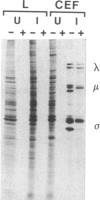
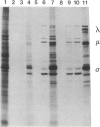
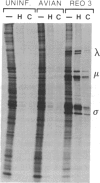
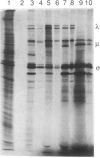
Abstract
Avian reovirus S1133 penetrates and uncoats in suspension cultures of mouse L cells. The multiple species of viral transcripts are produced in the cytoplasm of the infected cell, but they fail to associate with polysomes, consistent with the absence of viral protein synthesis. The selective block in avian virus mRNA translation is not overcome by coinfection with mammalian reovirus type 3, which replicates in mouse L cells, or by hypertonic shock or exposure to a low concentration of cycloheximide. Although the avian viral transcripts are inactive in vivo, RNA extracted from infected, nonpermissive L cells directs the synthesis of a normal spectrum of viral proteins in rabbit reticulocyte lysates. These results indicate that avian viral transcription is not restricted in mouse cells and that viral replication is prevented at the level of initiation of protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruzzi M., Shatkin A. J. Expression of reovirus p14 in bacteria and identification in the cytoplasm of infected mouse L cells. Virology. 1986 Aug;153(1):35–45. doi: 10.1016/0042-6822(86)90005-x. [DOI] [PubMed] [Google Scholar]
- DePhilip R. M., Rudert W. A., Lieberman I. Preferential stimulation of ribosomal protein synthesis by insulin and in the absence of ribosomal and messenger ribonucleic acid formation. Biochemistry. 1980 Apr 15;19(8):1662–1669. doi: 10.1021/bi00549a022. [DOI] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M. Fertilization triggers unmasking of maternal mRNAs in sea urchin eggs. Mol Cell Biol. 1987 Nov;7(11):3947–3954. doi: 10.1128/mcb.7.11.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Jacobs-Lorena M. Selective translational regulation of ribosomal protein gene expression during early development of Drosophila melanogaster. Mol Cell Biol. 1985 Dec;5(12):3583–3592. doi: 10.1128/mcb.5.12.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M. M., Kirk D. L. Translational regulation of protein synthesis, in response to light, at a critical stage of Volvox development. Cell. 1985 Jun;41(2):419–428. doi: 10.1016/s0092-8674(85)80015-5. [DOI] [PubMed] [Google Scholar]
- Kruys V., Wathelet M., Poupart P., Contreras R., Fiers W., Content J., Huez G. The 3' untranslated region of the human interferon-beta mRNA has an inhibitory effect on translation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6030–6034. doi: 10.1073/pnas.84.17.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Van Alstyne D., Berckmans R., Graham A. F. Synthesis of reovirus-specific polypeptides in cells pretreated with cycloheximide. J Virol. 1975 Sep;16(3):470–478. doi: 10.1128/jvi.16.3.470-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Cheng-Mayer C., Dina D., Luciw P. A. AIDS retrovirus (ARV-2) clone replicates in transfected human and animal fibroblasts. Science. 1986 May 23;232(4753):998–1001. doi: 10.1126/science.3010461. [DOI] [PubMed] [Google Scholar]
- Merits I. Actinomycin inhibition of "soluble" ribonucleic acid synthesis in rat liver. Biochim Biophys Acta. 1965 Dec 9;108(4):578–582. doi: 10.1016/0005-2787(65)90054-7. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C. D., Pech M., Robbins K. C., Aaronson S. A. The 5' untranslated sequence of the c-sis/platelet-derived growth factor 2 transcript is a potent translational inhibitor. Mol Cell Biol. 1988 Jan;8(1):284–292. doi: 10.1128/mcb.8.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. ACTINOMYCIN AND THE DIFFERENTIAL SYNTHESIS OF REOVIRUS AND L CELL RNA. Biochem Biophys Res Commun. 1965 May 3;19:506–510. doi: 10.1016/0006-291x(65)90154-3. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Nonpermissive infection of L cells by an avian reovirus: restricted transcription of the viral genome. J Virol. 1976 Sep;19(3):977–984. doi: 10.1128/jvi.19.3.977-984.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Graham A. F. Physical and chemical characterization of an avian reovirus. J Virol. 1976 Sep;19(3):968–976. doi: 10.1128/jvi.19.3.968-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N. M., Bray S. J., George E. L., Hunt T., Ruderman J. V. The small subunit of ribonucleotide reductase is encoded by one of the most abundant translationally regulated maternal RNAs in clam and sea urchin eggs. J Cell Biol. 1985 Jun;100(6):1968–1976. doi: 10.1083/jcb.100.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Walden W. E., Thach R. E. Translational control of gene expression in a normal fibroblast. Characterization of a subclass of mRNAs with unusual kinetic properties. Biochemistry. 1986 Apr 22;25(8):2033–2041. doi: 10.1021/bi00356a030. [DOI] [PubMed] [Google Scholar]
- Wiebe M. E., Joklik T. W. The mechanism of inhibition of reovirus replication by interferon. Virology. 1975 Jul;66(1):229–240. doi: 10.1016/0042-6822(75)90193-2. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Cereghini S., Krowczynska A., Brawerman G. Regulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxide. Mol Cell Biol. 1983 Jul;3(7):1197–1203. doi: 10.1128/mcb.3.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]