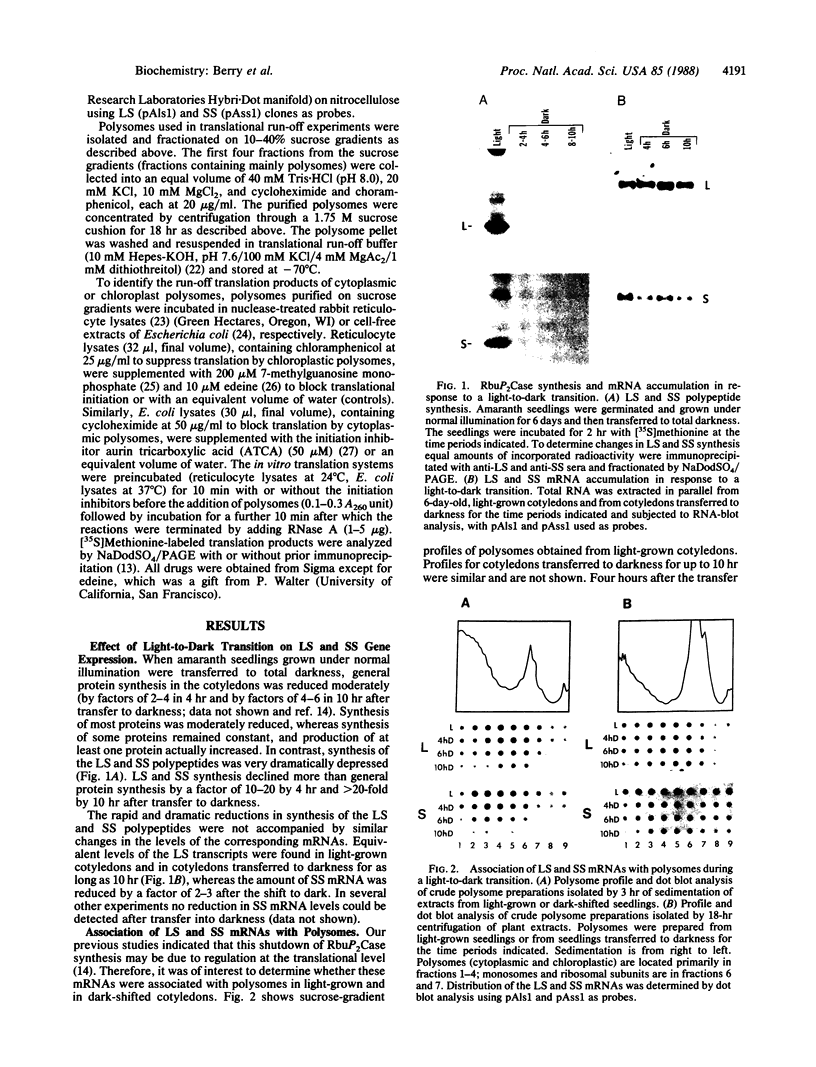


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Transcriptional and post-transcriptional regulation of ribulose 1,5-bisphosphate carboxylase gene expression in light- and dark-grown amaranth cotyledons. Mol Cell Biol. 1985 Sep;5(9):2238–2246. doi: 10.1128/mcb.5.9.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz E., Ekiel I., Lassota P., Tahara S. M. Inhibition of eukaryotic translation by analogues of messenger RNA 5'-cap: chemical and biological consequences of 5'-phosphate modifications of 7-methylguanosine 5'-monophosphate. Biochemistry. 1987 Jul 14;26(14):4372–4380. doi: 10.1021/bi00388a028. [DOI] [PubMed] [Google Scholar]
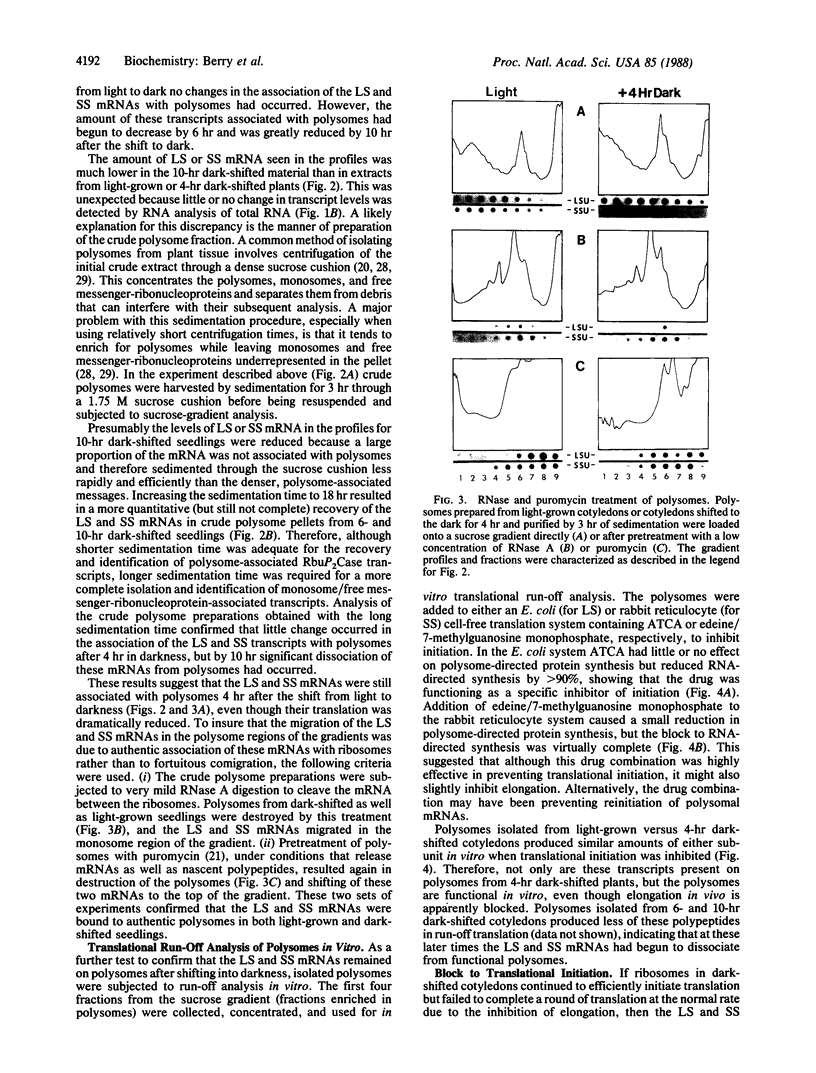
- Ferreira R. B., Davies D. D. Protein degradation in lemna with particular reference to ribulose bisphosphate carboxylase: I. The effect of light and dark. Plant Physiol. 1987 Apr;83(4):869–877. doi: 10.1104/pp.83.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A. P., Stewart M. L. Inhibition of the attachment of messenger ribonucleic acid to ribosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):719–725. doi: 10.1073/pnas.61.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanij V., Chua N. H., Siekevitz P. Synthesis and turnover of ribulose biphosphate carboxylase and of its subunits during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1975 Mar;64(3):572–585. doi: 10.1083/jcb.64.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze M. G., DeCorato D., Krug R. M. Cellular mRNA translation is blocked at both initiation and elongation after infection by influenza virus or adenovirus. J Virol. 1986 Dec;60(3):1027–1039. doi: 10.1128/jvi.60.3.1027-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Migration of 40 S ribosomal subunits on messenger RNA in the presence of edeine. J Biol Chem. 1978 Sep 25;253(18):6568–6577. [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Dyer J. A. Caution in the interpretation of plant ribosome studies. Biochem J. 1974 Oct;144(1):165–167. doi: 10.1042/bj1440165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Miziorko H. M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry. 1980 Nov 11;19(23):5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H. Ribulosebisphosphate carboxylase: amino acid sequence of a peptide bearing the activator carbon dioxide. Biochemistry. 1981 Mar 3;20(5):1236–1240. doi: 10.1021/bi00508a028. [DOI] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Nikolau B. J., Klessig D. F. Coordinate, Organ-Specific and Developmental Regulation of Ribulose 1,5-Bisphosphate Carboxylase Gene Expression in Amaranthus hypochondriacus. Plant Physiol. 1987 Sep;85(1):167–173. doi: 10.1104/pp.85.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Salvucci M. E., Ogren W. L. Activation of Ribulosebisphosphate Carboxylase/Oxygenase at Physiological CO(2) and Ribulosebisphosphate Concentrations by Rubisco Activase. Plant Physiol. 1986 Dec;82(4):967–971. doi: 10.1104/pp.82.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. Alterations of transcription and translation in HeLa cells exposed to amino acid analogs. Mol Cell Biol. 1984 Jun;4(6):1063–1072. doi: 10.1128/mcb.4.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]