Abstract
Cyclic adenosine monophosphpate (cAMP) signaling is thought to be involved in the pathophysiology of major depressive disorder and antidepressant action; however, relatively little is known about the possible role of cyclic guanosine monophosphate (cGMP) signaling. Accumulating evidence suggests that crosstalk occurs between cAMP and cGMP pathways. There is a need to clarify the trajectory of cAMP and cGMP concentrations, their synthesis by cyclases, and degradation by phosphodiesterases (PDEs) to understand the role of cyclic mononucleotide signaling in the effect of chronic antidepressant therapy. We used quantitative real-time PCR and enzyme immunoassay to systematically investigate the expression of intracellular signaling cascade elements in the hippocampus of rats chronically treated with the antidepressants fluoxetine and amitriptyline. We found increased cGMP levels, which were consistent with our findings of decreased PDE gene expression. Immunoassay results showed unchanged cAMP levels. We conclude that increased cGMP signaling might underlie the efficacy of chronic antidepressant treatment.
Keywords: Antidepressant; Hippocampus; Nucleotides, Cyclic; Phosphodiesterase; Rat
Introduction
Several intracellular signaling pathways have been implicated in the pathophysiology of major depressive disorder (MDD) including two intracellular messaging systems related to cyclic mononucleotides [7, 24]. These signaling cascade elements therefore represent potential targets for the study of how antidepressants operate to relieve the clinical symptoms of MDD. Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are second messengers that send membrane signals to the nucleus to affect gene expression. Cyclic nucleotides are compartmentalized within cells and their levels are subject to temporal and spatial regulation via the activity of cyclase synthetic enzymes and phosphodiesterase (PDE) degradative enzymes.
Cyclic AMP is produced by adenylate cyclase (AC) and cGMP by guanylyl cyclase (GC). Whereas AC is activated by membrane bound G-protein complexes, GC exists in both membrane bound (pGC) and soluble (sGC) forms. There are 9 isoforms of adenylate cyclases (AC) classified into 4 groups based on sensitivities to calcium/calmodulin, βγ subunit, and PKC phosphorylation [8]. All AC isoforms are expressed in the brain, but their distribution is variable. Both AC1 and AC2 isoforms are expressed in the rodent hippocampus [12]. Soluble guanylyl cyclase (sGC) is a heterodimer consisting of α and β subunits, with two isoforms for each subunit (α1, α2, β1, and β2). The α2, β1 sGC heterodimer is expressed in the hippocampus [14].
By degrading cyclic mononucleotides, PDE enzymes play an essential regulatory function in these complex intracellular messaging systems. Recently, the role of PDEs in psychiatric disorders including MDD has attracted more attention as PDEs are expressed throughout the brain and as commercially available PDE inhibitor drugs have shown some potential as cognitive enhancers [1, 21, 22]. PDE genes are categorized based on their specificity to degrade cAMP (PDE4, PDE7, PDE8), cGMP (PDE5, PDE6, PDE9) or both (PDE1, PDE2, PDE3, PDE10, and PDE11). Each PDE gene may be subdivided into isoforms designated with different letters (PDE4A, PDE4B, PDE4C, and PDE4D) with further division of these isoforms into splice variants (PDE4A1, PDE4A5, PDE4A8, and PDE4A10) [13]. There are 11 different PDE families with 21 genes and 50 functional enzymes via alternative splicing.
As second messengers, cAMP and cGMP regulate the activity of several downstream effectors, including protein kinase A (PKA) and protein kinase G (PKG), respectively, as well as PDEs, cyclic nucleotide gated channels, and guanine exchange factors to impact various central nervous system functions such as learning, memory, and mood. Cyclic AMP and cGMP possibly regulate each other’s concentrations via crosstalk signaling [17]. Hormone and neurotransmitter signaling is mediated by cAMP and nitric oxide (NO) signaling is mediated by cGMP, as sGC is activated by NO. The cAMP signaling cascade is thought to play a critical role in MDD pathophysiology and antidepressant mediated improvement [16, 24]. Specifically, cAMP binds to and activates PKA and then PKA phosphorylates the transcription factor cAMP response-element binding protein (CREB) to activate CREB and initiate the expression of many genes such as brain-derived neurotrophic factor (BDNF).
The therapeutic actions of antidepressants have been related to increased cAMP signaling as BDNF and CREB expression has been found to increase following antidepressant administration. These findings have led to indirect assumptions of increased cAMP levels following chronic antidepressant administration [16]. In a recent study, we unexpectedly found that 8 weeks of imipramine treatment resulted in a significant 46% decrease in cAMP levels with increased expression of several PDE genes and guanylyl cyclase subunits, and decreased expression of adenylate cyclase, consistent with the hypothesis that chronic antidepressants desensitize the beta-adrenergic receptor/adenylate cyclase system beyond the receptor level [11, 20]. To test the hypothesis that common intracellular signaling cascades, such as the cAMP and cGMP signaling pathways, are altered by antidepressant treatment, we chose to study the effects of two other prototypic antidepressants, fluoxetine (selective serotonin reuptake inhibitor) and amitriptyline (tricyclic antidepressant). We analyzed changes in gene expression in the PDE and cyclase gene families and assayed cAMP and cGMP levels in rat hippocampi to understand the alterations that occur in the cyclic mononucleotide messenger systems as a result of sustained antidepressant treatment.
Materials and Methods
Animals and drug treatment
Virus- and antibody-free adult, male Sprague Dawley rats (150–200 g, Harlan, Indianapolis, IN) were housed 2/cage 24°C with lights on from 06:00 to 18:00 h in a stress free environment for at least 5 days before the initiation of experimental procedures. Ten rats were randomly assigned to each experimental group: 1) control (0.9% saline), 2) fluoxetine (FLX, selective serotonin reuptake inhibitor), and 3) amitriptyline (AMI, tricyclic). All animals received daily 0.5 mL intraperitoneal (i.p.) injections of either 0.9% saline (Hospira Inc., Lake Forest, IL), 5.0 mg/kg of fluoxetine (generously provided by Eli Lilly, Indianapolis, IN) or 10.0 mg/kg amitriptyline (Sigma-Aldrich, St Louis, MO) dissolved in 0.9% saline for eight weeks. The 5.0 mg/kg dose for fluoxetine and 10 mg/kg dose for amitriptyline is based on previous reports [3, 29]. We chose the rigorous duration of eight weeks antidepressant treatment as the time course of administration to be sure that any results we observed in the rat brain would accurately reflect the effect of long-term, repeated antidepressant treatment. Recent evidence from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) clinical trials indicates that at least 8 weeks of continuous therapy is required for antidepressant efficacy in patients that will respond to therapy [26]. Furthermore, maintenance antidepressant therapy is prescribed for months to years to prevent relapse.
Animals received their last treatment 24 hours before termination of experiments. Animals were euthanized by decapitation and the hippocampus was dissected, snapfrozen, and stored at −80°C. All animals were sacrificed between 10:00–12:00 h to avoid confounding effects of circadian rhythms. This experimental protocol was approved by The University of Miami Institutional Animal Care and Use Committee.
Quantitative real-time RT-PCR
Total RNA was isolated from hippocampi using RNeasy Lipid Mini Kit including optional DNase digestion (Qiagen, Germantown, MD), quantified using a Nanodrop ND-1000 spectrophotometer (Thermo-Fisher Scientific, Waltham, MA) and reverse transcribed to cDNA using OMNISCRIPT RT Kit (Qiagen), random hexamer primers, and 1 µg RNA. We selected to study the expression of isoforms of the PDE3, PDE4, and PDE5 genes as they are expressed in the hippocampus and currently pharmacologic inhibitors selective for these 3 PDEs are available for use clinically. We selected to study AC1, AC2, GCα2, and GCβ1 isoforms as these cyclase genes are expressed in the hippocampus. PDE, cyclase, β-Actin (housekeeping gene) primers were designed in different exons to amplify cDNA using Primer Express Software (Applied Biosystems, Foster City, CA) as previously described [20]. PDE4A (NM_013101) primers were: forward (5'-GAAGTCTCAGGTGGGCTTCATC-3') and reverse (5'-CAGTCCCGGTTGTCTTCCA-3'). PDE4D (NM_017032) primers were: forward (5'-CCTCCAGAATATGGTGCACTGT-3') and reverse (5'-TATCTCCATGCCACGCTCA-3')[28]. AC2 (NM_031007) primers were: forward (5'-CGCCTTGCAAGCGATTG-3') and reverse (5'-TGCATTCATTCTCCTTTGCTATTT-3'). A standard curve of pooled, serially diluted cDNA was run for each PDE or cyclase gene and for a housekeeping gene, rat β-Actin, using the 7900HT Fast Real-Time PCR System (Applied Biosystems). cDNA samples were diluted 1:500 and run in triplicate for both rat β-Actin and the PDE or cyclase gene of interest. The three SYBR cycle threshold (Ct) values were averaged for each sample, and the RNA input for the target gene was calculated from the standard curve. Fold change in hippocampal target gene expression was expressed as a ratio to β-Actin expression for each sample. We confirmed that the expression of β-Actin was stable across treatment groups by comparing Ct values of the β-Actin housekeeping gene among the different experimental conditions (SAL, FLX, AMI) and found no significant variations (p= 0.6327).
Enzyme immunoassay (EIA)
Cyclic nucleotide (cAMP and cGMP) levels in the hippocampus were determined using direct, competitive enzyme immunoassay kits (Assay Designs, Ann Arbor, MI). Hippocampi were homogenized in 10 volumes of 0.1 M HCl and centrifuged ≥ 600 × g at room temperature. The supernatant was acetylated to improve signal detection and samples were run in duplicate. The concentration of cAMP or cGMP is expressed as pmol/mL.
Statistical Analysis
The data were analyzed using one-way analysis of variance with Neuman-Keuls post hoc tests with the significance level set at p<0.05.
Results
Quantitative real-time RT-PCR
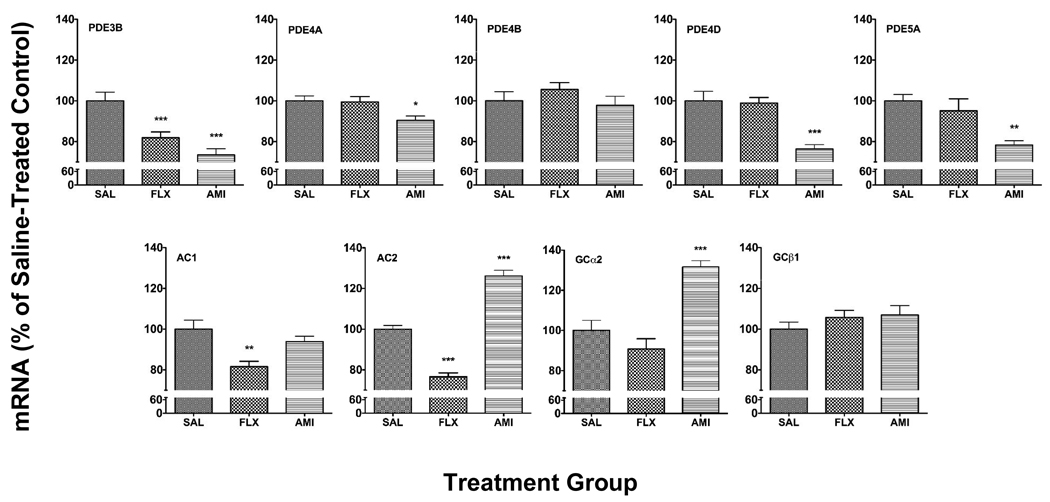
Chronic treatment with FLX and AMI decreased the expression of PDE genes studied. Both antidepressants decreased the dual substrate gene, PDE3B (−18%, p<0.01, FLX; −27%, p<0.001, AMI). Chronic AMI decreased the cAMP specific PDE genes, PDE4A (−10%, p<0.01) and PDE4D (−24%, p<0.001) and the cGMP specific PDE gene, PDE5A (−21%, p<0.001). The expression of PDE4B was not affected by these antidepressant treatments. The transcription of the AC1 gene was decreased by FLX (−18%, p<0.01). The AC2 gene was also decreased by FLX (−23%, p<0.001), whereas it was increased by AMI (+26%, p<0.001). GCα2 was increased by AMI (+32%, p<0.001), but not by FLX. The expression of GCβ1 was not affected by the antidepressant treatments (Fig. 1).
Fig. 1.
Effect of chronic fluoxetine (FLX) and amitriptyline (AMI) treatment on PDE and cyclase gene expression in the rat hippocampus. Results are expressed as mean + s.e.m. for FLX and AMI as percentage of saline-treated control (SAL) (n=9–10 animals/group). Asterisks indicate significant differences versus SAL (* p<0.05, ** p<0.01, *** p<0.001 one-way analysis of variance with Neuman-Keuls post hoc analyses).
Cyclic Nucleotide Enzyme immunoassays
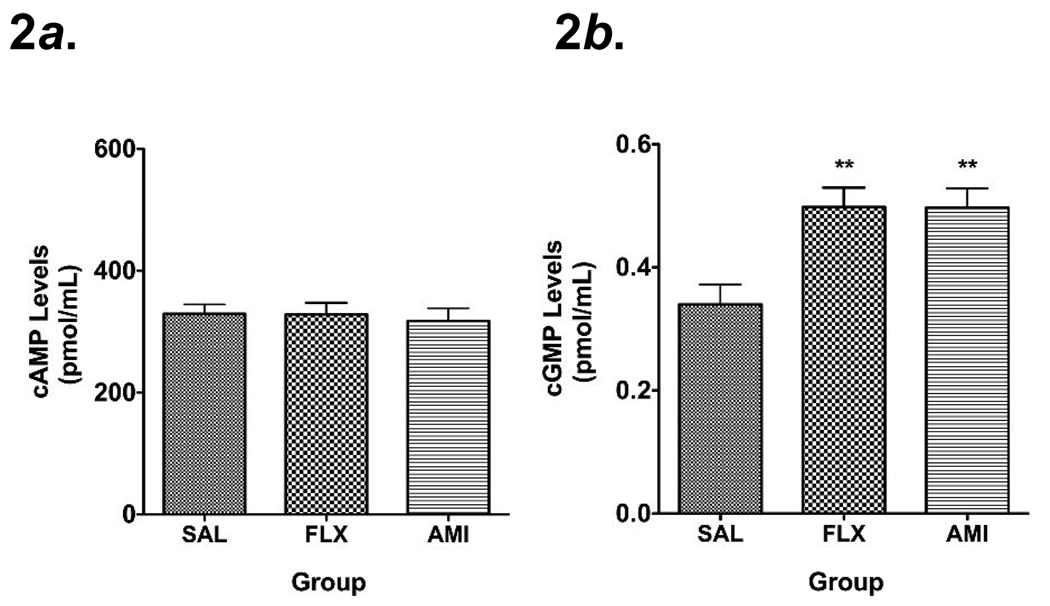
Hippocampal cGMP levels were increased following chronic FLX and AMI (Fig. 2b). In contrast, hippocampal cAMP levels were unchanged following chronic antidepressant treatment (Fig. 2a).
Fig. 2.
Mean + s.e.m. concentrations of cAMP (Fig. 2a) and cGMP (Fig. 2b) (expressed as pmol/mL) in the hippocampus of rats treated chronically with saline (SAL), fluoxetine (FLX) and amitriptyline (AMI) (n=9–10 animals/group). Asterisks indicate significant differences versus SAL (** p<0.01 one-way analysis of variance with Neuman-Keuls post- hoc analyses).
Discussion
In the present study, we have found that chronic, 8 week treatment with fluoxetine and amitriptyline resulted in decreased PDE gene expression, increased cGMP levels, and did not change cAMP levels in the rat hippocampus. These antidepressants displayed differential effects on cyclase gene expression. Fluoxetine decreased expression of two adenylate cyclase isoforms, AC1 and AC2, whereas amitriptyline increased expression of the AC2 isoform. Amitriptyline also increased expression of one of the subunits for the guanylyl cyclase gene, GCα2. These results are in contrast with our previous findings of increased PDE, decreased AC, decreased cAMP levels, and unchanged cGMP levels in the hippocampus of rats chronically treated with imipramine [20]. We hypothesized that if the regulation of cyclic nucleotide levels is relevant to the therapeutic antidepressant effect, then different prototypic antidepressants should cause similar changes after chronic treatment. However, we have showed this may not appear to be the case. It is possible that different mechanisms of actions of these drugs at the synapse might account for these results. Imipramine and amitriptyline are both tricyclic antidepressants that non-selectively block monoamine transporters at the synapse and both have a greater binding affinity for the serotonin transporter (imipramine, K i=7.7 nM; amitriptyline, K i=14.7 nM) than for the norepinephrine transporter (imipramine, K i= 67 nM; amitriptyline, K i=100 nM). However, desipramine, the active metabolite of imipramine, has a much higher binding affinity for the norepinephrine transporter (K i = 4 nM) than for the serotonin transporter (K i = 61 nM). Therefore, amitriptyline may have mainly serotonin effects while imipramine has mainly norepinephrine effects.
The PDE and cyclase gene expression results are congruent with our findings of increased cGMP levels for fluoxetine (+47%, p<0.01) and amitriptyline (+46%, p<0.01). Amitriptyline treatment decreased two cGMP degradative enzymes, the cGMP specific PDE5A and the dual substrate PDE3B and increased one of the subunits of the cGMP synthetic enzyme, GCα2 to increase cGMP levels. Although fluoxetine treatment did not affect the expression of the guanylyl cyclase genes, it did decrease a cGMP degradative enzyme, the dual substrate PDE3B to increase cGMP levels. The PDE and cyclase gene expression results for fluoxetine are congruent with our finding of unchanged cAMP for fluoxetine, but not for amitriptyline. Fluoxetine treatment decreased cAMP degradative enzyme, the dual substrate PDE3B, and decreased two isoforms of the cAMP synthetic enzyme, AC1 and AC2; therefore, synthesis and degradation of cAMP are balanced and cAMP is unchanged with chronic fluoxetine. Amitriptyline decreased two isoforms of cAMP degradative enzymes, the cAMP specific PDE4A and PDE4D, and increased one isoform of the cAMP synthetic enzyme, AC2. These PDE and cyclase changes we would suggest that cAMP levels would be increased; however, we found cAMP levels unchanged with chronic amitriptyline. It is important to note that the gene expression results we observed might not be reflected in changes in PDE and cyclase protein expression. Instead of using western blotting to determine PDE and cyclase protein levels, we used cyclic nucleotide enzyme immunoassay as an indirect measure of PDE enzyme activity. Future studies will be necessary to determine whether changes in PDE and cyclase gene expression following chronic antidepressant treatment are directly related to changes in the expression and activity of these proteins. Although we limited our analysis to the expression of PDE3, PDE4, and PDE5 because of the clinical availability of selective inhibitor drugs, it is possible that the expression of other PDEs that are present in the hippocampus, such as PDE2, could have been altered with chronic antidepressant treatment.
The effects of chronic antidepressant treatment have been presumed to occur through increased cAMP signaling via increased intracellular cAMP, but most studies have not directly measured cyclic nucleotides. We quantified hippocampal cAMP and cGMP content and unexpectedly found increased cGMP and unchanged cAMP with fluoxetine and amitriptyline. These results suggest that increased hippocampal cGMP might underlie the efficacy of chronic antidepressant treatment. A role for the NO/cGMP signaling pathway in MDD and antidepressant action is just beginning to be understood. Previous studies have shown that drugs that increase NO/cGMP signaling are anxiogenic whereas drugs that decrease NO/cGMP signaling display antidepressant effects in behavioral tests of anxiety and depression in rodents following acute administration [9, 10, 27]. However, drugs that increase NO demonstrate neuroprotective effects in cultured hippocampal cells in vitro and enhance long-term potentiation following low frequency stimulation in rat hippocampal slices [2, 5]. The cGMP specific PDE5 inhibitor sildenafil displays antidepressant effects in a behavioral test of depression in rodents only when combined with atropine, a muscarinic acetylcholine receptor (mAchR) antagonist [4]. Sildenafil also decreases scopolamine-induced deficits in memory in a T-maze passive avoidance task and has been shown to improve object recognition memory in rats, suggesting a cognitive enhancing effect of PDE5 inhibition [6, 19]. Clinically used for the treatment of erectile dysfunction (ED), sildenafil is safe and effective in male patients in remission from MDD and self-reports suggest a lowering of depressive symptoms with sildenafil treatment in ED patients [15, 25]. These studies point to the possibility that the NO/cGMP cascade might be dysregulated in MDD, and that antidepressant efficacy could be related to the normalization of dysfunctional NO/cGMP signaling in the brain.
We have measured global levels of cAMP and cGMP representative of several sub compartments using enzyme immunoassay in the hippocampus of rats euthanized by decapitation. A previous study comparing the effects of two different methods of euthanasia, microwave irradiation (MWI) of the brain versus decapitation, found that in some brain areas, such as the striatum, a rapid decrease in cAMP occurs following decapitation but not following MWI [23]. In other brain areas, such as the cortex, cAMP levels did not differ following decapitation versus MWI. This study did not evaluate differences in hippocampal cAMP levels comparing decapitation versus MWI. Our data was collected from the hippocampi of rats euthanized by decapitation and therefore the cyclic nucleotide level changes we observed might differ from those using the microwave irradiation technique. Although we have chosen to focus on antidepressant-mediated changes in the hippocampus in the present study, it is known that other brain areas, such as the cortex, have been implicated in the pathophysiology of MDD and antidepressant response. Therefore, future studies should address whether chronic antidepressants also alter cGMP signaling in the frontal cortex. The activity of cyclases and PDEs in time and space compartmentalizes cyclic nucleotides within cells. We have measured the global content of cAMP and cGMP in the hippocampus, and these relative levels do not necessarily reflect localized changes in cAMP and cGMP in specific hippocampal cell types or areas such as Cornu Ammonis (CA) fields.
Based on our PDE, cyclase, and cGMP findings, we suggest that these results may support a role for increased activation of cGMP signaling pathways relative to cAMP signaling pathways in the action of antidepressants. This hypothesis would also be compatible with our recent report on decreased cAMP levels after chronic imipramine treatment [20]. Further studies utilizing animal models of depression are necessary to determine whether an overall increase in hippocampal cGMP content following chronic fluoxetine and amitriptyline has an impact on antidepressant treatment response. While cAMP is present in much higher concentrations (~1000×) in the hippocampus, cGMP is thought to serve important roles in memory and cognition [18]. There is also evidence of crosstalk between the cAMP and cGMP signaling cascades [17]. In summary, these results indicate a possible role for intracellular cGMP signaling in MDD and antidepressant action.
Acknowledgements
This study was supported in part by a National Institutes of Health Grant K24RR017365 to MLW and K24RR016996 to JL and by institutional funds from the Department of Psychiatry and Behavioral Sciences at The University of Miami Miller School of Medicine. Fluoxetine hydrochloride was generously provided by Eli Lilly. The authors would like to thank Eridania Valdes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest: None
References
- 1.Blokland A, Schreiber R, Prickaerts J. Improving memory: a role for phosphodiesterases. Current pharmaceutical design. 2006;12:2511–2523. doi: 10.2174/138161206777698855. [DOI] [PubMed] [Google Scholar]
- 2.Bon CL, Garthwaite J. Exogenous nitric oxide causes potentiation of hippocampal synaptic transmission during low-frequency stimulation via the endogenous nitric oxide-cGMP pathway. The European journal of neuroscience. 2001;14:585–594. doi: 10.1046/j.0953-816x.2001.01680.x. [DOI] [PubMed] [Google Scholar]
- 3.Brady LS, Gold PW, Herkenham M, Lynn AB, Whitfield HJ., Jr The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain research. 1992;572:117–125. doi: 10.1016/0006-8993(92)90459-m. [DOI] [PubMed] [Google Scholar]
- 4.Brink CB, Clapton JD, Eagar BE, Harvey BH. Appearance of antidepressant-like effect by sildenafil in rats after central muscarinic receptor blockade: evidence from behavioural and neuro-receptor studies. J Neural Transm. 2008;115:117–125. doi: 10.1007/s00702-007-0806-5. [DOI] [PubMed] [Google Scholar]
- 5.Culmsee C, Gerling N, Landshamer S, Rickerts B, Duchstein HJ, Umezawa K, Klumpp S, Krieglstein J. Nitric oxide donors induce neurotrophin-like survival signaling and protect neurons against apoptosis. Molecular pharmacology. 2005;68:1006–1017. doi: 10.1124/mol.105.013086. [DOI] [PubMed] [Google Scholar]
- 6.Devan BD, Sierra-Mercado D, Jr, Jimenez M, Bowker JL, Duffy KB, Spangler EL, Ingram DK. Phosphodiesterase inhibition by sildenafil citrate attenuates the learning impairment induced by blockade of cholinergic muscarinic receptors in rats. Pharmacology, biochemistry, and behavior. 2004;79:691–699. doi: 10.1016/j.pbb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Reierson GW, Rong Luo H, Sheng Wu G, Licinio J, Wong ML. Phosphodiesterase genes and antidepressant treatment response: A review. Annals of medicine. 2008:1–9. doi: 10.1080/07853890802441169. [DOI] [PubMed] [Google Scholar]
- 8.Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, Suzuki Y, Defer N. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Molecular and cellular endocrinology. 1997;128:179–194. doi: 10.1016/s0303-7207(97)04013-6. [DOI] [PubMed] [Google Scholar]
- 9.Heiberg IL, Wegener G, Rosenberg R. Reduction of cGMP and nitric oxide has antidepressant-like effects in the forced swimming test in rats. Behavioural brain research. 2002;134:479–484. doi: 10.1016/s0166-4328(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 10.Kurt M, Bilge SS, Aksoz E, Kukula O, Celik S, Kesim Y. Effect of sildenafil on anxiety in the plus-maze test in mice. Pol J Pharmacol. 2004;56:353–357. [PubMed] [Google Scholar]
- 11.Manier DH, Shelton RC, Sulser F. Noradrenergic antidepressants: does chronic treatment increase or decrease nuclear CREB-P?, J Neural Transm. 2002;109:91–99. doi: 10.1007/s702-002-8239-6. [DOI] [PubMed] [Google Scholar]
- 12.Matsuoka I, Suzuki Y, Defer N, Nakanishi H, Hanoune J. Differential expression of type I, II, and V adenylyl cyclase gene in the postnatal developing rat brain. Journal of neurochemistry. 1997;68:498–506. doi: 10.1046/j.1471-4159.1997.68020498.x. [DOI] [PubMed] [Google Scholar]
- 13.Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–670. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- 14.Mergia E, Russwurm M, Zoidl G, Koesling D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003;15:189–195. doi: 10.1016/s0898-6568(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 15.Muller MJ, Benkert O. Lower self-reported depression in patients with erectile dysfunction after treatment with sildenafil. Journal of affective disorders. 2001;66:255–261. doi: 10.1016/s0165-0327(00)00295-0. [DOI] [PubMed] [Google Scholar]
- 16.Nair A, Vaidya VA. Cyclic AMP response element binding protein and brain-derived neurotrophic factor: molecules that modulate our mood? Journal of biosciences. 2006;31:423–434. doi: 10.1007/BF02704114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Progress in neurobiology. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 18.Prickaerts J, Sik A, van Staveren WC, Koopmans G, Steinbusch HW, van der Staay FJ, de Vente J, Blokland A. Phosphodiesterase type 5 inhibition improves early memory consolidation of object information. Neurochemistry international. 2004;45:915–928. doi: 10.1016/j.neuint.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Prickaerts J, van Staveren WC, Sik A, Markerink-van Ittersum M, Niewohner U, van der Staay FJ, Blokland A, de Vente J. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113:351–361. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 20.Reierson GW, Mastronardi CA, Licinio J, Wong ML. Chronic imipramine downregulates cyclic AMP signaling in rat hippocampus. Neuroreport. 2009;20:307–311. doi: 10.1097/wnr.0b013e328321b5a0. [DOI] [PubMed] [Google Scholar]
- 21.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose GM, Hopper A, De Vivo M, Tehim A. Phosphodiesterase inhibitors for cognitive enhancement. Current pharmaceutical design. 2005;11:3329–3334. doi: 10.2174/138161205774370799. [DOI] [PubMed] [Google Scholar]
- 23.Schneider HH. Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochemical pharmacology. 1984;33:1690–1693. doi: 10.1016/0006-2952(84)90295-8. [DOI] [PubMed] [Google Scholar]
- 24.Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Annals of medicine. 2007;39:531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- 25.Tignol J, Furlan PM, Gomez-Beneyto M, Opsomer R, Schreiber W, Sweeney M, Wohlhuter C. Efficacy of sildenafil citrate (Viagra) for the treatment of erectile dysfunction in men in remission from depression. International clinical psychopharmacology. 2004;19:191–199. doi: 10.1097/01.yic.0000117902.43995.b0. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 27.Volke V, Wegener G, Vasar E. Augmentation of the NO-cGMP cascade induces anxiogenic-like effect in mice. J Physiol Pharmacol. 2003;54:653–660. [PubMed] [Google Scholar]
- 28.Wang H, Edens NK. mRNA expression and antilipolytic role of phosphodiesterase 4 in rat adipocytes in vitro. Journal of lipid research. 2007;48:1099–1107. doi: 10.1194/jlr.M600519-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Yau JL, Olsson T, Morris RG, Meaney MJ, Seckl JR. Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience. 1995;66:571–581. doi: 10.1016/0306-4522(94)00612-9. [DOI] [PubMed] [Google Scholar]