Abstract
In this review, we summarize our research program that has as its goal charting the typical and atypical development of the social brain in children, adolescents, and adults with and without autism. We highlight recent work using virtual-reality stimuli, eye tracking, and functional magnetic resonance imaging that has implicated the superior temporal sulcus (STS) region as an important component of the network of brain regions that supports various aspects of social cognition and social perception. Our work in typically developing adults has led to the conclusion that the STS region is involved in social perception via its role in the visual analysis of others’ actions and intentions from biological-motion cues. Our work in adolescents and adults with high-functioning autism has implicated the STS region as a mechanism underlying social perception dysfunction in this neurodevelopmental disorder. We also report novel findings from a study of biological-motion perception in young children with and without autism.
Keywords: autism, fMRI, superior temporal sulcus
INTRODUCTION
Humans are deeply social creatures. They have existed for millennia in highly collective environments in which each person is dependent upon other individuals, including larger families and societal entities. Social cognition, broadly construed, is the term we use to refer to the fundamental abilities to perceive, categorize, remember, analyze, reason with, and behave toward other conspecifics. The extent to which such processes work successfully helps determine the fate of individual humans. A potential path to examine the neural basis of social cognition is to understand what separates those individuals who “do” social cognition effectively from those who do not. Our research program adopts this approach in two ways. We first study typically developing children, adolescents, and adults at different points along the developmental pathway toward mature social cognition abilities. Just as biology is gaining immeasurably from examinations of organisms in development, so it is with mental phenomena. In order to understand the neural basis of typical and atypical social cognition, it is important to understand the normative development of social cognition in children, because such a focus can provide the crucially needed early view of intricate mental processes during their formation. Advances in techniques for imaging the developing brain have provided exciting opportunities for studying the mechanisms involved in the development of social cognition abilities. We also compare brain function and brain development in individuals with and without autism, a disorder that limits social cognition. The use of functional neuroimaging to study abnormal brain function provides an approach in which brain differences can inform us about disease processes and also help us to better understand normal brain functioning and development.
For much of the twentieth century, psychologists produced theories and a wealth of empirical evidence concerning the basic building blocks of social cognition. This understanding is beginning to benefit from the use of noninvasive brain imaging techniques, including functional magnetic resonance imaging (fMRI), to identify a network of brain regions that supports various facets of social cognition in humans. In the past decade, studies of how we think about ourselves and other minds, how we mimic and change, and how we regulate our emotions and perceive emotions in other people have allowed us to learn about the various components of social cognition. However, this work has focused on the mature minds of adult humans almost exclusively. Little work has been conducted to explore patterns of brain development as they relate to the emergence and refinement of social cognition abilities, leaving this area wide open for investigation.
Through the lens of recent social neuroscience research, it is increasingly clear that social cognition abilities depend upon specialized brain systems for processes which include rapidly recognizing the faces of others, interpreting the actions of others through an analysis of biological-motion cues, and determining others’ emotional states via inspection of facial expression. Various proposals have been put forth describing the brain regions involved in social cognition. One early and influential model was outlined by Brothers (1990) who conceived of social cognition as the processing of information that culminates in the accurate analysis of the dispositions and intentions of other individuals and proposed the involvement of the amygdala, orbitofrontal cortex (OFC), and superior temporal sulcus (STS) region as key nodes of the primate “social brain.” In this and other models of the social brain, components of the neural circuitry supporting social cognition consist of mechanisms that are relatively old in evolutionary terms. The social world in which the human brain carries out its functions, however, has changed dramatically over a relatively short period. For example, a child born 50,000 years ago would have had similar mental powers as a child today, but a decidedly different set of demands, aspirations, and opportunities, not to mention rights, responsibilities, chances of survival, and definitions of success.
Work in our laboratory has focused on identifying and characterizing the development of psychological and brain mechanisms supporting a relatively simple aspect of social cognition, social perception, that refers to the initial stages of evaluating the emotions, actions, and intentions of others using their gaze direction, body movements, hand gestures, facial expressions, and other biological-motion cues (Allison, Puce, & McCarthy, 2000). We have employed virtual-reality character animation, behavioral, eye-tracking, and fMRI techniques to explore normal and abnormal development of brain mechanisms for social perception. In particular, we have focused on the use of biological-motion cues to interpret others’ actions. We will describe some of the key, recent findings from this research program regarding the role of the STS region in neurologically normal adults and in typically developing children. Then, we consider how these advances have informed, and have been informed by, our understanding of the brain mechanisms underlying social-perception dysfunction in autism.
DEFINING THE SOCIAL BRAIN
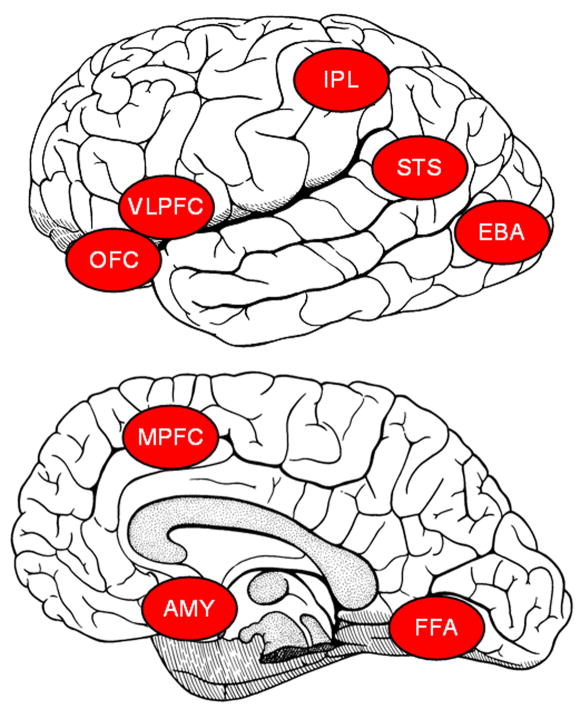
As illustrated in Figure 1, neuroscientists have described several brain regions that comprise the neural circuitry supporting various aspects of social cognition and social perception. These include: (1) the lateral fusiform gyrus or “fusiform face area”, which is important for structural encoding of faces in the environment and for rapid face recognition (Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996). (2) The posterior STS region, which has been implicated in processing dynamic expressions of emotion and in the interpretation of the actions and intentions of others through visual analysis of biological-motion cues (Bonda, Petrides, Ostry, & Evans, 1996; LaBar, Crupain, Voyvodic, & McCarthy, 2003; Pelphrey et al., 2003a; Pelphrey, Morris, & McCarthy, 2004a; Pelphrey, Singerman, Allison, & McCarthy, 2003b; Pelphrey, Viola, & McCarthy, 2004b). In humans, the STS region is a term used to describe the STS proper, portions of the superior and middle temporal gyri, and areas of the angular gyrus near the ascending limb of the STS (Allison et al., 2000). (3) The amygdala and interconnected frontal-limbic regions have been implicated in determining the emotional states of others through analysis of facial expressions (Adolphs, Tranel, Damasio, & Damasio, 1995; Morris et al., 1996) and plays a central and complex role in multiple aspects of emotion (Davis & Whalen, 2001; Kluver & Bucy, 1997; LeDoux, 1992). (4) The extrastriate body area (EBA), which has been implicated in the visual perception of human bodies (Downing, Jiang, Shuman, & Kanwisher, 2001). (5) A set of regions involving portions of the parietal cortex, including the inferior and superior parietal lobules, the anterior intraparietal sulcus, and frontal cortical regions, including the inferior frontal gyrus (IFG), has been dubbed the “mirror system” in humans because they activate equally to the execution of a motor action and the observation of a motor action by another person (Buccino et al., 2001; Iacoboni et al., 2001; Iacoboni et al., 1999; Rizzolatti, Fadiga, Gallese, & Fogassi, 1996). (6) The orbitofrontal cortex (OFC) has been implicated in the processing of reward and social reinforcement (Bechara, Damasio, Damasio, & Anderson, 1994; Rolls, 2000). While the OFC has been most commonly implicated in “low-level” representation of reward or punishment values (O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001), the more lateral (i.e. ventral and dorsal) prefrontal cortices (VLPFC) have been implicated in facilitating relatively more complex adaptive behavioral responses to changes in reward or punishment values (Cools, Clark, Owen, & Robbins, 2002). (7) Finally, the medial prefrontal cortex (MPFC) has been implicated in making inferences about other people’s intentions and mental states (Castelli, Frith, Happe, & Frith, 2002; Frith & Frith, 1999; Gregory et al., 2002) as well as the attribution of emotion to self and other (Ochsner et al., 2004). To date, cognitive neuroscientists have emphasized the identification of the unique contribution of each region to social cognition and social perception. This analytic perspective has helped provide a framework for organizing our emerging understanding of the social brain, but this approach does not fully reflect the complexity of interactions among these neuroanatomical structures. It is likely that as our field evolves, these structures will be better understood as components in a network of regions subserving social cognition and social perception.
Figure 1.
Some of the brain regions involved in various aspects of social cognition and social perception. VLPFC = ventral lateral prefrontal cortex, IPL = inferior parietal lobule, STS = superior temporal sulcus, OFC = orbital frontal cortex, MPFC = medial prefrontal cortex, EBA = extrastriate body area, AMY = amygdala, FFA = fusiform face area.
BRAIN STUDIES OF SOCIAL PERCEPTION IN ADULTS
We have established a program of research that has as its major goal the identification and definition of the neural systems involved in social perception and the study of those systems in typically and atypically developing children, adolescents, and adults. We began our research on the neurobiology of social perception in humans with the intention of further specifying the role of the STS region. This work was initiated to test the hypothesis that the STS region plays an important role in social perception via its involvement in interpreting the actions and social intentions of other people from an analysis of biological-motion cues (Allison et al., 2000). This proposition was based on the available human neuroimaging evidence as well as elegant work in nonhuman primates demonstrating the sensitivity of neurons in the STS to various socially relevant cues, including head and gaze direction (Perrett et al., 1985). Here, we describe selected experiments that addressed this question using fMRI and virtual-reality techniques with typically developing, adult participants.
Are there Specialized Brain Regions for the Perception of Biological Motion?
The STS region was identified early on as playing a role in biological motion perception (for a review, see Allison et al., 2000). We define biological motion as the visual perception of a living being performing a recognizable action or movement. Examples of this include people dancing, waving, and talking. Our visual system can identify others engaging in such activities even in the absence of visual form detail. For example, many researchers have created stimuli by affixing a number of small lights to the joints of actors and filming these individuals while they perform various movements. Experimental participants viewing these stimuli are able to describe correctly the actors, their emotions, and their activities (Johannson, 1973). Most of these prior studies used point-light displays as stimuli. For instance, Bonda and colleagues (1996) demonstrated that the perception of point-light displays conveying recognizable hand movements activated the STS region more than random motion of lights. This left open the possibility that the response from the STS region was being driven by the fact that biological motion was more familiar, recognizable, and nameable than random motion. It is possible that coordinated and meaningful nonbiological motion might also activate the STS region and this called into question the specificity of this region for processing biological motion.
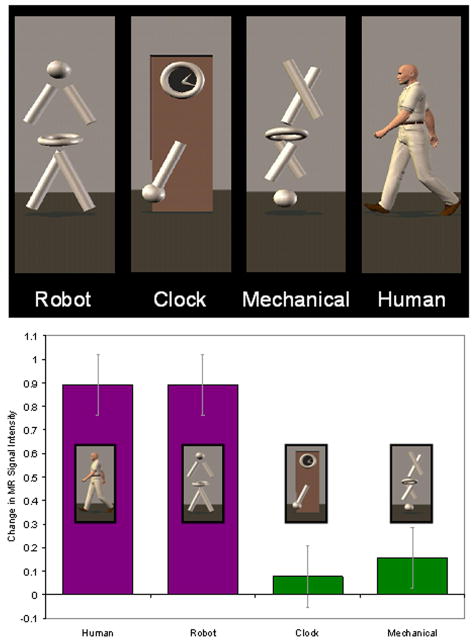
To address this issue, we conducted an event-related fMRI study to compare the responses from the STS region to four different types of motion conveyed via animated virtual-reality characters. As illustrated in the top panel of Figure 2, participants viewed walking, a biological motion conveyed by robot (Robot) or a human (Human). They also viewed a nonmeaningful but complex nonbiological motion in the form of a disjointed mechanical figure (Mechanical) and a complex, meaningful, and nameable, nonbiological motion involving the movements of a grandfather clock (Clock) (Pelphrey et al., 2003a). Our design addressed the critical question of whether the STS region is specialized for the perception of biological motion. As shown in the bottom panel of Figure 2, we observed strong and equivalent activity in the right hemisphere STS region to the Human and Robot conditions. This result ruled out the possibility that the STS region was merely responding to the presence of a human form. Overall, the response to biological motion was far greater than that to the moving clock and the mechanical figure. Critically, not every brain region showed this pattern of effects. For instance, MT or V5 (MT/V5), which is known to respond to various kinds of motion (Puce et al., 1995; Watson et al., 1993; Zeki et al., 1991), responded strongly to all four types of motion. From these results, we concluded that biological motion selectively activates the STS region. Thus, we began to view the STS region as node of the neural system supporting social perception via its role in the detection and perception of human actions.
Figure 2.
Stimuli (top panel) and results (bottom panel) from a study of biological-motion perception in typically developing adults. The STS region responded more strongly to the biological than to nonbiological motion (bottom panel).
Is the STS Region involved in Representing the Intentions of other People?
Our prior study demonstrated that the STS region is involved selectively in the perception of biological motion. Given this finding, we next sought to determine whether the STS region represents the intentions of other people. That is, we evaluated whether the STS region is sensitive to the goals and intentions of observed actions – in this case, the movement of a character’s eyes to be consistent or inconsistent with a subject’s expectation about what the virtual character “ought” to do in a particular context.
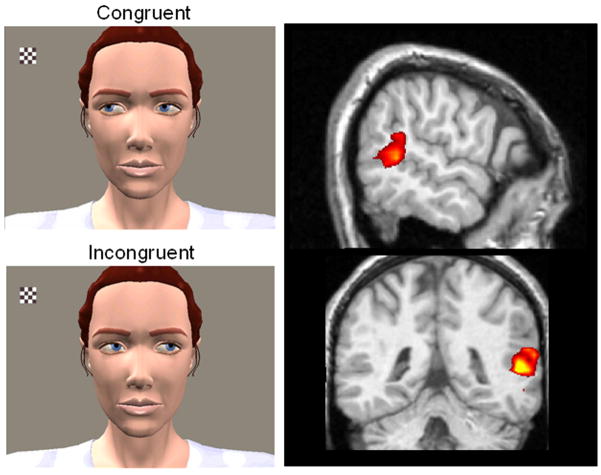
Inside the MRI scanner, our subjects watched an animated character as a small checkerboard appeared and flickered in her visual field (see left panels of Figure 3). On congruent trials, the character looked toward the checkerboard (Figure 3, top left), acting in accordance with the subject’s presumed expectation. On incongruent trials, the character looked away from the checkerboard at a different part of her visual field (Figure 3, bottom left), thereby violating expectations. We had suspected that the STS region would be sensitive to these differences in intentionality and that this region would therefore differentiate between the congruent and incongruent conditions. This would suggest that this area is involved in monitoring expectations about the goals of others. Activity in the STS region was greater for incongruent than for congruent gaze shifts, demonstrating a need for different levels of processing for observed goal-directed and non-goal-directed observed actions.
Figure 3.
Experiment to determine brain activation in response to expected and unexpected gaze shifts on the part of another person (left panel) and corresponding brain activation to biological motion (observed human movements; right panel). Incongruent trials evoked greater right hemisphere STS activity than did congruent trials, demonstrating the sensitivity of the STS region to the intentions conveyed by eye-gaze shifts. Reprinted with permission from Pelphrey and Morris (2006).
This study advanced our understanding of the social brain by demonstrating that the activity in the STS region is modulated by the perceived intentions of actions. Thus, we concluded that the STS region participates in social perception beyond its role in the perception of biological motion: it is also involved in the visual analysis of other people’s actions and intentions. We note that the pattern of effects (incongruent > congruent) is not specific to eye movements, because it is also observed when subjects view congruent and incongruent reaching-to-grasp movements of the hand and arm (Pelphrey et al., 2004a).
Does the STS Region serve as a Mechanism for the Detection of Socially Relevant Gaze Shifts?
Our prior fMRI study of gaze processing established that the STS region is sensitive to at least one aspect of the context within which an action is observed (i.e., goal-directedness vs. non-goal-directedness). Here, we conducted an fMRI study to evaluate whether the STS region is also sensitive to overtly social messages regarding approach and avoidance conveyed via mutual and averted gaze. Gaze direction can serve as a powerful social cue, with mutual gaze often signaling threat or approach and averted gaze conveying submission or avoidance (Argyle & Cook, 1976). Of all the primate species, humans have the most prominent eyes (largest and brightest sclera) which facilitate the determination of gaze direction (Kobayashi & Kohshima, 1997). This and other findings support the “cooperative eye” hypothesis put forward by Tomasello and colleagues (Tomasello, Hare, Lehmann, & Call, 2007). They suggest that particularly visible eyes have made it increasingly easy throughout evolution to coordinate close-range collaborative activities, promoting the understanding the attentional focus and plans of others. One prediction of this hypothesis is that the primate social brain should include mechanisms for the detection of socially relevant gaze shifts.
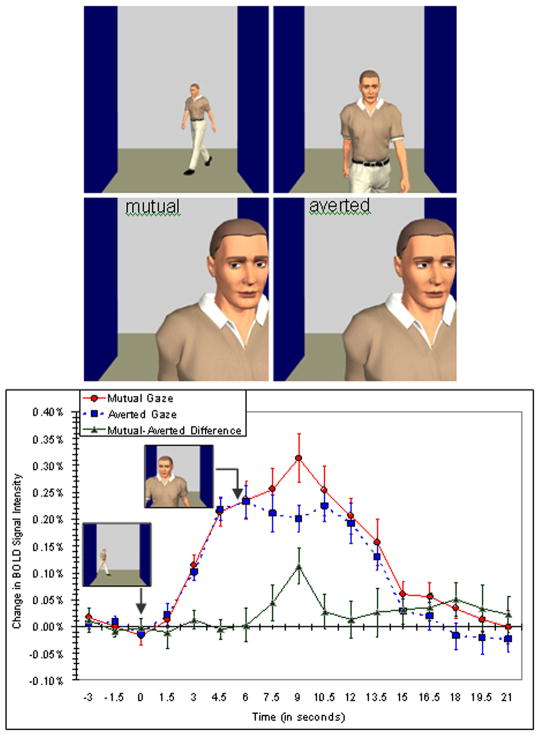
In order to further examine the roles played by gaze in social interactions, we created a virtual-reality scenario wherein characters would approach the participant and either meet or avoid his or her eyes (top panel of Figure 4). Participants viewed these stimuli through virtual-reality goggles in an MRI scanner. We predicted greater activity for mutual compared to averted gaze, reflecting the demand for greater social processing in the case of mutual gaze. As illustrated in the bottom panel of Figure 4, we saw this pattern of response for the approach and associated gaze shift in the STS region. However, there was a functional dissociation between the STS and the right fusiform gyrus wherein the fusiform gyrus did not differentiate between mutual and averted gaze. This suggests a more straightforward role of the right fusiform gyrus in face detection, but a more specific function for the STS region in gaze comprehension.
Figure 4.
Stimuli (top panel) and findings (bottom panel) from an experiment measuring brain activation in response to a stranger initiating or avoiding social interaction. Participants viewed an animated character approaching down a virtual hallway, who shifted his gaze either toward or away from the subject. In both situations, the animated sequence evoked activation in the right STS region. The graph at bottom shows the time courses of activation (indicated as average blood-oxygenation-level-dependent contrast, or BOLD, signal changes) from the right STS region in response to the passerby’s gaze movements. The mutual and averted gaze conditions are plotted along with a plot of their difference (mutual minus averted gaze). Note that the change in activity begins with the appearance of the character in the hallway and increases again at the moment the gaze shift occurs. Reprinted with permission from Pelphrey and Morris (2006).
These two studies of eye-gaze perception illustrate a role for the STS region in the detection and processing of eye movements in order to interpret the attentional foci and goals of others during social interactions. They also support the hypothesis that a social brain component can be influenced by the context of an action, even during passive observation of others. Also, this research suggests that such processing occurs prior to such higher-level executive functions as decision making, response selection, and the perception of novelty, which largely take place in prefrontal regions. More broadly, our findings regarding contextual influences on brain activity fit well within social psychology findings regarding the behavioral effects that result from situational and contextual factors. Thus, the principles of situational and contextual influence operate at multiple levels of the organism, including the individual’s behavior in social situations and localized brain activity.
Summary of the Characteristics of the STS Region
In summary, the studies reviewed above, together with others not discussed here, have allowed us to characterize the STS region as a node in social brain. To date, we know that: The STS region demonstrates a preferential response to biological motion relative to non-biological motion (Pelphrey et al., 2003a). The STS region is multimodal, responding to auditory and visual stimuli and exhibiting polysensory interactions (Wright, Pelphrey, Allison, McKeown, & McCarthy, 2003). Also, this region is sensitive to the goals or intentions (social and nonsocial) conveyed by biological-motion cues (Mosconi, Mack, McCarthy, & Pelphrey, 2005; Pelphrey et al., 2004a; Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005b; Pelphrey et al., 2003b; Pelphrey et al., 2004b). The STS region exhibits a roughly somatotopic organization (Pelphrey et al., 2005b). Given the emerging portrait of the role of the STS region in social perception, it is not surprising that this neuroanatomical structure has become a focus of research for several groups seeking to understand brain mechanisms in autism, a disorder that features profound deficits in several aspects of social perception and social cognition. We now turn to a discussion of our laboratory’s work in this area.
NEURAL BASIS OF SOCIAL PERCEPTION DYSFUNCTION IN AUTISM
Defining the Phenotype: Social Perception Dysfunction in Autism
Autism is a complex, behaviorally defined, neurodevelopmental disorder characterized by severe and pervasive deficits in social functioning and communication as well as restricted, repetitive behaviors and a distinctive developmental course (APA, 2000). Affected individuals differ in the extent to which they demonstrate each of these impairments, but the core disability appears to revolve around social functioning (Kanner, 1943; Waterhouse et al., 1996). As highlighted by Kanner (1943), the “inability to interact in the ordinary way” is a cardinal feature of autism and is pathognomonic of this neurodevelopmental disorder. In Kanner’s (1943) first description of 11 children with “infantile autism”, he described the children as lacking the necessary skills for normal social functioning, with a particular impairment in social interactions. To wit, “We must, then, assume that these children have come into the world with innate inability to form the usual, biologically provided affective contact with other people, just as other children come into the world with innate physical or intellectual handicaps.” (p.250). In his view, the most notable social difficulties were a lack of variation in emotional expression, withdrawn interest in other people, detachment and inaccessibility in social interactions, and the preference for objects over people. Although each individual varied along a spectrum, they all shared an inherent lack of understanding of social situations and an inability to offer an emotional connection when interacting with others. The uniqueness of social behavior in autism led Kanner (1943) to characterize this disorder as a “Disturbance of Affective Contact”. In a 30-year follow-up of these same children, Kanner (1973) found little improvement in affective contact with people and he concluded that they lacked the appropriate biological mechanisms. These brief highlights from Kanner’s original observations of autism confirm the same spectrum of autism observed today and the multiple facets of social dysfunction in autism.
Viewing the World through the Eyes of Autism
Our laboratory’s first foray into the study of autism provided a striking illustration of the social perception deficits that characterize this neurodevelopmental disorder. As displayed in Figure 5, we used eye tracking to evaluate the visual scanpaths of high-functioning adults with autism while they viewed faces displaying various expressions of emotion. Neurologically normal adults (right panel of Figure 5) spent most of their time viewing the core, socially informative features of the faces (i.e., the eyes, nose and mouth). In stark contrast, individuals with autism (see left panel of Figure 5) did not look at the eyes or the other core features of the faces. Instead, they scanned the faces in a seemingly random fashion, suggesting a failure to understand the significance of the features for social understanding.
Figure 5.
Sample scanpaths from an eye-tracking study of high functioning adults with autism (left column) and IQ, gender, and age-matched, typically developing comparison subjects (right column). Reprinted with permission from Pelphrey et al. (2002).
The STS Region and Social Dysfunction in Autism
Along with the scanpath differences we reported, individuals with autism have been characterized as possessing deficits in using gaze information to understand the intentions and mental states of others as well as to coordinate joint attention (Simon Baron-Cohen, 1995; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Leekam, Hunnisett, & Moore, 1998; Leekam, Lopez, & Moore, 2000; Loveland & Landry, 1986; Mundy, Sigman, Ungerer, & Sherman, 1986; Baron-Cohen et al., 1999; Frith & Frith, 1999). Such deficits are seen very early in development. Joint attention can be learned in some cases, using gaze information to infer mental states and intentions and is consistently impaired even in high-functioning adults with autism (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). Note that it is not that these individuals cannot detect gaze direction, but rather that they cannot use such information to infer others’ mental states and behaviors. Based on our prior findings and knowledge of these deficits, we decided to explore the role of the STS in gaze processing dysfunction in autism. To do so, we utilized our checkerboard paradigm. We knew that typically developing individuals should show an increased response for incongruent versus congruent gaze shifts in the STS and other social brain regions, showing expectations of intentionality. However, although we found activity in the same brain regions in individuals with autism, we saw no such differentiation in brain activity based on condition. This suggests an absence of contextual influence on the STS region, which is a possible mechanism underlying the gaze processing deficits reported behaviorally in autism. Hypoactivation of the STS and reduced functional connectivity between the STS and portions of the inferior occipital gyrus (visual area V3) have also been reported in individuals with autism during tasks involving attribution of intentions to moving geometric figures (Castelli, Frith, Happe, & Frith, 2002).
One of the most interesting aspects of our findings was the observation that dysfunction in the STS region was strongly and specifically correlated with the level of social impairment exhibited by individual subjects. Recall that as a group, activity in the STS region in the subjects with autism did not differ significantly for incongruent and congruent gaze shifts. However, just as autism is heterogeneous in severity, there were clear individual differences in the degree of STS dysfunction. To explore whether these individual differences were related to the severity of autism, we computed correlations between the scores on several algorithmic domains of the Autism Diagnostic Interview – Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994), which is used to support the diagnosis of autism, and the magnitude of the incongruent versus congruent differentiation in the right STS region. We assumed that lower levels of incongruent versus congruent differentiation (i.e., incongruent minus congruent difference scores) in the STS region would indicate greater cortical dysfunction. Higher scores on aspects of the ADI-R can indicate greater severity of autism. Strikingly, the magnitude of the incongruent versus congruent difference score was strongly, negatively correlated with scores in the Reciprocal Social Interaction Domain (r = −0.78, p = 0.004), However, it was not significantly correlated with impairments in the Communication Domain or the Restricted, Repetitive and Stereotyped Patterns of Behavior Domain. These findings suggest that the degree of neurofunctional impairment in the right STS region is related to the severity of specific core features of the autism phenotype.
Another deficit reported in the behaviors of individuals with autism is that of speech and language processing difficulties, including speech perception. The STS has also been shown to play a role in these processes. Reports from neuroimaging studies have suggested abnormal laterality during speech perception (Boddaert et al., 2002) as well as abnormal responses in the STS region to human voices (Gervais et al., 2004) and a lack of response to vocal sounds (Belin, Zatorre, Lafaille, Ahad, & Pike, 2000) in individuals with autism. Note, however, that these individuals showed typical auditory cortex responses to nonvocal sounds. We decided to further explore speech perception deficits by using audiovisual speech displays in individuals with autism and neurologically normal controls (Collins and Pelphrey, unpublished thesis data). When perceiving the simultaneous auditory and visual speech displays, the STS region showed bilateral activation. In typically developing individuals, there was a greater level of activity in this region when the auditory and visual displays matched than when they did not match or when auditory or visual information was presented in isolation. However, the individuals with autism exhibited hypoactivation in the STS region for all conditions and no differentiation between matching and mismatching speech presentations. These results suggest failed integration of auditory and visual speech.
NEUROBIOLOGY OF SOCIAL PERCEPTION DEVELOPMENT IN CHILDREN WITH AND WITHOUT AUTISM
While cognitive neuroscientists have generated a wealth of information regarding the brain regions involved in social perception and social cognition, very little work has been conducted to evaluate the development of the social brain in typically or atypically developing children. Several early studies focused on the amygdala and its response to fearful faces. First, Baird and colleagues (1999) demonstrated amygdala activation to fearful faces in children ages 12 to 17 years. Thomas and colleagues (2001) reported that adults demonstrated greater amygdala activation to fearful facial expressions, whereas 11-year-old children showed greater amygdala activation to neutral faces. It may be that the neutral faces were seen as more ambiguous than fearful facial expressions, with resulting increases in amygdala activation. Sex differences in amygdala development have been reported in children and adolescents (Killgore, Oki, & Yurgelun-Todd, 2001). Whereas the left amygdala responded to fearful facial expressions in all children, its activity decreased over the adolescent period in females but not in males. In a follow-up study, Killgore and Yurgelun-Todd (2004) compared children, adolescents, and adults during the perception of fearful faces. They reported that males and females differed in the asymmetry of activation of the amygdala and prefrontal cortex (PFC) across the three age groups. For males, activation within the dorsolateral PFC was bilateral in childhood, right lateralized in adolescence, and bilateral in adulthood, whereas females showed a monotonic relationship with age, with older females showing more bilateral activation than younger ones. In contrast, amygdala activation was similar for both sexes, with bilateral activation in childhood, right-lateralized activation in adolescence, and bilateral activation in adulthood. Finally, Lobaugh, Gibson, and Taylor, 2006 reported that fear, disgust, and sadness recruit distinct neural systems in 10-year-old children similarly to adults (Phan, Wager, Taylor, & Liberzon, 2002). Other studies have examined the role of the lateral fusiform gyrus (FFG) in face processing in children, with studies using fMRI and ERPs beginning to provide important information. Thus far, it is known that some degree of FFG specialization for faces is apparent early in development (e.g., Tzourio-Mazoyer et al., 2002), and this specialization continues to develop throughout infancy and into late childhood and adolescence (Aylward et al., 2005; Mosconi et al., 2005; Taylor, Edmonds, McCarthy, & Allison, 2001). Studies have found similar mirror neuron activity in children between 10 and 12 years of age as had been identified previously in adults, but patterns of activity in these areas have not yet been explored in younger children (Dapretto et al., 2006; Ohnishi et al., 2004).
Acquisition of neuroimaging data from children involves a number of methodological challenges. Perhaps the most noteworthy of these is the child’s compliance with the requirement to remain motionless during the scanning session. A key methodological advance in our laboratory’s efforts to establish a program of child functional neuroimaging research was been to develop “mock scanning” facilities. We completed construction of an MRI simulator for use in acclimating children to the scanner environment and for training these subjects to minimize head motion. Specifically, children are trained using operant conditioning procedures implemented with custom-written software that receives input from a head motion sensor and uses this input to direct the operation of a video player. The child watches a movie, and the movie is halted whenever the child exhibits head motion above a progressively stricter threshold. We have successfully used this system to prepare children ages 4 to 12 years for fMRI sessions (Cantlon, Brannon, Carter, & Pelphrey, 2006; Carter & Pelphrey, 2006; Mosconi et al., 2005).
The neural basis of gaze perception in typically developing children
Building upon our work on the role of the STS region in adults with and without autism, we have initiated a series of studies to examine the neurofunctional development of this region in children with and without autism. We first examined the neural circuitry involved in school-aged children (Mosconi et al., 2005). Using our incongruent versus congruent gaze paradigm (left panel of Figure 2), we investigated the sensitivity of brain regions involved in processing gaze and the intentions conveyed by shifts in eye gaze in typically developing children between 7 and 10 years old. Based on our prior findings using this and similar fMRI paradigms in adults (Pelphrey et al., 2003b, 2004b, 2005b), we hypothesized that STS activity would differentiate congruent and incongruent trials, reflecting the ability of typically developing 7-year-old children to link the perception of the gaze shift with its mentalistic significance. Our results confirmed our prediction and indicated that the STS region was sensitive to the intentions underlying the stimulus character’s eye movements. These findings suggest that the neural circuitry underlying the processing of eye gaze and the detection of intentions from gaze in children in this age range is very similar to that of adults.
Functional Neuroimaging of Biological Motion Perception in Children with and without Autism
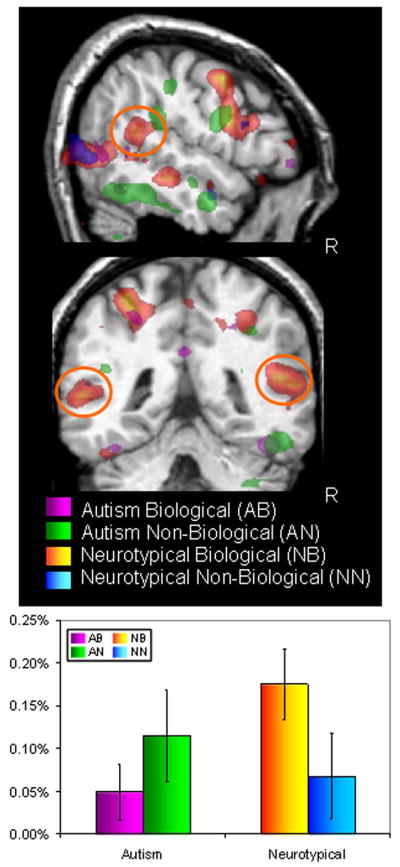
More recently, we examined the degree to which the STS region is selective for biological as compared to non-biological motion in 6- to 10-year-old children with and without autism. In an elegant behavioral study, Blake, Turner, Smoski, Pozdol, and Stone (2003) demonstrated that children (ages 8–10 years) with autism are significantly impaired at recognizing biological motion from point-light displays relative to IQ-matched neurologically normal children. We sought to identify the brain correlates of these biological motion perception deficits in children with autism. We employed our prior design with four different motion conditions: a walking man, a walking robot, a disjointed mechanical figure with the same components as the robot, and a grandfather clock (Pelphrey et al., 2003a). In this way, we were able to control for whether the figure was biological, whether the motion was biological, and whether the motion was organized. As reviewed above, we had previously shown that the STS region in neurologically normal adults was activated more by the biological motion conditions (human and robot) than by the nonbiological motion conditions (clock and mechanical figure). As illustrated in the left panel of Figure 6, we identified a network of brain regions in our sample of 7- to 10-year-old typically developing children that had greater responses evoked by biological than by non-biological motion, including the STS region and portions of the purported human mirror neuron system, including the inferior frontal gyri, the precentral gyri, and middle and superior frontal gyri (Carter & Pelphrey, 2006). Additionally, we found a developmental change that suggested increasing specificity for biological motion with age in the STS region. Specifically, the magnitude of the biological greater than non-biological difference score was positively correlated with age in the right STS region (r = .64, p < .03, two-tailed), with age accounting for approximately 41% of the variance in the biological greater than non-biological difference scores.
Figure 6.
Summary activations from participants with and without autism viewing biological and non biological motion (top panel). The graph in the bottom panel shows the response from the STS region as a function of condition and participant group.
As shown in the right panel of Figure 6, when we used this same paradigm with 7- to 10-year-old high functioning children with autism (N = 5) we found that they did not have different STS activity for biological and non-biological motion. Critically, not every brain region showed these patterns of effects: the motion-sensitive visual area MT/V5 was equally activated by both motion types in children with and without autism. This functional dissociation suggests that the STS and other social brain regions are specific for biological motion in typically developing children but not in children with autism. We did not observe reduced activity in all brain areas investigated, indicating that the effect we observed was not being driven by a general reduction in the fMRI signal.
These findings are particularly interesting in light of theoretical perspectives that emphasize the importance of biological motion in the development of theory of mind abilities. For example, Frith and Frith (1999) suggested that the ability to distinguish between biological and nonbiological figures and their actions is one of the likely evolutionary and developmental precursors to theory of mind. Thus, early biological motion detection abilities could allow children to develop the ability to use knowledge about the actions and intentions of others to infer mental states and thus to develop full-fledged theory of mind abilities. The findings from our fMRI study of biological motion perception in children with and without autism suggest that children with autism do not possess one of the basic building blocks believed to underlie theory of mind. It is noteworthy that these individuals also show pronounced deficits on theory of mind tasks (Baron-Cohen, Leslie, & Frith, 1985; Perner, Frith, Leslie, & Leekam, 1989).
CONCLUSION
Prior functional neuroimaging studies of autism have focused on the adolescent and adult age ranges, with the average age of samples in fMRI studies of adults with autism at 21 years and the youngest age group at 12 years. There are several reasons why it is critical to adopt a developmental perspective for studies of the brain mechanisms underlying emotion-processing deficits in autism. First, many of the neuroimaging findings from adults with autism could represent either actual causes of autism and/or compensatory changes in the brains of people with autism. Group differences in imaging data from adults might represent a causal factor or an effect of having autism on the brain. Recognizing the manifestations of compensatory effects in people with autism will be critical to understanding neuroimaging findings and may lead to novel treatments. Second, autism has its onset in the earliest years of childhood and its symptoms change over ontogeny. Neuroimaging studies of autism that take into account these psychological and behavioral continuities and discontinuities will better inform us of the neurobiological mechanisms in autism than studies that provide only a static picture in adults. Third, functional brain correlates of autism in children may prove useful in early diagnosis, and the elucidation of developmental trajectories of the neural circuitry supporting emotion processing might inform the design of more effective treatments. Fourth, a developmental perspective can be a useful tool for unraveling the interaction between seemingly disparate levels of organization, such as those among the molecular biology of gene expression, the structure and function of the brain, and the development of cognitive abilities. Ultimately, only prospective, longitudinal studies of children at risk for developing autism will be able to clarify the relationship between brain abnormalities and the course of the disorder.
Acknowledgments
The studies reviewed herein were supported in part by grants from the National Institute of Mental Health, the John Merck Scholars Fund, National Institute of Child Health and Human Development, the Veterans Affairs Administration, the National Alliance for Autism Research/Autism Speaks, and the National Institute for Neurological Disorders and Stroke. Kevin Pelphrey is supported by a Career Development Award from the National Institutes of Health, NIMH Grant MH071284. Elizabeth Carter is supported by a predoctoral fellowship from the National Alliance for Autism Research/Autism Speaks. We gratefully acknowledge our collaborators, especially Gregory McCarthy, James Morris, and Truett Allison. We are grateful to Jaime Doyle for assistance with manuscript preparation.
References
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- APA. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Argyle M, Cook M. Gaze and mutual gaze. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- Baron-Cohen S. Mindblindness: an essay on autism and “theory of mind”. Cambridge, Mass: MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Barthelemy C, Bourgeois M, Poline JB, Brunelle F, et al. Bitemporal lobe dysfunction in infantile autism: positron emission tomography study. Journal deRadiologie. 2002;83(12 Pt 1):1829–1833. [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Campbell R, MacSweeney M, Surguladze S, Calvert G, McGuire P, Suckling J, et al. Cortical substrates for the perception of face actions: an fMRI study of the specificity of activation for seen speech and for meaningless lower-face acts (gurning) Brain Research. Cognitive Brain Research. 2001;12(2):233–243. doi: 10.1016/s0926-6410(01)00054-4. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 2006;4(5):e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(Pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293(5539):2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, et al. Abnormal cortical voice processing in autism. Nature Neuroscience. 2004;7(8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125(Pt 4):752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, et al. Reafferent copies of imitated actions in the right superior temporal cortex. Proceedings of the National Academy of Sciences of the Unites States of America. 2001;98(24):13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Johannson G. Visual perception of biological motion and a model for its analysis. 1973;14 [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2(2):217–230. [PubMed] [Google Scholar]
- Kanner L. Historical perspective on developmental deviations. Journal of Autism and Childhood Schizophrenia. 1973;3(3):187–198. doi: 10.1007/BF01538280. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. 1939. Jouranl of Neuropsychiatry and Clinical Neuroscience. 1997;9(4):606–620. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kohshima S. Unique morphology of the human eye. Nature. 1997;387(6635):767–768. doi: 10.1038/42842. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Crupain MJ, Voyvodic JT, McCarthy G. Dynamic perception of facial affect and identity in the human brain. Cerebral Cortex. 2003;13(10):1023–1033. doi: 10.1093/cercor/13.10.1023. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Current Opinion in Neurobiology. 1992;2(2):191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. Journal of Child Psychology and Psychiatry. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: a functional neuroimaging study of social perception in children. Neuroimage. 2005;27(1):247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: the contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. Journal of Neuroscience. 2003a;23(17):6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004a;16(10):1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005a;(128):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an FMRI study of eye, mouth and hand movements. Cerebral Cortex. 2005b;15(12):1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003b;41(2):156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004b;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, et al. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Royal Society (Great Britian) 1985;223(1232):293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16(16):5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Constable RT, Luby ML, McCarthy G, Nobre AC, Spencer DD, et al. Functional magnetic resonance imaging of sensory and motor cortex: comparison with electrophysiological localization. Journal of Neurosurgery. 1995;83(2):262–270. doi: 10.3171/jns.1995.83.2.0262. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Research. Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Experimental Brain Research. 1996;111(2):246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Hare B, Lehmann H, Call J. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. Journal of Human Evolution. 2007;52(3):314–320. doi: 10.1016/j.jhevol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Waterhouse L, Morris R, Allen D, Dunn M, Fein D, Feinstein C, et al. Diagnosis and classification in autism. Journal of Autism and Developmental Disorders. 1996;26(1):59–86. doi: 10.1007/BF02276235. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, et al. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cerebral Cortex. 1993;3(2):79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G. Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cerebral Cortex. 2003;13(10):1034–1043. doi: 10.1093/cercor/13.10.1034. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]