Abstract
Mothers of children with developmental delays may experience poorer psychological well-being than other mothers; however, little research has examined how delayed development in children might predict mothers’ perceived physical well-being. Children with delayed development manifest heightened behavior problems, which may negatively affect maternal well-being. We examined the associations between developmental delay and behavior problems at child age 3 and mothers’ self-perceived physical health at child ages 3, 4, and 5, in families of 218 children with and without developmental delays. The study sample comprised 218 families from central Pennsylvania and Southern California, USA who were recruited through community agencies that provide diagnostic and intervention services for individuals with development difficulties. We found that mothers of children with delayed development at age 3 reported poorer concurrent and later physical health than mothers of children with typical development. Broadening the analyses to include not only child development status (delayed development versus typical development) but also child behavior problems at age 3, only child behavior problems and the interaction of development status and behavior problems, but not development status itself, predicted maternal health. Early child behavior problems contributed to later maternal health above and beyond early maternal health, suggesting a possible causal association between child behavior problems and mothers’ physical health. The relation between child behavior problems and maternal health was moderated by mothers’ parenting stress and mediated by depressive symptoms. Mothers of children with both delayed development and high behavior problems are a particular risk group that may be especially in need of early intervention. Further research should examine the behavioral and biological pathways by which these child-related stressors influence mothers’ physical health.
Keywords: USA, Mothers, Developmental delay, Behavior problems, Maternal well-being, Physical health, Preschool-age children
Extensive research has shown that mothers of children with developmental delays experience poorer mental health compared to mothers of children with typical development (e.g. Baker, Blacher, Kopp, & Kraemer, 1997). Further, children with developmental delays experience higher rates of behavior problems than typically developing children, which are also tied to poorer maternal well-being (Pearson et al., 2000). However, little is known about the impact of raising a child with delayed development on maternal physical health. Research on chronic stress suggests that, over time, the stressors these mothers face may negatively affect their physical well-being (Taylor, Repetti, & Seeman, 1997). The current study examines how child developmental delay, behavior problems, and maternal well-being indicators (parenting stress and depressive symptoms) interact to predict mothers’ self-perceived physical health across child ages 3 through 5 years.
Psychological well-being among mothers of children with delayed development
While most studies of mothers’ psychological well-being have linked direct caring for a child with delayed development with greater parenting stress and depressive symptoms (Hauser-Cram, Warfield, Shonkoff, & Krauss, 2001; Olsson & Hwang, 2001), there is also evidence for other challenges to well-being, including difficulty finding reliable child care, disruption of family plans, financial burden, and restrictions on social or employment activities (Baker et al., 1997; Brandon, 2007). In addition to these negative outcomes, parents may also report fewer positive outcomes (Veisson, 1999).
Interestingly, the heightened distress experienced by parents of children with delayed development appears to be more attributable to child behavior problems than to the presence of cognitive delays per se (Baker, Blacher, Crnic, & Edelbrock, 2002; Baker et al., 2003; Floyd & Phillippe, 1993). Individuals with intellectual disability are at heightened risk for developing mental disorder, and in children this risk is often manifested as heightened behavior problems. Children with delayed development are three to four times as likely to have a psychological disorder as children without delayed development (e.g. Dekker, Koot, van der Ende, & Verhulst, 2002; Emerson, 2003a).
These increased rates of psychopathology are evident at an early age. Baker et al. (2002, 2003), examining 3-year-olds in the present sample, found odds ratios of greater than 3:1 for clinical level behavior problems in children with delayed development versus children with typical development, and found behavior problems to be highly related to parents’ stress and depressive symptoms. Moreover, parents of school-age children with delayed development spend more time issuing commands and working to gain compliance, and they experience more behavior management struggles and coercive parent-child interactions than other parents (Floyd & Phillippe, 1993). Thus, the thrust of recent studies has been that although raising children with delayed development can be particularly stressful, the primary source of this additional stress is the children’s increased level of behavior problems rather than the presence of the delayed development itself.
Behavior problems, especially externalizing ones, tend to be stable across childhood, especially when they emerge early in childhood (Shaw, Lacourse, & Nagin, 2005). Associated with heightened parenting stress in families of both typically developing children (e.g. Deater-Deckard, 1998) and children with delayed development (Hastings, Daley, Burns, & Beck, 2006), child behavior problems can be viewed as a chronic stressor for parents. Moreover, the relation between child behavior problems and family adjustment is often transactional (Baker et al., 2003; Sameroff & Chandler, 1975). It may be, as well, that poorer maternal health is predictive of increased child behavior problems over time. Thus we will explore the evidence for a transactional relationship.
Physical well-being among mothers of children with delayed development or behavior problems
The current study assesses mothers’ perceptions of their own physical health, in line with many other studies that also rely on mothers’ subjective, self-reported health (e.g. Belzeval, 1998; Chen, Ryan-Henry, Heller, & Chen, 2001; Neises & Gruneberg, 2005; Waters et al., 2000). Despite extensive research documenting poor psychological outcomes facing parents raising children with delayed development, little research has examined these parents’ physical health. The related research has tended to group together mothers raising children with a range of disabilities, or has focused on the health of aging mothers caring for adult offspring with intellectual disability (Chen et al., 2001; Epel et al., 2004). Studies examining the health of older mothers caring for adult offspring with intellectual disability generally have not found poorer physical health compared to mothers of adults without intellectual disability (Chen et al., 2001; Seltzer & Krauss, 1989). These studies, however, focused on selected families who chose to have the adult son or daughter continue to live at home; in many cases such parents derive many positive benefits from the relationship (Hastings, Beck, & Hill, 2005; Heller, Miller, & Factor, 1997). To our knowledge, no study has focused on the physical health of mothers raising young children with or without delayed development in the context of behavior problems; this population of mothers is the focus of the present study.
The relationship between rearing a difficult child and mothers’ physical health is likely to be stronger in some families than others. Weiss (2002) highlighted the importance of emotional and social resources; mothers’ positive marital adjustment, sense of accomplishment in parenting, self-reported hardiness, social support, and higher educational achievement were protective factors for stress-related somatic symptoms. Epel et al. (2004) examined biomarkers of cellular aging that are strong predictors of longevity, among mothers aged 20–50 years raising children with various physical or developmental disabilities (e.g. autism, cerebral palsy, chronic illnesses) compared to mothers of children with typical development. Overall, there was no difference in cellular aging between mothers of children with and without disabilities. However, mothers reporting high perceived parenting stress had aged 9–17 years more than those who perceived low parenting stress according to their biomarkers of cellular aging. In fact, mothers’ self-reported perceptions of stress were the strongest predictor of cellular aging, more so than objective measures of stress, such as presence of a child with a disability or years of caregiving. Overall, these studies suggest that raising a young child with challenging behaviors, especially when associated with chronically high perceptions of stress, may affect physical health. Thus, in the current study, we consider stress and depressive symptoms as potential moderators of the relation between early child characteristics (developmental status, behavior problems) and later perceived maternal health; we expect that high maternal stress or depressive symptoms may exacerbate the effect of child characteristics on maternal health.
Pathways of influence between chronic stress and physical health
Considerable research has linked both chronic stress and depressive symptoms to poorer physical health (Taylor, Lerner, Sage, Lehman, & Seeman, 2004), including suppressed immune function (Herbert & Cohen, 1993) and increased rates of mortality (Martin et al., 1995). There are multiple pathways by which these mothers’ stress and depressive symptoms may get “under the skin” to affect immune function and physical health (Taylor et al., 1997). Stress and depressive symptoms may impact mothers’ health habits and behaviors, and may lead to prolonged arousal and dysregulation of multiple aspects of the neuroendocrine stress response system, which can compromise immune function and health (see McEwen, 2000 for a review). Both of these potential pathways are also likely to affect mothers’ self-perceived health, in addition to affecting objective, biological indicators of health. Thus, we examine the potential mediating role of perceived stress and depressive symptoms, as mechanisms through which child delayed development or behavior problems may affect mothers’ later perceived physical health.
The current study considers how child delayed development, child behavior problems, and maternal psychological factors (parenting stress, depressive symptoms) interact to predict mothers’ self-reported physical health across child ages 3–5 years. We considered the following questions:
Do mothers of children with delayed development report poorer physical health across child ages 3, 4, and 5 compared to mothers of children with typical development? Is this relationship attributable to the higher behavior problems in childrenwith delayed development?
Do early child delayed development and/or behavior problems at age 3 predict changes in maternal physical health two years later? To determine whether this relationship is transactional, we also ask: does maternal health at age 3 predict changes in child behavior problems two years later?
Do maternal depressive symptoms and parenting stress at child ages 3, 4, and 5 moderate the relationship between early child-related stressors (delayed development, behavior problems) at age 3 and later maternal physical health at child age 5?
Do maternal depressive symptoms and parenting stress at child age 4 mediate the relationship between early child-related stressors (delayed development, behavior problems) at age 3 and later maternal physical health at child age 5?
Method
Participants
Participants were 218 families with a 3-year-old child, 126 boys and 92 girls. They were recruited to participate in a longitudinal study of preschoolers, with samples drawn from Central Pennsylvania (25%) and Southern California (75%). Children were classified as having developmental delays (delayed development; n = 91) or typical development (typical development; n = 127). Families of children with delayed development were recruited through community agencies that provide diagnostic and intervention services for individuals with developmental disabilities. In California, practically all families of children with delayed development register for services with these Regional Centers. The selection criteria were that the child: (a) be 30–40 months of age; (b) score 40–84 on the Bayley Scales of Infant Development-II (BSID-II); (c) be ambulatory; and (d) not be diagnosed with autism. These inclusion criteria allow children with borderline, mild, or moderate levels of intellectual disability, as indicated by their IQ, to be included in the delayed development group. The inclusion of children with borderline intellectual functioning reflects findings that these children face heightened risk of dual diagnosis comparable to that of children with other levels of delayed development (Fenning, Baker, Baker, & Crnic, 2007). Like other levels of intellectual disability, borderline intellectual functioning is recognized as a psychological disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition – Text Revision (American Psychiatric Association, 2000).
Families for the typical development group were recruited through preschools and daycare programs. The selection criteria were that the child: (a) be 30–40 months of age; (b) score 85 or above on the BSID-II; and (c) not be born prematurely or have a developmental disability. Table 1 shows demographic characteristics by developmental status at age 3. In the combined sample, child age at intake averaged 35.2 months; 60% of children were Caucasian, 16.5% Hispanic, 6.9% African American, 2.8% Asian American, and 13.8% Mixed/other. Recruitment initially focused on intact families, so most (85%) participants were married; 47% of mothers and 48% of fathers had graduated from college, and 52% of families had an annual income of $50,000 or more. Within the delayed development group, most children (46 children; 51.7%) were diagnosed with undifferentiated developmental delays, although 13 children had cerebral palsy, 11 had Down syndrome, 7 had other genetic syndromes, and 12 children received diagnoses of autism after their age 3 assessments.
Table 1.
Demographics by developmental status group (developmental delays, typical development)
| Variable | Delayed development (n = 91) | Typical development (n = 127) | t or χ2 | P |
|---|---|---|---|---|
| Child sex: % boys | 65.9 | 52.0 | χ2 = 3.69 | ns (.055) |
| Child race: % Caucasian | 58.2 | 61.4 | χ2 = 0.11 | ns |
| Child age at assessment: mean mo. | 35.6 | 34.9 | t = 1.53 | ns |
| Bayley mental development index | 60.4 | 104.2 | t = 26.01 | <.001 |
| Mother age in years: mean | 32.5 | 34.1 | t = 2.05 | .041 |
| Mother grade completed: mean | 14.3 | 15.8 | t = 4.34 | <.001 |
| Mother employment: % employed | 48.4 | 61.4 | χ2 = 3.16 | ns |
| Marital status: % married | 80.2 | 89.0 | χ2 = 2.58 | ns |
| Father age in years: mean | 36.3 | 36.6 | t = 0.32 | ns |
| Father grade completed: mean | 14.0 | 15.8 | t = 4.28 | <.001 |
| Family income: % > $50,000 | 41.8 | 58.7 | χ2 = 5.43 | .02 |
Delayed development and typical development groups differed on mother age and on key socioeconomic variables (mother’s highest grade completed in school, father’s highest grade completed in school, family income). Both maternal education and family income also related to maternal health, our outcome variable. Father education data was not available for many of our participants; because it was highly correlated with maternal education and its effect on our key variables was not significant once maternal education was accounted for, father education was not used as a covariate. Thus, z scores of maternal education and family income at child age 3 were entered as covariates in all analyses.
Procedures
Data were obtained through child assessments (age 3) and mother-completed questionnaires (child ages 3, 4, and 5). All procedures were approved by the Institutional Review Boards of the universities involved. Initial measures of child cognitive ability and behavior problems were obtained at a home assessment at about child age 3. Prior to this visit, parents received an informed consent form and completed a telephone interview. Two examiners visited the family’s home; after reviewing study procedures and obtaining parental informed consent, they administered the Bayley Scales of Infant Development-II (Bayley, 1993) to the child. During this testing, the child’s mother, and father if present, completed the Child Behavior Checklist (CBCL; Achenbach, 2000) privately in written form. Only mothers’ responses were included in the present study.
Measures
Maternal health
A family demographic assessment, administered to mothers at child ages 3, 4, and 5, included an item assessing mothers’ global physical health: “How is your physical health in general?” The response scale is: 1 = Poor; 2 = Fair; 3 = Good; 4 = Excellent. This single-item measure was used to assess mothers’ perceptions of their own physical health. Despite consisting of only a single item, this health measure has been shown to predict morbidity and mortality across a range of diseases and populations (Idler & Benyamini, 1997), and has correlated 0.70 with a physical examination (Multidimensional functional assessment, 1978). This measure and similar 3-point and 5-point global health measures have become widely accepted as measures of self-perceived physical health. Previous research has demonstrated its construct validity among ethnically diverse individuals (e.g. Bzostek, Goldman, & Pebley, 2007; Mulvaney-Day, Alegria, & Sribney, 2007), parents in general (e.g. Belzeval, 1998; Neises & Gruneberg, 2005; Waters et al., 2000), and parents of individuals with intellectual disability (e.g. Chen et al., 2001; Seltzer & Krauss, 1989).
Bayley Scales of Infant Development-II (BSID-II; Bayley, 1993)
The Bayley is a widely used assessment of mental and motor development in children aged 1–42 months. Only mental development items were administered. The mental development index (MDI) is normed with a mean of 100 and a standard deviation of 15. Bayley (1993) reported high short-term test-retest reliability for the mental development index (r = 0.91). With children aged 36–42 months, the mental development index related to the Full-Scale IQ of the Wechsler Preschool and Primary Scale of Intelligence – Revised, r = 0.73 (Bayley, 1993).
Child Behavior Checklist for ages 1.5–5 (CBCL; Achenbach, 2000)
The CBCL assesses behavior problems in children with or without delayed development. This preschool form has 99 items that indicate child problems. The respondent indicates whether each item is (0) not true, (1) somewhat or sometimes true, or (2) very true or often true, now or in the past two months. The present study utilized only total CBCL problem scores; these are converted to T scores with a mean of 50 and SD of 10. For some analyses, behavior problems groups were determined following Achenbach’s (2000) suggested groupings of non-clinical (T score < 60) and clinical (T score ≥ 60, indicating borderline or clinical range). In the present sample, total score alpha for mothers at 36 months was 0.94.
Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977)
This is a 20-item self-report measure of depressive symptoms including mood, somatic complaints, and cognitions, which was completed by mothers at child ages 3, 4, and 5. Total scores can range from 0 to 60, with a cutoff of ≥16 for the clinical range. Alpha in the present sample for mothers was 0.89. The CES-D has four subscales – somatic symptoms (7 items), depressed affect (7 items), positive affect (reverse scored; 4 items), and interpersonal functioning (2 items) – each with good reliability and validity (e.g. Williams et al., 2007). Alphas for the four subscales in our sample ranged from 0.70 to 0.92.
Family Impact Questionnaire (FIQ; Donenberg & Baker, 1993)
This 50-item questionnaire asks about the “child’s impact on the family compared to the impact other children his/her age have on their families” (e.g. Item 1: “My child is more stressful”), endorsed on a 4-point scale from “not at all” to “very much.” The questionnaire was completed by mothers at child ages 3, 4, and 5. It has six subscales. Of interest here is a combined negative impact (or parenting stress) score, derived from scales measuring negative impact on feelings about parenting (9 items) and social relationships (11 items). Alpha in the present sample for mothers’ negative impact was 0.92.
Results
Preliminary analyses
Our two child characteristics were developmental status and behavior problems. Developmental status groups were children with developmental delays (n = 91) and typical development (n = 127). Behavior problems were considered continuously and also as a two-group variable, including borderline and clinical level behavior problems (n = 45) and non-clinical problems (n = 173). These two child characteristics were significantly related; children with developmental delays were significantly more likely to have clinical behavior problems than children with typical development, X2 (1) = 14.49, p < .001. Bivariate correlations of total mother-reported behavior problems revealed high correlations between behavior problems at ages 3–4 years (r = 0.74), 3–5 (r = 0.74), and ages 4–5 (r = 0.74), all p < .001. The outcome variable was mothers’ self-reported global physical health at child ages 3, 4, and 5. Mean scores of maternal health on the 1–4 scale were 3.23 (SD = 0.67) at age 3, 3.12 (SD = 0.70) at age 4, and 3.11 (SD = 0.77) at age 5. Means of the maternal well-being variables at each timepoint are shown in Tables 2a and b. Bivariate correlations revealed moderate correlations between health at ages 3–4 years (r = 0.55), ages 3–5 (r = 0.55) and ages 4–5 (r = 0.59), all p < .001. The maternal health variables met the normality assumptions required for analyses of variance and regression; the skewness statistics fell within the acceptable range between −1 and +1 at all three ages.
Table 2.
| Table 2a. Mean (SD) Maternal well-being by developmental status group (developmental delays, typical development) at age 3 | ||||
|---|---|---|---|---|
| Variable | Delayed development (n = 91) | Typical development (n = 127) | F | P |
| Maternal health at age 3 | 3.07 (.7) | 3.36 (.6) | 5.83 | .02 |
| Maternal health at age 4 | 2.96 (.7) | 3.24 (.7) | 4.81 | .03 |
| Maternal health at age 5 | 2.97 (.8) | 3.21 (.7) | 1.72 | .19 |
| Maternal stress at age 3 | 18.09 (13.1) | 11.30 (7.7) | 19.90 | <.001 |
| Maternal stress at age 4 | 17.90 (12.3) | 12.11 (8.7) | 14.46 | <.001 |
| Maternal stress at age 5 | 15.42 (11.0) | 9.99 (7.1) | 15.00 | <.001 |
| Maternal depressive symptoms at age 3 | 11.27 (8.9) | 9.58 (8.9) | .42 | .52 |
| Maternal depressive symptoms at age 4 | 12.82 (10.7) | 10.02 (10.3) | 1.80 | .18 |
| Maternal depressive symptoms at age 5 | 12.52 (11.6) | 9.92 (9.4) | 1.71 | .19 |
| Table 2b. Mean (SD) maternal well-being by developmental status (developmental delays, typical development) and behavior problems groups (BP; non-clinical versus borderline or clinical level behavior problems) at age 3 | ||||||
|---|---|---|---|---|---|---|
| Variable | Delayed development, low BP (n = 61) | Delayed development, High BP (n = 30) | Typical development, low BP (n = 111) | Typical development, high BP (n = 15) | F (4-level variable) | p |
| Maternal health at age 3 | 3.16 (.8) | 2.87 (.6) | 3.39 (.6) | 3.13 (.6) | 3.37 | .02 |
| Maternal health at age 4 | 3.16 (.6) | 2.53 (.8) | 3.31 (.6) | 2.73 (.9) | 10.33 | <.001 |
| Maternal health at age 5 | 3.21 (.7) | 2.47 (.9) | 3.22 (.7) | 3.13 (.8) | 5.82 | .001 |
| Maternal stress at age 3 | 12.31 (.7.7) | 29.83 (14.1) | 10.03 (6.8) | 20.60 (8.0) | 44.75 | <.001 |
| Maternal stress at age 4 | 13.33 (7.7) | 27.03 (14.7) | 10.94 (.7.8) | 20.33 (10.3) | 25.25 | <.001 |
| Maternal stress at age 5 | 12.60 (8.3) | 21.82 (13.7) | 9.51 (7.1) | 13.38 (6.7) | 12.65 | <.001 |
| Maternal depressive symptoms at age 3 | 7.90 (5.8) | 18.13 (10.3) | 8.93 (8.3) | 14.33 (11.7) | 10.63 | <.001 |
| Maternal depressive symptoms at age 4 | 8.43 (6.7) | 21.60 (11.9) | 8.61 (8.7) | 20.07 (14.8) | 19.36 | <.001 |
| Maternal depressive symptoms at age 5 | 7.97 (8.9) | 21.77 (11.2) | 9.19 (8.9) | 15.13 (11.5) | 15.38 | <.001 |
Age 3 family income and maternal education were covaried in all analyses.
Early child-related stressors in relation to maternal health over time
Child developmental delay
We examined whether developmental status at age 3 related to maternal health across ages 3, 4, and 5. A repeated measure ANCOVA was conducted, with age 3 family income and maternal education as covariates, age 3 developmental status (delayed versus typical) as the independent variable, and maternal health at ages 3, 4, and 5 as the dependent variables. Maternal health varied by child developmental status [F(1, 211) = 3.77, p = .05, partial η2, or Eta squared, a measure of effect size = .018], child age [F(2, 422) = 4.41, p = .01, partial η2 = .020], and family income [F(1, 211) = 13.49, p < .001], and was marginally associated with maternal education [F(1, 211) = 3.64, p = .06, partial η2 = .017]. Maternal health was poorer when children had developmental delay or when families had lower incomes, was marginally poorer when mothers were less educated, and was poorer as children grew older. Interactions were non-significant.
Child developmental delay and behavior problems
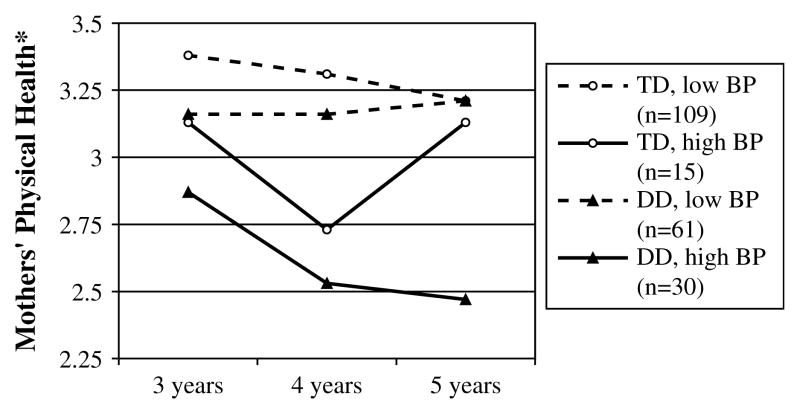
We next examined how both child developmental status and behavior problems at age 3 related to maternal health across ages 3, 4, and 5. A repeated measure analysis of covariance was conducted, with age 3 family income and maternal education as covariates, age 3 child developmental status and child behavior problems group (non-clinical versus clinical) as the independent variables, and maternal health at ages 3, 4, and 5 as the dependent variables. Maternal health had a significant relationship with child behavior problems group [F(1, 209) = 15.65, p < .001, partial η2 = .070], age [F(2, 418) = 6.12, p = .002, partial η2 = .028], family income [F(1, 209) = 9.65, p = .002, partial η2 = .044], and maternal education [F(1, 209) = 4.14, p = .04, partial η2 = .019], but did not vary by developmental status [F(1, 209) = 2.12, p = .15, partial η2 = .010]. Maternal health was poorer when children had clinical behavior problems and as children got older, and when families had fewer socioeconomic resources. Two-way interactions involving developmental status were non-significant, but the interaction of age by child behavior problems was significant [F(2, 418) = 4.00, p = .019, partial η2 = .019]. Notably, there was a significant three-way interaction of age by child behavior problems by child developmental status [F(2, 418) = 3.81, p = .023, partial η2 = .018]. As shown in Fig. 1, high behavior problems and child developmental status together were associated with the poorest maternal health, which became poorer over time.
Fig. 1.
Maternal physical health over time based on child developmental status [Developmental delay (DD) versus typical development (TD)] and behavior problems (BP) at age 3. * Maternal physical health score was based on mothers’ self-reported global physical health on a 4-point scale at child age 5, where 1 = Poor, 2 = Fair, 3 = Good, and 4 = Excellent.
We conducted follow-up analyses of covariance to understand the nature of this three-way interaction effect. As Fig. 1 suggests, level of behavior problems significantly predicted mother-reported health only within the delayed group [F(3, 87) = 14.50, p < .001] after covarying family income and maternal education, and not within the typical group (p = .72). Further, in line with our expectation that child-related stressors would have a delayed effect on mother-reported health, these follow-up analyses of covariance revealed that the interaction of developmental status × behavior problems at age 3 significantly predicted poorer maternal health by child age 5 but not at child ages 3 and 4. There is also an increase in maternal health from child age 4 to child age 5 within the typical, high behavior problems group; this unexpected finding may reflect unrelated variance due to the small cell size (n = 15) within this subgroup of mothers.
Change in maternal health over time
We next addressed the hypothesis that raising a child with delayed or high behavior problems leads to a decline in maternal health from child age 3 to child age 5. We conducted a 2 (delayed versus typical) by 2 (clinical versus non-clinical behavior problems) ANCOVA, to determine whether age 3 child characteristics predict maternal health above and beyond baseline maternal health at child age 3. Our dependent variable was maternal health at child age 5. We entered family income, maternal education, and maternal health at child age 3 as covariates, and age 3 developmental status and age 3 child behavior problems group as independent variables. After controlling for age 3 maternal health in this way, child behavior problems group continued to exert a significant detrimental main effect on later maternal health [F(1, 210) = 4.32, p = .039, partial η2 = .020]. The main effect of developmental status was non-significant. The developmental status by behavior problems interaction effect on later maternal health remained significant [F(1, 210) = 6.49, p = .012, partial η2 = .030]; high behavior problems and child delayed status together were associated with increasingly poor maternal health. Thus, even after controlling for age 3 maternal health, child behavior problems and the interaction term at age 3 both exerted a significant effect on maternal health at child age 5.
Testing a transactional model
The finding that child behavior problems, especially when associated with developmental delay, predict adverse change in maternal health from ages 3 to 5 is consistent with a unidirectional relationship. However, there also may be a transactional relationship, with child characteristics and maternal health mutually influencing one another over time. To test this possibility, we conducted a hierarchical linear regression to determine whether maternal health at child age 3 predicted child behavior problems at age 5, above and beyond age 3 behavior problems. In the final model, maternal health at child age 3 only accounted for a non-significant 0.3% of the variance in age 5 behavior problems; thus a transactional model was not supported.
Maternal factors as moderators
We next considered the extent to which the observed relations between early child behavior problems and later maternal health were moderated by maternal well-being; in other words, the extent to which there was a significant interaction between early child behavior problems and maternal stress or depressive symptoms in predicting later maternal health. We first examined whether the relation between child behavior problems at age 3 and maternal health at age 5 was moderated by mothers’ perceptions of parenting stress at child ages 3, 4, and 5. A parenting stress composite variable was created as the sum of the z scores of mother-reported parenting stress on the Family Impact Questionnaire (negative impact sum score) at child ages 3, 4, and 5. The behavior problems by parenting stress interaction term were calculated as the product of the z score of age 3 mother-reported total behavior problems T score by the z score of the parenting stress composite. This regression is shown in Table 3.
Table 3.
Regression analyses: mothers’ perceived parenting stress across ages 3, 4, and 5 moderates the relation between child behavior problems at age 3 and mothers’ perceived health at age 5
| Unstand. Beta (SE) | Stand. Beta | |
|---|---|---|
| STEP 3 (Final Model) | ||
| Family income, age 3 | .20 (.06) | .27** |
| Maternal education, age 3 | .04 (.06) | .05 |
| Child behavior problems, age 3 | −.13 (.07) | −.17† |
| Maternal stress composite, ages 3, 4, & 5 | .09 (.08) | .11 |
| Behavior problems × Maternal stress interaction | −.13 (.05) | −.22** |
Dependent variable: mothers’ self-reported health at child age 5.
Step 1: R2 Δ= .104, p < .001. Step 2: R2 Δ= .024, p = .06. Step 3: R2 Δ= .030, p = .007.
p < .10;
p < .01; df = 1203.
In the final model, explaining 13.7% of the variance in maternal health, behavior problems contributed marginally to maternal health and the interaction term contributed significantly. That is, higher parenting stress was associated with worse maternal health when children had behavior problems. When parenting stress as a moderator was examined separately within the delayed and typical groups, the effect did not reach significance in either.
To examine maternal depressive symptoms as a moderator, the same regression analysis was conducted with maternal depressive symptoms (mother-reported CES-D total scores) in place of parenting stress. As with parenting stress, a depressive symptoms composite variable was created as the sum of the z scores of mother-reported CES-D total depression at child ages 3, 4, and 5. In the combined sample and in the typical sample, maternal depressive symptoms did not moderate the relationship between child behavior problems and later maternal health. However, in the delayed sample, depressive symptoms significantly moderated this relationship. Mothers who had both clinical levels of depressive symptoms and high child behavior problems reported the poorest physical health. The results for the combined sample and the delayed sample are shown in Table 4.
Table 4.
Regression analyses: mothers’ depressive symptoms across ages 3, 4, and 5 as a moderator of the relation between age 3 child behavior problems and age 5 mothers’ perceived health
| Combined sample (df = 1204) |
Delayed sample (df = 184) |
|||
|---|---|---|---|---|
| Unstand. Beta (SE) | Stand. Beta | Unstand. Beta (SE) | Stand. Beta | |
| STEP 3 (Final Model) | ||||
| Family income, age 3 | .15 (.06) | .22** | .21 (.08) | .26* |
| Maternal education, age 3 | .03 (.06) | .04 | .05 (.09) | .05 |
| Child behavior problems, age 3 | .0002 (.05) | .002 | −.16 (.09) | .20† |
| Maternal depressive symptoms composite, ages 3, 4, & 5 | −.29 (.05) | −.38*** | .01 (.11) | .01 |
| Behavior problems × depressive symptoms interaction | −.003 (.04) | −.006 | −.22 (.09) | −.33* |
Combined sample: Step 1: R2 Δ= .104, p < .001. Step 2: R2 Δ= .14, p < .001. Step 3: R2 Δ= .000, p = .9.
Sample of children with developmental delays: Step 1: R2 Δ= .125, p = .003; at Step 2, R2 Δ= .148, p < .001; at Step 3, R2 Δ= .050, p = .015.
Dependent variable: mothers’ self-reported health at child age 5.
p < .10;
p < .05;
p < .01;
p < .001.
Maternal factors as mediators
We next examined whether maternal stress or depressive symptoms mediated the association between child behavior problems and later maternal health using Baron and Kenny (1986) procedure. Parenting stress analyses indicated that it was not a mediator. For depressive symptoms, the three prerequisites for mediation were met for the combined and delayed samples. Hierarchical linear regressions shown in Table 5 indicate that child behavior problems contributed significantly to maternal health in Step 2, but dropped to non-significance when maternal depressive symptoms was added in Step 3 (Sobel z = −3.79, p < .001). Thus, in the combined sample, full mediation was supported.
Table 5.
Regression analyses: mothers’ depressive symptoms at child age 4 mediate the relation between age 3 child behavior problems and age 5 maternal physical health
| Combined sample (df = 1207) |
Delayed sample (df = 185) |
|||
|---|---|---|---|---|
| Unstand. Beta (SE) | Stand. Beta | Unstand. Beta (SE) | Stand. Beta | |
| STEP 1 | ||||
| Family income, age 3 | .22 (.06) | .28*** | .26 (.09) | .33** |
| Maternal education, age 3 | .06 (.06) | .08 | .04 (.10) | .04 |
| STEP 2 | ||||
| Family income, age 3 | .20 (.06) | .26** | .19 (.09) | .24* |
| Maternal education, age 3 | .04 (.06) | .06 | .06 (.09) | .07 |
| Child behavior problems, age 3 | −.12 (.05) | −.16* | −.29 (.08) | −.35*** |
| STEP 3 | ||||
| Family income, age 3 | .16 (.06) | .21** | .16 (.09) | .20† |
| Maternal education, age 3 | .05 (.06) | .06 | .08 (.09) | .09 |
| Child behavior problems, age 3 | −.02 (.05) | −.03 | −.19 (.09) | −.24* |
| CES-D depressive symptoms, age 4 | −.27 (.05) | −.25*** | −.20 (.09) | −.25* |
Combined Sample: Step 1: R2 Δ= .108, p < .001. Step 2: R2 Δ= .024, p = .018. Step 3: R2 Δ= .010, p < .001.
Sample of children with developmental delays: Step 1: R2 Δ= .125, p = .003. Step 2: R2 Δ= .118, p < .001. Step 3: R2 Δ= .044, p = .024.
Dependent variable: mothers’ self-reported health at child age 5.
p < .10;
p < .05;
p < .01;
p < .001.
In the delayed sample, partial mediation was supported. As shown in Table 5, depressive symptoms contributed significantly to maternal health in the final model and the relation between child behavior problems and maternal health dropped, although still significant (Sobel z = −2.10, p = .035).
Further examination of depressive symptoms as a mediator
We next examined the subscales of the CES-D – including somatic symptoms, positive affect (reverse scored), depressed affect, and interpersonal functioning – as mediators with the combined sample and the delayed sample. The somatic symptoms, positive affect (reverse scored), and depressive affect subscales were full mediators of the relation between child behavior problems at age 3 and maternal health at child age 5 in the combined sample. Within the delayed sample, the somatic symptoms and depressive affect subscales were partial mediators of the relation between child behavior problems and maternal health.
Discussion
Child-related stressors and maternal health
Mothers raising young children with delayed development reported significantly poorer physical health than mothers raising children with typical development. This finding complements research documenting poorer psychological well-being for mothers raising young children with developmental delays (e.g. Baker et al., 2003), as well as research showing poorer physical health among older mothers of adults with intellectual disability (e.g. Seltzer, Greenberg, Floyd, Pettee, & Hong, 2001).
Consistent with other research on mothers’ psychological well-being (Baker et al., 2003; Floyd & Phillippe, 1993), the observed difference in perceived physical health between mothers of children with versus without delays was primarily accounted for by the heightened behavior problems in the delayed sample, not their cognitive delay per se. Thus mothers’ experiences of both psychological and physical health are impacted by behavior problems, and as early as child age 3 years. Further, the interactions between developmental status and behavior problems and between age, developmental status, and behavior problems revealed that mothers of children who have both high behavior problems and developmental delays reported the poorest physical health, which became poorer over time. This age-related finding is consistent with biological models, which suggest that the effects of chronic stress on health are delayed and cumulative over time (McEwen & Seeman, 1999). The findings also suggest that the behavior problems of children with delays have a more negative effect on mothers’ perceived health than behavior problems in children with typical development. This result is in line with findings that children’s social skills or adaptive behaviors may offset the negative effects of child behavior problems on maternal well-being, especially at high levels of behavior problems (e.g. Neece & Baker, in press). Among children with typical development, the detrimental effects of a child’s behavior problems on maternal well-being may be counteracted by the positive effects of his/her social skills or other strengths; meanwhile, children with delays may have fewer social skills to offset the negative effects of their behavior problems on mothers’ well-being.
Our results supported a unidirectional model, in which child behavior problems and their interaction with developmental status influenced change in maternal health over time, as there was no evidence that early maternal health influenced change in child behavior problems over time. Thus, the relation between early child characteristics and mothers’ self-reported health does not appear to be transactional, but unidirectional and child-driven.
Parenting stress and depressive symptoms
The effects of behavior problems on mothers’ perceived health were stronger when mothers were more stressed. This finding demonstrates the importance of perceptions of stress, rather than only objective stressors, in predicting mothers’ perceived health. It corroborates the conclusions of Epel et al. (2004), who found that perceived stress was a stronger predictor of cellular aging than mothers’ actual number of years of caregiving. Too, depressive symptoms moderated the relation between behavior problems and perceived health among mothers of children with developmental delays; for these mothers, those who reported both high depressive symptoms and high child behavior problems reported markedly poorer health than all other groups of mothers.
Other psychological factors such as coping style and perceived control might also moderate the impact of child-related stressors on mothers’ health (Kemeny, 2003; Olff, 1999). Further, in the current study, socioeconomic factors including family income and parents’ education level exerted a main effect on maternal health, in line with substantial research showing a connection between economic resources and health (Emerson, 2003b). Subsequent studies could explore contextual factors – such as cultural background, social and instrumental support, and social roles beyond parenting – that may moderate the relation between child-related stressors and maternal health (Eisenhower & Blacher, 2006).
Mothers’ depressive symptoms mediated the association between early child behavior problems and later self-reported maternal health, consistent with research linking chronic stress to depression (e.g. Kiecolt-Glaser, Dura, Speicher, Trask, & Glaser, 1991) and depression to health (Herbert & Cohen, 1993). Further studies should examine the extent to which physical symptoms of depression (e.g. sleep disturbance, fatigue, other somatic complaints) versus psychological symptoms (e.g. hopelessness, anhe-donia) mediate the stress-health relationship. In our sample, both of these factors – somatic symptoms as well as depressive affect and lack of positive affect – emerged as mediators.
Implications for intervention
Our results identify mothers of children with developmental delays and high behavior problems as a particular risk group that may be especially in need of early intervention. Programs may be effective in improving parents’ physical well-being when they focus not only on increasing children’s cognitive skills but also on reducing behavior problems. Reductions in behavior problems could, in turn, reduce parents’ stress and depression and thus have health benefits for mothers. This is corroborated by research indicating that reductions in child behavior problems following parent training mediated improvements in parenting attitudes and stress (Feinfield & Baker, 2004). On the other hand, child behavior problems are highly stable and for a substantial percentage of children, parent training programs do not lead to long-term improvements. Our findings suggest that, for these high-risk parents of children with delayed development and behavior problems, interventions focused on reducing negative affect, increasing positive affect, or enhancing stress coping skills might also lead to improved health. A recent review of stress intervention research for parents of children with delays found strong empirical support for cognitive behavioral group interventions in reducing stress among these mothers (Hastings & Beck, 2004). Likewise, parent training programs that focus on improving parental coping or reducing parent stress and discord may have an indirect effect on child behavior as well, especially for children facing multiple systemic and familial risks (Appleyard, Egeland, van Dulmen, & Sroufe, 2005; Webster-Stratton, Reid, & Hammond, 2004).
Our findings also suggest that interventions ought to focus directly on protecting mothers’ physical health, promoting health behaviors, and helping mothers cope with stress and depressive symptoms. Future research should examine how evidence-based interventions for stress management, depression, and health behavior can be adapted to address the specific stresses facing parents of children with delayed development and behavior problems.
Limitations and future directions for research
The current study examined health over a short period (2 years) and relied on a single-item, self-report measure of health. This measure is well suited to the current study’s focus on self-perceptions of health, but limits our ability to assess health objectively or in detail. Further, our health measure shares method variance with our measures of psychological well-being and child behavior problems, as these also rely on mother-report; too, the health measure exhibits some positive skewness, though within the acceptable range. Mothers’ affect, reflected in their scores of depression and stress, may also influence their memory and judgments when reporting on health. There is a need to replicate the current findings using an in-depth measure of subjective reports of health, as well as more objective indicators of health, including health behaviors, doctor visits, physical examinations, and biological measures of immune function. Such research would help to isolate the specific systems and pathways responsible for compromised health.
Further study is needed to understand the behavioral and biological pathways by which child-related stressors affect mothers’ physical health. One potential pathway is through mothers’ health behaviors and habits. Raising a challenging child may affect mothers’ engagement in healthy living habits, such as regular exercise, a healthy diet, routine medical care, and undisrupted sleep. These behaviors all modulate the immune response and are lower in depressed individuals (Irwin, Mascovich, Gillin, & Willoughby, 1994).
Second, biological pathways may account for the link between child-related stress and maternal health. Chronic stress and depression have been shown to lead to prolonged arousal and dysregulation of the neuroendocrine system, including the sympathetic–adrenal–medullary system and the hypothalamic–pituitary–adrenocortical system. Activation of the former system releases catecholamines, which lead to increased heart rate and blood pressure and constriction of the peripheral blood vessels (McEwen & Seeman, 1999; Olff, 1999). Activation of the latter system is manifested in the prolonged release of cortisol and other stress hormones (Plotsky, Owens, & Nemeroff, 1998). Over time, prolonged arousal of these systems leads to cardiovascular risk, suppressed immune function, susceptibility to illness, and chronic inflammation (McEwen & Seeman, 1999; Plotsky et al., 1998). Future research could examine mothers’ catecholamine levels and cortisol reactivity to determine the extent to which a pathway of: child stressor – depression – sympathetic system – cardiovascular health (Kvetnansky et al., 1995) or: child stressor – depression –hypothalamic system – immune function (Stein, Miller, & Trestman, 1991) explains their poorer health. Overall, our findings highlight the need for research examining the biological and behavioral pathways that account for the perceived poorer health of mothers raising children with developmental delays and/or elevated behavior problems.
Footnotes
This article is based on the activities of the Collaborative Family Study (CFS), supported by NICHD grant 34879-1459 (Dr. Keith Crnic, Principal Investigator, and Drs. Bruce L. Baker, Jan Blacher and Craig Edelbrock, co-PIs). The CFS is conducted at three sites: Pennsylvania State University, State College, PA, the Fernald Child Study Center at the University of California, Los Angeles, CA, and the Vernon Eady Center at the University of California, Riverside, CA. We appreciate the assistance of our colleagues on the CFS. We are especially grateful for the participation of the families who made this research possible.
Contributor Information
Bruce L. Baker, Email: baker@psych.ucla.edu.
Jan Blacher, Email: jan.blacher@ucr.edu.
References
- Achenbach TM. Manual for the child behavior checklist 1 1/2–5 2000 [Google Scholar]
- Burlington VT University of Vermont, Department of Psychiatry. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington DC: Author; 2000. (text revision) [Google Scholar]
- Appleyard K, Egeland B, van Dulmen MH, Sroufe LA. When more is not better: the role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry. 2005;46:235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Baker BL, Blacher J, Crnic KA, Edelbrock C. Behavior problems and parenting stress in families of three year old children with and without developmental delays. American Journal on Mental Retardation. 2002;107:433–444. doi: 10.1352/0895-8017(2002)107<0433:BPAPSI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Baker BL, Blacher J, Kopp CB, Kraemer B. Parenting children with mental retardation. International Review of Research in Mental Retardation. 1997;20:1–45. [Google Scholar]
- Baker BL, McIntyre LL, Blacher J, Crnic K, Edelbrock C, Low C. Preschool children with and without developmental delay: behaviour problems and parenting stress over time. Journal of Intellectual Disability Research. 2003;47:217–230. doi: 10.1046/j.1365-2788.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bayley N. Manual. 2. San Antonio, TX: Psychological Corp; 1993. Bayley scales of infant development. [Google Scholar]
- Belzeval M. The self-reported health status of lone parents. Social Science & Medicine. 1998;46:1337–1353. doi: 10.1016/s0277-9536(97)10083-1. [DOI] [PubMed] [Google Scholar]
- Brandon P. Time away from “smelling the roses”: where do mothers of children with disabilities find time to work? Social Science & Medicine. 2007;65:667–679. doi: 10.1016/j.socscimed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Bzostek S, Goldman N, Pebley A. Why do Hispanics in the USA report poor health? Social Science & Medicine. 2007;65:990–1003. doi: 10.1016/j.socscimed.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Chen SC, Ryan-Henry S, Heller T, Chen EH. Health status of mothers of adults with intellectual disability. Journal of Intellectual Disability Research. 2001;45:439–449. doi: 10.1046/j.1365-2788.2001.00352.x. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Parenting stress and child adjustment: some old hypotheses and new questions. Clinical Psychology: Science and Practice. 1998;5:314–332. [Google Scholar]
- Dekker MC, Koot HM, van der Ende J, Verhulst FC. Emotional and behavioral problems in children and adolescents with and without intellectual disability. Journal of Child Psychology and Psychiatry. 2002;43:1087–1098. doi: 10.1111/1469-7610.00235. [DOI] [PubMed] [Google Scholar]
- Donenberg G, Baker BL. The impact of young children with externalizing behaviors on their families. Journal of Abnormal Child Psychology. 1993;21:179–198. doi: 10.1007/BF00911315. [DOI] [PubMed] [Google Scholar]
- Eisenhower A, Blacher J. Mothers of adults with intellectual disability: multiple roles, ethnicity and well-being. Journal of Intellectual Disability Research. 2006;50:905–916. doi: 10.1111/j.1365-2788.2006.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson E. Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. Journal of Intellectual Disability Research. 2003a;47:51–58. doi: 10.1046/j.1365-2788.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- Emerson E. Mothers of children and adolescents with intellectual disability: social and economic situation, mental health status, and the self-assessed social and psychological impact of the child’s difficulties. Journal of Intellectual Disability Research. 2003b;47:385–399. doi: 10.1046/j.1365-2788.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabgar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinfield KA, Baker BL. Empirical support for a treatment program for families of young children with externalizing problems. Journal of Clinical Child and Adolescent Psychology. 2004;33:182–195. doi: 10.1207/S15374424JCCP3301_17. [DOI] [PubMed] [Google Scholar]
- Fenning RM, Baker JK, Baker BL, Crnic KA. Parenting children with borderline intellectual functioning: a unique risk population. American Journal on Mental Retardation. 2007;112:107–121. doi: 10.1352/0895-8017(2007)112[107:PCWBIF]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd FJ, Phillippe KA. Parental interactions with children with and without mental retardation: behavior management, coerciveness, and positive exchange. American Journal on Mental Retardation. 1993;97:673–684. [PubMed] [Google Scholar]
- Hastings RP, Beck A. Practitioner review: stress intervention for parents of children with intellectual disabilities. Journal of Child Psychology and Psychiatry. 2004;45:1338–1349. doi: 10.1111/j.1469-7610.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- Hastings RP, Beck A, Hill C. Positive contributions made by children with an intellectual disability in the family: mothers’ and fathers’ perceptions. Journal of Intellectual Disabilities. 2005;9:155–165. doi: 10.1177/1744629505053930. [DOI] [PubMed] [Google Scholar]
- Hastings RP, Daley D, Burns C, Beck A. Maternal distress and expressed emotion: cross-sectional and longitudinal relationships with behavior problems of children with intellectual disabilities. American Journal on Mental Retardation. 2006;111:48–61. doi: 10.1352/0895-8017(2006)111[48:MDAEEC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hauser-Cram P, Warfield ME, Shonkoff JP, Krauss MW. Children with disabilities: a longitudinal study of child development and parent well-being. Monographs of the Society for Research in Child Development. 2001;66:1–131. [PubMed] [Google Scholar]
- Heller T, Miller AB, Factor A. Adults with mental retardation as supports to their parents: effects on parental caregiving appraisal. Mental Retardation. 1997;35:338–346. doi: 10.1352/0047-6765(1997)035<0338:AWMRAS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychological Bulletin. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Irwin M, Mascovich A, Gillin JC, Willoughby R. Partial sleep deprivation reduced natural killer cell activity in humans. Psychosomatic Medicine. 1994;56:493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. The psychobiology of stress. Current Directions in Psychological Science. 2003;12:124–129. [Google Scholar]
- Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosomatic Medicine. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, et al. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenal system. Annals of the New York Academy of Sciences. 1995;771:131–158. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- Martin LR, Friedman HS, Tucker JS, Schwartz JE, Criqui MH, Wingard DL, et al. An archival prospective study of mental health and longevity. Health Psychology. 1995;14:381–387. doi: 10.1037//0278-6133.14.5.381. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Multidimensional functional assessment. The OARS methodology: A manual. 2. Durham, NC: Duke University, Center for the Study of Aging and Human Development; 1978. [Google Scholar]
- Mulvaney-Day NE, Alegria M, Sribney W. Social cohesion, social support, and health among Latinos in the United States. Social Science & Medicine. 2007;64:477–495. doi: 10.1016/j.socscimed.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neece CL, Baker BL. Predicting maternal parenting stress in middle childhood: The roles of child intellectual status, behaviour problems and social skills. Journal of Intellectual Disability Research. doi: 10.1111/j.1365-2788.2008.01071.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neises G, Gruneberg C. Socioeconomic situation and health outcomes of single parents. Journal of Public Health. 2005;13:270–278. [Google Scholar]
- Olff M. Stress, depression, and immunity: the role of defense and coping styles. Psychiatry Research. 1999;85:7–15. doi: 10.1016/s0165-1781(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Olsson MB, Hwang CP. Depression in mothers and fathers of children with intellectual disability. Journal of Intellectual Disability Research. 2001;45:535–543. doi: 10.1046/j.1365-2788.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Lachar D, Loveland KA, Santos CW, Faria LP, Azzam PN, et al. Patterns of behavioral adjustment and maladjustment in mental retardation: comparison of children with and without ADHD. American Journal on Mental Retardation. 2000;105:236–251. doi: 10.1352/0895-8017(2000)105<0236:POBAAM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneur-oimmunology of depression: hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sameroff AJ, Chandler MJ. Reproductive risk and the continuum of caretaking casualty. In: Horowitz FD, Hetherington M, Scarr-Salapatek S, Siegel G, editors. Review of child development research. Vol. 4. Chicago: University of Chicago; 1975. [Google Scholar]
- Seltzer MM, Greenberg JS, Floyd FJ, Pettee Y, Hong J. Life course impacts of parenting a child with a disability. American Journal on Mental Retardation. 2001;106:265–286. doi: 10.1352/0895-8017(2001)106<0265:LCIOPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW. Aging parents with adult mentally retarded children: family risk factors and sources of support. Amer-ican Journal on Mental Retardation. 1989;94:303–312. [PubMed] [Google Scholar]
- Shaw DS, Lacourse E, Nagin DS. Developmental trajectories of conduct problems and hyperactivity from ages 2 to 10. Journal of Child Psychology and Psychiatry. 2005;46:931–942. doi: 10.1111/j.1469-7610.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- Stein M, Miller AH, Trestman RL. Depression, the immune system, and health and illness. Archives of General Psychiatry. 1991;48:171–177. doi: 10.1001/archpsyc.1991.01810260079012. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality Special Issue on Emotions, Personality, and Health. 2004;72:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annual Review of Psychology. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- Veisson M. Depression symptoms and emotional states in parents of disabled and non-disabled. Social Behavior & Personality. 1999;27:87–97. [Google Scholar]
- Waters E, Doyle J, Wolfe R, Wright M, Wake M, Salmon L. Influence of parental gender and self-reported health and illness on parent-reported child health. Pediatrics. 2000;106:1422–1428. doi: 10.1542/peds.106.6.1422. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ, Hammond M. Treating children with early-onset conduct problems: intervention outcomes for parent, child, and teacher training. Journal of Clinical Child and Adolescent Psychology. 2004;33:105–124. doi: 10.1207/S15374424JCCP3301_11. [DOI] [PubMed] [Google Scholar]
- Weiss MJ. Hardiness and social support as predictors of stress in mothers of typical children, children with autism, and children with mental retardation. Autism. 2002;6:115–130. doi: 10.1177/1362361302006001009. [DOI] [PubMed] [Google Scholar]
- Williams CD, Taylor TR, Makambi K, Harrell J, Palmer JR, Rosenberg L, et al. CES-D four-factor structure is confirmed, but not invariant, in a large cohort of African American women. Psychiatry Research. 2007;150:173–180. doi: 10.1016/j.psychres.2006.02.007. [DOI] [PubMed] [Google Scholar]