Abstract
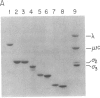
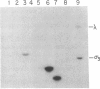
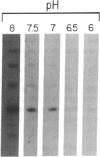
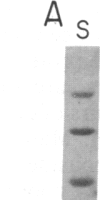
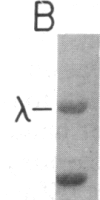
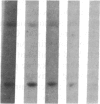
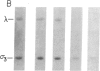
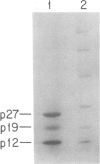
We have characterized a simple method that uses 65ZnCl2 to detect zinc-binding proteins that have been immobilized on nitrocellulose. Conditions have been identified that permit the detection of as little as 1 microgram of some zinc-binding proteins. The specificity of the binding is indicated by the ability of other divalent metal ions to compete with 65Zn(II) in this assay. We have used this technique to provide evidence that the nucleic acid-binding gag protein of retroviruses also binds zinc. This technique can be applied to biological mixtures of proteins and may be used in proteolytic mapping studies to identify protein fragments that have zinc-binding activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E., Gettins P. Alkaline phosphatase, solution structure, and mechanism. Adv Enzymol Relat Areas Mol Biol. 1983;55:381–452. doi: 10.1002/9780470123010.ch5. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Biochemical studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):161–170. doi: 10.1099/0022-1317-63-1-161. [DOI] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedroc D. P., Keating K. M., Williams K. R., Konigsberg W. H., Coleman J. E. Gene 32 protein, the single-stranded DNA binding protein from bacteriophage T4, is a zinc metalloprotein. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8452–8456. doi: 10.1073/pnas.83.22.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Houts G. E., Miyagi M., Ellis C., Beard D., Beard J. W. Reverse transcriptase from avian myeloblastosis virus. J Virol. 1979 Feb;29(2):517–522. doi: 10.1128/jvi.29.2.517-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huismans H., Joklik W. K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976 Apr;70(2):411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. The role of zinc in alcohol dehydrogenase. V. The effect of metal-binding agents on thestructure of the yeast alcohol dehydrogenase molecule. J Biol Chem. 1960 Nov;235:3188–3192. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Quiocho F. A., Reeke G. N., Jr The structure of carboxypeptidase A. IX. The x-ray diffraction results in the light of the chemical sequence. Proc Natl Acad Sci U S A. 1969 Sep;64(1):28–35. doi: 10.1073/pnas.64.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Mikawa T., Ebashi S. Detection of calcium binding proteins by 45Ca autoradiography on nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem. 1984 Feb;95(2):511–519. doi: 10.1093/oxfordjournals.jbchem.a134633. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblas B., Boyd N. D., Singer R. H. Analysis of receptor-ligand interactions using nitrocellulose gel transfer: application to Torpedo acetylcholine receptor and alpha-bungarotoxin. Anal Biochem. 1983 Apr 1;130(1):1–8. doi: 10.1016/0003-2697(83)90641-3. [DOI] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- Vincent A. TFIIIA and homologous genes. The 'finger' proteins. Nucleic Acids Res. 1986 Jun 11;14(11):4385–4391. doi: 10.1093/nar/14.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff H. W., Handschumacher M., Murthy H. M., Sowadski J. M. The three dimensional structure of alkaline phosphatase from E. coli. Adv Enzymol Relat Areas Mol Biol. 1983;55:453–480. doi: 10.1002/9780470123010.ch6. [DOI] [PubMed] [Google Scholar]