Abstract
Using targeted mouse mutants and pharmacologic inhibition of αCaMKII, we demonstrate that the αCaMKII protein, but not its activation, autophosphorylation or its ability to phosphorylate synapsin I, is required for normal short-term presynaptic plasticity. Furthermore, αCaMKII regulates the number of docked vesicles independent of its ability to be activated. These results indicate that αCaMKII has a nonenzymatic role in short-term presynaptic plasticity at hippocampal CA3-CA1 synapses.
The α isoform of Ca2+/calmodulin-dependent protein kinase II (αCaMKII) was originally identified as synapsin I kinase1. Subsequent studies showed that αCaMKII is abundantly associated with presynaptic vesicles by binding to synapsin I (ref. 2). Together with the observation that αCaMKII is one of the most abundant proteins of the hippocampus3, these results suggest that αCaMKII also has a nonenzymatic function, but such a function has not directly been demonstrated yet. Analysis of αCaMKII knockout (KO) mice confirmed a presynaptic role for αCaMKII in short-term presynaptic plasticity4,5, but these experiments did not address whether this role is mediated by αCaMKII as a kinase, as a structural protein, or as both.
To study the enzymatic requirements of αCaMKII in presynaptic plasticity, we used four different lines of αCaMKII mutants. Autophosphorylation at the threonine 286 (T286) and T305/T306 sites was prevented by using αCaMKII-T286A (T286 is mutated to alanine) mice, which lack αCaMKII autonomous (Ca2+/CaM independent) activity6, and αCaMKII-T305V/T306A (T305 and T306 are mutated to valine and alanine, respectively) mice, which lack αCaMKII inhibitory autophosphorylation7. Furthermore, we used αCaMKII-T305D (T305 is mutated to an aspartic acid) mice, in which constitutive autophosphorylation at the T305 site in the Ca2+/CaM domain is mimicked, preventing αCaMKII in these mice from becoming activated7. The fourth line (αCaMKII-KO) lacks the entire αCaMKII protein7 (for an overview of all phenotypes see8).
Because these mutants were backcrossed in C57BL/6, we first tested whether the originally reported long-term potentiation (LTP) deficits (in hybrid 129Sv/C57BL/6 mice) were still present6,7,9. We confirmed that αCaMKII activation and its subsequent autophosphorylation at T286 are absolute requirements for LTP, but that loss of αCaMKII can partially be compensated for (Supplementary Fig. 1 online). In contrast, loss of inhibitory phosphorylation in αCaMKII-T305V/T306A mice reduced the threshold for LTP induction as reported previously7 (Supplementary Fig. 1). Western blots of isolated synaptosomes of all the mutants did not reveal changes in the levels of the β, γ and δ CaMKII isoforms, nor was the amount of calmodulin affected in these mutants (Supplementary Fig. 2 online).
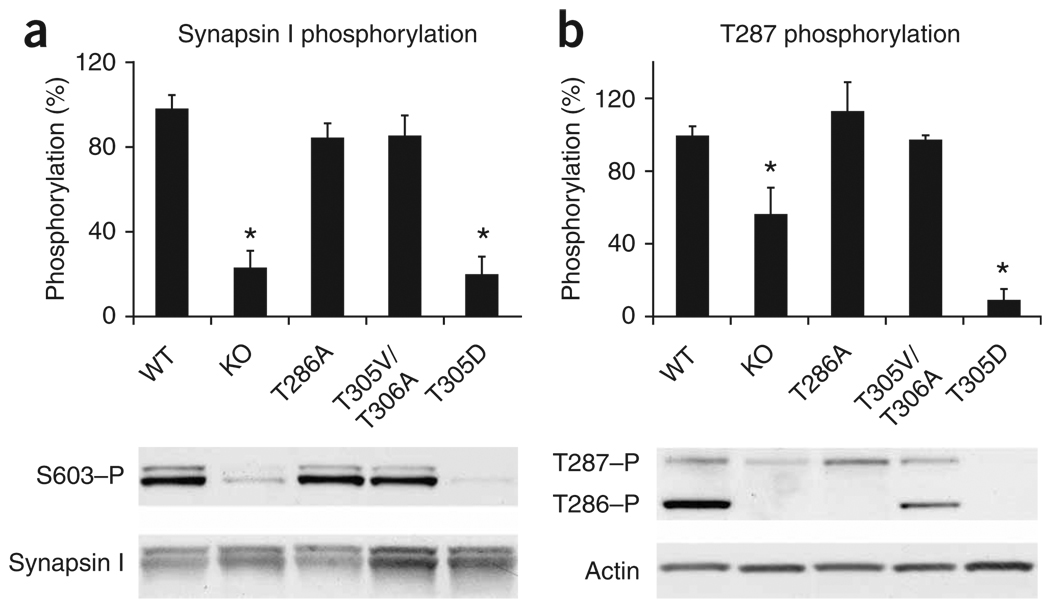
We looked at the ability of these mutants to phosphorylate synapsin I at serine 603 (S603, site 3), which is an exclusive CaMKII site. αCaMKII-KO mice showed a marked decrease of synapsin I phosphorylation compared to wild-type mice (P < 0.001, ANOVA, Fig. 1), suggesting that none of the other CaMKII isoforms could efficiently compensate for the loss of αCaMKII phosphorylation of synapsin I in vivo. Notably, steady-state levels of phosphorylated synapsin I were not affected in αCaMKII-T286A (P = 0.2) mice and in αCaMKII-T305V/306A mice (P = 0.3), indicating that loss of autonomous activity or self-inhibition does not have a large effect on synapsin I phosphorylation in vivo. In contrast, activation of αCaMKII is an absolute requirement for synapsin I phosphorylation, as S603 phosphorylation in the αCaMKII-T305D mutant was not significantly above background level (P = 0.2; Fig. 1a). The dominant-negative nature of the αCaMKII-T305D mutation was further illustrated by the fact that this was also the only mutant inwhich autophosphorylation at both αCaMKII-T286 and βCaMKII-T287 was indistinguishable from background level (both P > 0.8; Fig. 1b), further suggesting that αCaMKII is inactive in this mutant. Taken together, these results show that these mutants provide an ideal tool for dissecting the requirements of αCaMKII activation, αCaMKII autophosphorylation and synapsin I S603 phosphorylation in short-term presynaptic plasticity.
Figure 1.
Phosphorylation of synapsin I and CaMKII-T286/T287 in synaptosomes obtained from αCaMKII mutants. (a) Phosphorylation of synapsin I S603 was not affected by impaired αCaMKII autophosphorylation, but required αCaMKII protein and its activation by Ca2+/calmodulin. (b) Phosphorylation of αCaMKII-T286 and βCaMKII-T287 was absent in αCaMKII-T305D mice. Graph represents data from βCaMKII-T287 only. Error bars indicate s.e.m. Each sample contains pooled fractions from four independent isolations.
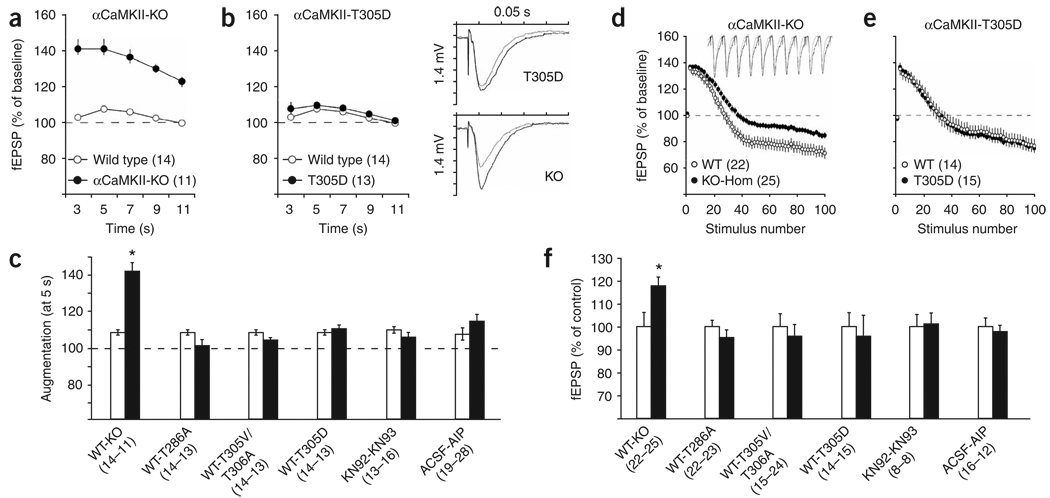
Previous studies from multiple laboratories4,5,8, using independently generated targeted deletions of αCaMKII5,9, have demonstrated that the loss of αCaMKII results in enhanced augmentation and decreased synaptic fatigue, which are both measures of presynaptic plasticity. Augmentation is an increase in the evoked postsynaptic response that is observed several seconds after high-frequency afferent stimulation, caused by facilitated exocytosis10. We measured augmentation at hippocampal CA3-CA1 synapses using extracellular field recording (Supplementary Methods online). αCaMKII-KO mice showed a striking increase in synaptic augmentation (during 3–11 s: F4,92 = 13, P < 0.0001, repeated measures ANOVA; Fig. 2), confirming that αCaMKII critically regulates this form of presynaptic plasticity4. Activation of presynaptic αCaMKII may be important in synaptic augmentation, as transiently elevated presynaptic calcium is thought to be a major factor underlying this process. However, although αCaMKII activity is regulated by autophosphorylation, augmentation was not affected in the T286A and T305V/T306A autophosphorylation–deficient mutants (Fig. 2c). The lack of a phenotype in these mutants could reflect the short-term nature of this kind of plasticity, and/or the fact that phosphorylation of synapsin I S603 was unaffected in these mutants (Fig. 1). Unexpectedly however, augmentation was also unaffected in αCaMKII-T305D mutants (P = 0.5, ANOVA; Fig. 2b,c), where αCaMKII activation was blocked and synapsin I phosphorylation was absent (Fig. 1). These results strongly suggest that synaptic augmentation does not depend on the ability of αCaMKII to phosphorylate synapsin I, nor on the activation of αCaMKII.
Figure 2.
Presynaptic short-term plasticity requires αCaMKII protein, but not its autophosphorylation, activation or activity. (a–c) Increased synaptic augmentation in αCaMKII-KO mutant mice was not caused by the lack of CaMKII kinase activity. fEPSP responses (normalized to pretetanus baseline) of CaMKII-KO (a) and CaMKII-T305D (b) mice were recorded at the indicated time after a 10 theta-burst tetanus. Traces are from baseline response (gray) and the response 5 s post-tetanization (black). Augmentation summary of responses obtained 5 s post-tetanus normalized to baseline is shown in c. Black bars represent mutants or drug-treated slices, white bars represent control slices as indicated. (d–f). Decreased synaptic fatigue during repetitive stimulation in αCaMKII-KO mice was not caused by the lack of αCaMKII kinase activity. (d,e) fEPSP responses (normalized against baseline) of CaMKII-KO (d) and CaMKII-T305D (e) mice were recorded during a 10-Hz tetanus. Only the first and even numbered stimuli are shown for clarity. Traces are from wild-type (gray) and αCaMKII-KO slices (black) recorded from stimulus number 21–30. Depletion summary of the last (100) stimulus of the 10-Hz train is shown in f. Black bars represent mutant or drug-treated slices, normalized against the controls as indicated (white bars, set at 100%). Numbers between brackets indicate the number of slices. Error bars indicate s.e.m.
Because activation of both αCaMKII and βCaMKII seem to be impaired in the αCaMKII-T305D mutant (Fig. 1b), we would expect that a similar result should be obtained if the augmentation experiment is carried out in the presence of the membrane-permeable CaMKII inhibitor KN-93 (ref. 11). This inhibitor competes with Ca2+/calmodulin binding and therefore prevents activation of both αCaMKII and βCaMKII. LTP experiments in the presence of this inhibitor showed that KN-93 was indeed able to block LTP (Supplementary Fig. 1). In contrast, we observed similar levels of augmentation in the presence of KN-93 or its inactive analog KN-92 (P = 0.5, ANOVA; Fig. 2c), confirming that CaMKII activation is not required for normal augmentation.
Our biochemical analyses (Fig. 1) indicated that CaMKII activity in the αCaMKII-T305D mutant was reduced to such an extent that phosphorylation of CaMKII substrates was undetectable. If CaMKII activity is indeed not required for augmentation, the potent CaMKII inhibitor AIP-II (autocamtide-2–related inhibitory peptide II) should not affect augmentation either. This inhibitor is 500 times more potent than KN-93, and it is noncompetitive for Ca2+/calmodulin and exogenous substrates, thus also blocking basal and autonomously active CaMKII12. Efficient penetration was achieved by making use of AIP that was fused to the antennapedia transport peptide, and by preincubating slices for 1 h with AIP (see Supplementary Methods). Indeed, LTP was fully blocked, indicating that the drug was able to penetrate the slice (Supplementary Fig. 1), and notably, Ant–AIP-II showed no discernable effect on synaptic transmission (Supplementary Fig. 3 online), which makes it suitable for use in these experiments. However, like KN-93, Ant–AIP-II did not affect augmentation (P = 0.1, ANOVA; Fig. 2c). Taken together, these results indicate that αCaMKII protein, but not αCaMKII activity, is required for normal augmentation.
Previous whole-cell patch-clamp recordings in CA3-restricted αCaMKII-KO mice showed a substantial enhancement of the excitatory postsynaptic current amplitude during repetitive stimulation of CA3-CA1 synapses5, suggesting that the fatigue rate of neurotransmitter release is regulated by αCaMKII. Likewise, extracellular field recordings from our global αCaMKII-KO mice also demonstrated reduced synaptic fatigue (Fig. 2d). The responses to repetitive 10-Hz stimulation reveal the competing processes of facilitation, vesicle depletion and vesicle mobilization10. αCaMKII-KO mutants had a similarly shaped curve as wild-type mice; however, there was a significant effect of genotype (F1,45 = 4.5, P < 0.05) and a significant interaction between genotype and stimulus number (F99,4455 = 4.5, P < 0.0001). αCaMKII-KO mutants were only significantly different from wild-type mice after 20 stimuli (first 20: F19,855 = 1.2, P = 0.3; last 80: F79,3555 = 2.6, P < 0.001), suggesting a differential rate of vesicle depletion and/or mobilization, the cellular processes primarily responsible for the maintenance of excitatory postsynaptic potential (EPSP) amplitude during prolonged stimulation10.
To test the αCaMKII autophosphorylation and synapsin I phosphorylation requirements in this presynaptic measure, we repeated this experiment in the αCaMKII point mutants. No significant effect of genotype was observed in either αCaMKII-T286A or αCaMKII-T305V/T306A autophosphorylation-defective mutants (both P > 0.3, ANOVA at stimulus number 100; Fig. 2f). Notably, the depletion rate was also not affected in αCaMKII-T305D mutants (P > 0.7; Fig. 2e,f), in which activation of αCaMKII was prevented and phosphorylation of synapsin I was absent. In addition, there was no discernable effect on depression in slices treated with KN-93 or Ant–AIP-II (P = 0.9 and P = 0.7, respectively; Fig. 2f). Taken together, these data strongly suggest that the αCaMKII protein plays a structural role rather than an enzymatic role, during this form of short-term presynaptic plasticity.
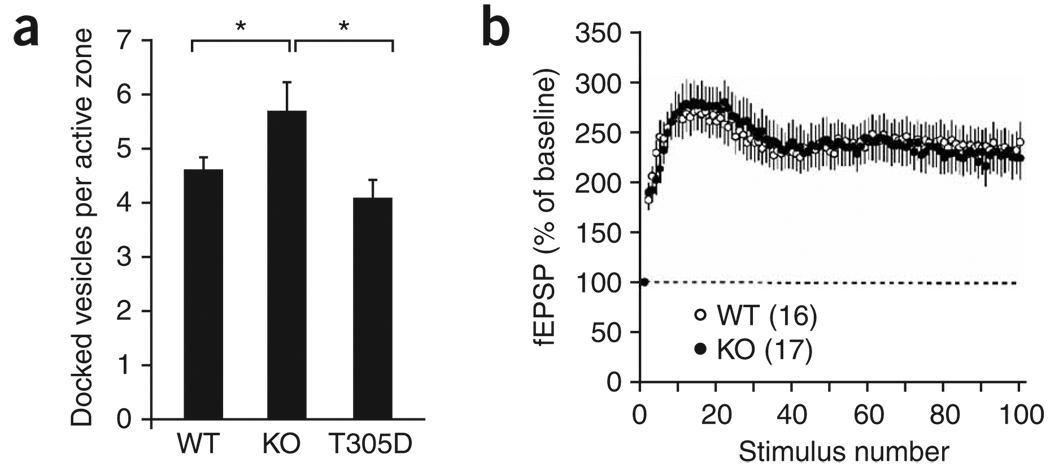
Mechanistically, enhanced synaptic augmentation and reduced synaptic depression could reflect changes in the pool sizes of the synaptic vesicles, in particular the size of the readily releasable pool (RRP). Therefore, we used electron microscopy to measure the number of docked vesicles, a morphological correlate of the RRP13,14. We obtained measurements at the active zone of excitatory synapses on CA1 spines of wild-type, αCaMKII-KO and αCaMKII-T305D mice. Indeed, there was a significant effect of genotype (F2,41 = 4.7, P < 0.05), with synapses in αCaMKII-KO mice showing a 20% increase in the total number of docked vesicles compared with wild-type (Fisher’s PLSD, P < 0.05) or αCaMKII-T305D mice (P < 0.01) (Fig. 3a). In contrast, no significant difference in the number of docked vesicles was observed between the αCaMKII-T305D mutant and wild-type mice (Fisher’s PLSD, P = 0.24). Additional measurements of the number of reserve pool vesicles, active zone length and presynaptic terminal area were similar between the mutants and wild-type mice (all measures P > 0.2; Supplementary Fig. 4 online). Thus, the absence of αCaMKII protein results in an increased number of docked vesicles, whereas the loss of αCaMKII activation and synapsin I S603 phosphorylation does not affect vesicle docking.
Figure 3.
αCaMKII protein regulates the number of docked vesicles. (a) Quantitative electron microscopy of asymmetric synapses on dendritic spines of CA1 pyramidal neurons showed a 20% increase in the number of docked vesicles in αCaMKII-KO mice. (b) Decreasing the depletion rate by lowering extracellular calcium reversed the phenotype of the αCaMKII-KO mice during repetitive stimulation. Error bars indicate s.e.m.
Together, these results suggest a model in which αCaMKII functions nonenzymatically to limit the size of the RRP, thereby modulating short-term presynaptic plasticity. If the observed increase in the size of EPSPs during repetitive stimulation in αCaMKII-KO mice is indeed mediated by a larger RRP, then presynaptic function should be normal under conditions that minimize depletion of the RRP. Accordingly, we decreased the extracellular calcium concentration, which limits the rate of depletion from the RRP15. Decreasing the extracellular calcium concentration from 2.5 to 0.8 mM normalized the responses during 10-Hz stimulation in αCaMKII-KO mice (F1,31 < 0.0001, P = 1, Fig. 3b), supporting the idea that αCaMKII limits the available pool of readily-releasable neurotransmitter vesicles.
Taken together, our experiments suggest that synapsin I S603 phosphorylation and αCaMKII activity are not required for short-term presynaptic plasticity measures such as augmentation and synaptic fatigue during repetitive stimulation. Specifically, we have demonstrated that, at hippocampal CA3-CA1 synapses, αCaMKII functions independently of its kinase activity to modulate short-term presynaptic plasticity by limiting the number of presynaptic docked neurotransmitter vesicles.
Supplementary Material
ACKNOWLEDGMENTS
We greatly appreciate the help of H. Beck, M. Merkens, R. Anwyl, K. Wang and R. Colbran for their (hands-on) advice on the use of CaMKII inhibitors, and from G. Borst and S. Kushner and members of the Silva and Elgersma lab for stimulating discussions and critically reading the manuscript. We thank M. Elgersma, H. van der Burg and E. Phillips for technical support. This work was supported by grants from NWO-ZonMW (TOP, VIDI) and Neuro-BSIK to Y.E. We also thank the University of Alabama, Birmingham Neuroscience Cores (P30-NS47466, P30-HD38985, P30-NS57098).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Published online at http://www.nature.com/natureneuroscience
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Bennett MK, Erondu NE, Kennedy MB. J. Biol. Chem. 1983;258:12735–12744. [PubMed] [Google Scholar]
- 2.Benfenati F, et al. Nature. 1992;359:417–420. doi: 10.1038/359417a0. [DOI] [PubMed] [Google Scholar]
- 3.Erondu NE, Kennedy MB. J. Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PF, Frenguelli BG, Smith A, Chen CM, Silva AJ. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 5.Hinds HL, Goussakov I, Nakazawa K, Tonegawa S, Bolshakov VY. Proc. Natl. Acad. Sci. USA. 2003;100:4275–4280. doi: 10.1073/pnas.0530202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 7.Elgersma Y, et al. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 8.Elgersma Y, Sweatt JD, Giese KP. J. Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 10.Zucker RS, Regehr WG. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 11.Sumi M, et al. Biochem. Biophys. Res. Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- 12.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. Biochem. Biophys. Res. Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 13.Schikorski T, Stevens CF. Nat. Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- 14.Tyler WJ, Pozzo-Miller LD. J. Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borst JG, Sakmann B. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.