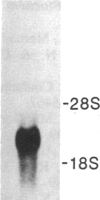
Abstract
Follistatin is a single-chain gonadal protein that specifically inhibits follicle-stimulating hormone release. By use of the recently characterized porcine follistatin cDNA as a probe to screen a human testis cDNA library and a genomic library, the structure of the complete human follistatin precursor as well as its genomic organization have been determined. Three of eight cDNA clones that were sequenced predicted a precursor with 344 amino acids, whereas the remaining five cDNA clones encoded a 317 amino acid precursor, resulting from alternative splicing of the precursor mRNA. Mature follistatins contain four contiguous domains that are encoded by precisely separated exons; three of the domains are highly similar to each other, as well as to human epidermal growth factor and human pancreatic secretory trypsin inhibitor. The genomic organization of the human follistatin is similar to that of the human epidermal growth factor gene and thus supports the notion of exon shuffling during evolution.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelt D. C., Shapanka R., Greene L. J. The primary structure of the human pancreatic secretory trypsin inhibitor. Amino acid sequence of the reduced S-aminoethylated protein. Arch Biochem Biophys. 1977 Feb;179(1):189–199. doi: 10.1016/0003-9861(77)90103-5. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Shimasaki S., Mercado M., Cooksey K., Ling N., Ying S., Ueno N., Guillemin R. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Mol Endocrinol. 1987 Nov;1(11):849–855. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- Eto Y., Tsuji T., Takezawa M., Takano S., Yokogawa Y., Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Miyamoto K., Hasegawa Y., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of bovine follicular fluid inhibin of about 32 kDa. Mol Cell Endocrinol. 1986 Jan;44(1):55–60. doi: 10.1016/0303-7207(86)90105-x. [DOI] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Esch F., Denoroy L., Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7217–7221. doi: 10.1073/pnas.82.21.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. A homodimer of the beta-subunits of inhibin A stimulates the secretion of pituitary follicle stimulating hormone. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1129–1137. doi: 10.1016/s0006-291x(86)80400-4. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986 Jun 19;321(6072):779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Ling N., Esch F., Ueno N., Ying S. Y., Guillemin R., Niall H., Seeburg P. H. Complementary DNA sequences of ovarian follicular fluid inhibin show precursor structure and homology with transforming growth factor-beta. Nature. 1985 Dec 19;318(6047):659–663. doi: 10.1038/318659a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Rivier J., Spiess J., McClintock R., Vaughan J., Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985 Nov 27;133(1):120–127. doi: 10.1016/0006-291x(85)91849-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Klein R., de Vos F. L., McLachlan R. I., Wettenhall R. E., Hearn M. T., Burger H. G., de Kretser D. M. The isolation of polypeptides with FSH suppressing activity from bovine follicular fluid which are structurally different to inhibin. Biochem Biophys Res Commun. 1987 Dec 16;149(2):744–749. doi: 10.1016/0006-291x(87)90430-x. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., de Vos F. L., Foulds L. M., McLachlan R. I., Burger H. G., Morgan F. J., Hearn M. T., de Kretser D. M. Isolation of a 31 kDa form of inhibin from bovine follicular fluid. Mol Cell Endocrinol. 1986 Mar;44(3):271–277. doi: 10.1016/0303-7207(86)90133-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Ling N., Ying S. Y., Esch F., Shimasaki S., Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35,000 monomeric protein that inhibits the release of follicle-stimulating hormone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakamura Y., Nishide J., Emi M., Ogawa M., Mori T., Matsubara K. Molecular cloning and nucleotide sequence of human pancreatic secretory trypsin inhibitor (PSTI) cDNA. Biochem Biophys Res Commun. 1985 Oct 30;132(2):605–612. doi: 10.1016/0006-291x(85)91176-3. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Swanson G., Tan P., Ling N., Esch F., Ueno N., Shimasaki S., Guillemin R. Follistatin specifically inhibits pituitary follicle stimulating hormone release in vitro. Biochem Biophys Res Commun. 1987 Nov 30;149(1):133–139. doi: 10.1016/0006-291x(87)91614-7. [DOI] [PubMed] [Google Scholar]