Abstract
Background. Patients with hypertrophic cardiomyopathy (HCM) and HCM mutation carriers are at risk of sudden cardiac death (SCD). Both groups should therefore be subject to regular cardiological testing – including risk stratification for SCD – according to international guidelines. We evaluated Dutch cardiologists' knowledge of and adherence to international guidelines on risk stratification and prevention of SCD in mutation carriers with and without manifest HCM.
Methods. A questionnaire was sent to 1109 Dutch cardiologists (in training) containing case-based questions.
Results. The response rate was 21%. Own general knowledge on HCM care was rated as insufficient by 63% of cardiologists. The percentage of correct answers (i.e. in agreement with international guidelines), on the case-based questions ranged from 37 to 96%, being lowest in cases with an unknown number of risk factors for SCD. A substantial portion of correct answers was based on the correct answer ‘ask an expert opinion’. Significantly more correct answers were provided in cases with manifest HCM. There was little difference between the answers of cardiologists with different self-reported levels of knowledge, with different numbers of HCM patients in their practice or with different numbers of carriers without manifest HCM.
Conclusion. Knowledge on risk stratification and preventive therapy was mediocre, and knowledge gaps exist, especially on HCM mutation carriers without manifest disease. Fortunately, experts are frequently asked for their opinion which might bring patient care to an adequate level. Hopefully, our results will stimulate cardiologists to follow developments in this field, thereby increasing quality of care for HCM patients and mutation carriers. (Neth Heart J 2009;17:464-9.).
Keywords: Hypertrophic cardiomyopathy, risk stratification, sudden cardiac death, questionnaire
With a prevalence of about one in 500, hypertrophic cardiomyopathy (HCM) is one of the most common genetic diseases.1 HCM is inherited as an autosomal dominant trait caused by a large variety of mutations – around 450 different mutations in more than 11 genes have been reported.2 HCM is clinically classified as a (usually asymmetric) thickening of the myocardial wall of the left ventricle, often involving the interventricular septum, which cannot be accounted for by other clinical diseases.3,4 Most patients experience few if any symptoms. If present, symptoms are diverse. Common symptoms are chest pain during exertion and dyspnoea.5
The most feared complication of HCM is sudden cardiac death (SCD) with an annual incidence of 1% in unselected HCM patients.6 Although SCD in HCM predominantly occurs in young asymptomatic adults (i.e. below 35 years of age), available evidence suggests the risk to continue throughout life.7,8 Currently, six major risk factors for SCD in individuals with a clinical diagnosis of HCM have been identified: (1) prior cardiac arrest (ventricular fibrillation) or spontaneous sustained ventricular tachycardia, (2) family history of premature sudden death, (3) unexplained syncope, (4) left ventricular (LV) wall thickness ≥30 mm, (5) abnormal exercise blood pressure and (6) nonsustained ventricular tachycardia.9
According to a consensus document from the American College of Cardiology (ACC) and the European Society of Cardiology (ESC), HCM patients (with manifest disease) and mutation carriers (with or without manifest disease) should be regularly evaluated (probably yearly) by 12-lead ECG, two-dimensional echocardiography, ambulatory (Holter) ECG and an exercise test.9 If a HCM patient has two or more of these risk factors, the estimated annual risk of SCD increases to 4 to 5%.10 In that case, according to guidelines on risk stratification, antiarrhythmic treatment with an implantable cardioverter defibrillator (ICD) should be considered.9 Recent guidelines on arrhythmia and the prevention of SCD also support ICD implantation in HCM patients with only one risk factor who are considered to be at high risk for SCD.11,12
The optimal treatment of mutation carriers with manifest HCM is probably the same as of any HCM patient with respect to risk stratification and preventive therapy. In mutation carriers without clinical diagnosis (based on LV hypertrophy or ECG abnormalities),13 left ventricular hypertrophy may still emerge at any point in life.14 Consequently repeated rather than singular clinical evaluation and testing is thought to be mandatory in carriers still without manifest HCM. However, it is uncertain if risk stratification for SCD and subsequent risk-dependent preventive therapy is effective in the prevention of SCD in mutation carriers without manifest disease. This is also reflected in our observation that not all mutation carriers known to us receive (regular) follow-up including risk stratification. Therefore, the aim of this study is to gain insight into and evaluate the current status on risk stratification in HCM mutation carriers based on the current knowledge of and adherence to the present guidelines, and the surveillance practice in everyday cardiology practice in the Netherlands.
Methods
Participants
Dutch cardiologists and cardiologists in training (n=1109) were contacted by use of the membership records of the Netherlands Society of Cardiology, the NVVC, in 2008.
Questionnaire
Data were collected by sending a questionnaire to home addresses of all cardiologists and cardiologists in training using regular mail. Additionally, a reminder was sent twice using two different editions of the NVVC newsletter. The questionnaire was developed for this particular study and a translation is available as supplementary material. A pilot study (n=13) had been conducted to optimise the questionnaire.
The first part of the questionnaire addressed standard sociodemographic and professional data. The next part addressed respondents' knowledge with respect to risk stratification and preventive therapy by eliciting responses on four hypothetical realistic patients (the vignette method). The approach was interactive, gradually disclosing more information.
First, two HCM mutation carriers without cardiac complaints who had not been cardiologically evaluated for HCM in the past were described, one a 25-year-old (case A) and the other a 50-year-old (case B). We asked each cardiologist (in training) what tests they would perform at a first visit of each of these patients. In the next questions, two additional cases were introduced, both HCM mutation carriers clinically diagnosed with HCM, again one 25-year-old (case C) and one 50-year-old (case D). The echocardiography results of case A and B were provided (no LV hypertrophy). We asked the cardiologists (in training) for each of the four cases what he or she would do when presented with: (1) an unknown number of risk factors for SCD (after ECG and echocardiography research, but before more extensive testing), (2) one risk factor for SCD present and (3) two risk factors for SCD present. We used multiple-choice response options. Cases of different ages were introduced for two reasons: (1) according to literature SCD occurs mainly in young patients and (2) early literature suggests that HCM does not become manifest after early adulthood.
Finally, cardiologists' opinion on the possible (dis)-advantages of frequent risk stratification of mutation carriers without manifest HCM was asked for.
Analysis
Data were analysed with SPSS (version 15.0) statistical software. The answers to the case-based questions were dichotomised into correct and incorrect according to the guidelines.9,11,12 These guidelines advise, irrespective of age, performing yearly risk stratification in HCM patients and mutation carriers with manifest disease and periodically (probably yearly) for mutation carriers without manifest disease.9 Although the guidelines advise yearly risk stratification, our clinical experience and previous research has shown that only a minority of HCM patients and mutation carriers received these yearly evaluations without any adverse events. We therefore relaxed the criterion for a correct answer: ‘extra diagnostics every two to three years’ was also considered a correct answer alongside ‘extra diagnostics every year’. Extra diagnostics involved ECG, echocardiography, Holter recording and exercise test. In cases with manifest HCM with one or more risk factors for SCD, answers supporting ICD implantation were considered correct according to the guidelines.9,11,12 HCM mutation carriers with manifest disease were regarded as HCM patients. There are no specific guidelines on HCM mutation carriers without manifest disease with risk factors and no studies on the prognostic significance of risk factors for SCD in this group. We therefore assumed the best and correct answer was to continue extra diagnostics on a yearly basis or once every two to three years. We also accepted the option of asking the opinion of an expert on HCM as a correct answer in all cases.
The association between correct answers and cardiologists' demographic and clinical variables was tested using χ2 and t-tests. We considered two-sided p values smaller than 0.05 to be significant.
Results
Out of the 1109 questionnaires that were sent to the members of the NVVC, 237 cardiologists and cardiologists in training responded (21% response rate). The sociodemographic and professional characteristics of the participating cardiologists and cardiologists in training are displayed in table 1, as well as their level of experience with HCM and their self-estimated knowledge of HCM. From the NVVC we obtained data on gender, age and affiliation from all Dutch cardiologists. There were no significant differences on these characteristics between the cardiologists who responded to our questionnaire and the cardiologists from the NVVC database.
Table 1.
Characteristics of participating cardiologists (in training), experience with hypertrophic cardiomyopathy (HCM) and own estimated knowledge of HCM.
| Characteristic | n (%) |
|---|---|
| Age (mean, SD) | 43.9±10.1 |
| - <30 | 8 (3) |
| - 30–39 | 82 (35) |
| - 40–49 | 71 (31) |
| - 50–59 | 55 (23) |
| -≥60 | 19 (8) |
| Male gender | 189 (80) |
| Affiliation | |
| - Academic hospital | 67 (28) |
| - General hospital | 164 (71) |
| - Other | 3 (1) |
| Working as | |
| - Cardiologist | 167 (71) |
| - Cardiologist in training | 60 (26) |
| - Other | 6 (3) |
| Years of experience (mean, SD) | 10.9±9.2 |
| Number of carriers/patients with manifest HCM under surveillance | |
| - 0 patients | 37 (16) |
| - 1–5 patients | 97 (42) |
| - 6–10 patients | 49 (21) |
| - 11–20 patients | 34 (15) |
| - >20 patients | 15 (7) |
| Number of HCM carriers with no manifest HCM seen | |
| - 0 carriers | 114 (49) |
| - 1–5 carriers | 77 (33) |
| - 6–10 carriers | 17 (7) |
| - 11–20 carriers | 6 (3) |
| - >20 carriers | 9 (4) |
| - Unknown | 11 (5) |
| Estimated knowledge of HCM* | |
| - Very poor | 60 (26) |
| - Poor | 89 (38) |
| - Sufficient | 68 (29) |
| - Good | 13 (6) |
| - Excellent | 5 (2) |
Data are reported as number (%) or mean (SD). *Estimated knowledge on prognosis, treatment, supporting care and the use of DNA test results in HCM.
Case-based questions
Of the cardiologists (in training), 52% provided a ‘correct’ answer when first presented with the 25-year-old carrier (case A), and 53% followed the guidelines when first presented with the 50-year-old carrier (case B). The respondents' clinical experience (i.e. trainee or senior, number of HCM patients and HCM mutation carriers seen) and self-reported level of knowledge did not affect the proportion of correct answers.
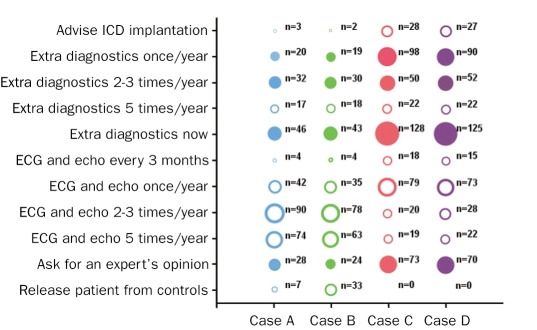
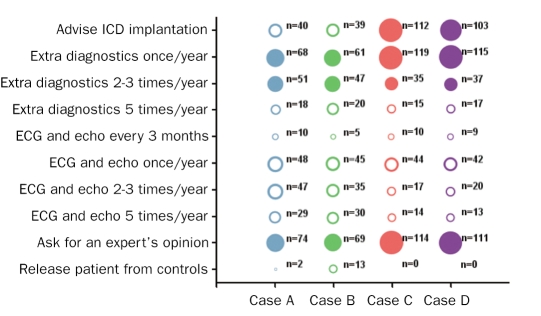
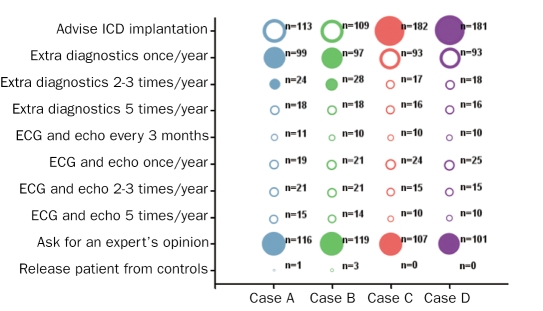
Figure 1 shows the responses to the question on what to do when confronted with case A (25-year-old mutation carrier without manifest HCM), B (50-year-old mutation carrier without manifest HCM), C (25-year-old mutation carrier with manifest HCM) and D(50-year-old mutation carrier with manifest HCM) with an unknown number of risk factors for SCD for each case (after ECG and echocardiography, but before more extensive cardiological evaluation). For case A, 40% of the answers were correct, for case B 37%, for case C 87% and for case D 85%. Figure 2 shows the answers given to the question on what to do when confronted with each of the four cases but now with one risk factor for SCD present. For case A, 67% of the answers were correct, for case B 63%, for case C 96% and for case D 95%. Finally, figure 3 shows the answers to the same question, however in each case two risk factors for SCD are present. For case A, 74% of the answers were correct, for case B 74%, for case C 94% and for case D 92%.
Figure 1 .
Answers from cardiologists (in training) to the question on how they would proceed when confronted with these four cases with an unknown number of risk factors. On x-axis: Case A: 25-year-old HCM mutation carrier without manifest HCM, Case B: 50-year-old HCM mutation carrier without manifest HCM, Case C: 25-year-old HCM mutation carrier with manifest HCM, Case D: 50-year-old HCM mutation carrier with manifest HCM. On y-axis: possible answers to this question. Fully coloured circles represent the correct answers. Extra diagnostics involves ECG, echocardiography, Holter recording and an exercise test.
Figure 2 .
Answers from cardiologists (in training) to the question on how they would proceed when confronted with these four cases with one risk factor. For explanation, see figure 1.
Figure 3 .
Answers from cardiologists (in training) to the question on how they would proceed when confronted with these four cases with two risk factors. For explanation, see figure 1.
Cardiologists (in training) provided significantly more correct answers for both carriers with manifest HCM (cases C, D) compared with both carriers without manifest HCM (cases A, B). No significant difference in correct answers was found when comparing the 25-year-old with the 50-year-old within the same group with or without manifest HCM (table 2).
Table 2.
Differences in proportion of correct answers between cases.
| Differences in correct answers between carrier and patient cases | Differences in correct answers between cases of different ages | ||||
|---|---|---|---|---|---|
| Cases | Correct answer (%) | Comparison A-C | Comparison B-D | Comparison A-B | Comparison C-D |
| Unknown number of risk factors | |||||
| A: carrier 25 yrs | 40% | ||||
| B: carrier 50 yrs | 37% | p<0.0001 | p<0.0001 | p=0.52 | p=0.59 |
| C: patient 25 yrs | 87% | ||||
| D: patient 50 yrs | 85% | ||||
| One risk factor | |||||
| A: carrier 25 yrs | 67% | ||||
| B: carrier 50 yrs | 63% | p<0.0001 | p<0.0001 | p=0.28 | p=0.83 |
| C: patient 25 yrs | 96% | ||||
| D: patient 50 yrs | 95% | ||||
| Two risk factors | |||||
| A: carrier 25 yrs | 74% | ||||
| B: carrier 50 yrs | 74% | p<0.0001 | p<0.0001 | p=0.92 | p=0.48 |
| C: patient 25 yrs | 94% | ||||
| D: patient 50 yrs | 92% | ||||
In general, the accuracy of response did not differ between cardiologists and cardiologists in training. Only in case C and D with two risk factors for SCD present did cardiologists in training more often provide the correct answer than cardiologists (p=0.018 for case C, and p=0.045 for case D). Response of cardiologists (in training) did not differ significantly according to experience with HCM patients (n <6 HCM patients vs. n ≥6). Similarly, there was no difference according to experience with mutation carriers (n <6 HCM carriers vs. n ≥6). There was no correlation between the accuracy of answers of cardiologists (in training) and their level of self-estimated knowledge of HCM in any of the cases.
Opinions on extensive and repeated medical evaluations
When asked their opinion on extensive and repeated medical testing of mutation carriers without manifest HCM in order to assess their risk of SCD, about half of the cardiologists and cardiologists in training (55%) indicated that these tests are absolutely necessary. Almost one third (31%) thought that these evaluations should be discussed with and advised to the patient, but that these evaluations are not absolutely necessary. Five percent indicated that these evaluations should be discussed with the patient, but not advised. Eleven percent thought that the risk of SCD did not justify extensive evaluations for carriers without manifest HCM: 6% responded that the patient's burden of extensive cardiological evaluations would be too large, while another 5% thought the stress on hospital resources would be too high.
Discussion
A 2003 questionnaire showed that Dutch cardiologists valued their own general knowledge of genetic heart disorders and of HCM as insufficient.15 Despite the fact that cardiologists in our study reported their level of knowledge as low, it appears that, in general, they provided reasonably good answers to the case-based questions with a range of 37 to 96% of correct answers. However, a substantial portion of the cardiologists gave the answer ‘Ask the opinion of an expert’. This answer could be an indication of insufficient knowledge of risk stratification, but we considered this to be a correct answer because this is common practice and will lead to good patient care. However, it probably covers up true knowledge of the guidelines and looking at answers that follow the guidelines, one should conclude that knowledge on risk stratification and preventive therapy is mediocre and that cardiologists are aware of that considering their self-reported levels of knowledge. The high frequency of the answer ‘Ask the opinion of an expert’ and the relatively low number of cardiologists who indicate having more than ten HCM patients or mutation carriers under surveillance plead for more specialised cardiologists or specialised centres who can function as an expert or expert centre for their colleagues.
The response rate of our questionnaire (21%) is in the same range as similar questionnaire studies among physicians.15-17 The specialised subject of our questionnaire could have contributed to the low response, but it is also likely that our study overestimates the average level of knowledge of cardiologists. Doctors are more likely to fill out a questionnaire on a subject that they are knowledgeable about. Although there were no differences in age, gender and affiliation between responding cardiologists and all NVVC cardiologists, selection bias cannot completely be excluded.
There is little difference in correct answers between cardiologists and cardiologists in training. Surprisingly, a higher self-reported level of knowledge did not correspond with an increased proportion of correct answers to the case-based questions. Respondents' clinical experience, i.e. the number of HCM mutation carriers with or without manifest HCM a cardiologist had under surveillance, made no difference to the proportion of correct answers either.
Our HCM cases differed on three levels: the number of risk factors for SCD present, the presence of LV hypertrophy (manifest HCM) and their age. With an increasing number of risk factors for SCD the proportion of correct answers also increased. Moreover, cardiologists' knowledge of risk stratification and preventive therapy was smaller in carriers without manifest disease compared with that in carriers or patients with manifest HCM. One possible explanation might be that most respondents had little or no experience with mutation carriers without manifest HCM (81% had less than six and 48% even had no mutation carriers without manifest HCM) and consequently may not read or search for information and thus may not be aware of the (latest) guidelines and studies. But answers are also in line with the fact that the evidence level of the guidelines is higher for manifest HCM. This is exemplified by the fact that there are no guidelines regarding carriers without manifest HCM and one or two risk factors for SCD. Although we do assume risk factors to be of prognostic significance in mutation carriers without manifest disease because we evaluate them, this has never been investigated. The risk for mutation carriers without manifest disease to die suddenly seems to be small but probably cannot be neglected completely. Prognostic significance of risk factors in this subgroup should be the subject of future research, especially since the number of mutation carriers without manifest HCM is rapidly increasing.
When comparing the answers for the 25-year-old carriers and patients to the answers for the 50-year-old carriers and patients, we found no significant difference. This could be a sign that the message in recent literature that HCM can become manifest at any age and SCD can also occur in advanced age, has come across. However, this message is still important, because our research shows that 14% would incorrectly discharge a 50-year-old mutation carrier without manifest HCM from further follow-up. In comparison, only 3% would discharge a 25-year-old mutation carrier without manifest HCM from follow-up.
Conclusions
Our study suggests that overall knowledge on risk stratification and preventive therapy in HCM is mediocre, and that knowledge gaps exist, especially with respect to HCM mutation carriers without manifest disease. Fortunately, experts are frequently asked for their opinion which might bring patient care to an adequate level. We hope our results will stimulate cardiologists (in training) to read and apply the guidelines on risk stratification for SCD in their clinics and to develop guidelines for carriers without manifest disease. Given the frequency of the answer ‘Ask the opinion of an expert’ to our case-based questions, specialised cardiologists or specialised centres should be open to every patient and doctor.
Acknowledgements
This research is financially supported by ZorgOnderzoek Nederland (ZonMw), grant number 62000010 and the Netherlands Heart Foundation (NHS), grant number 2003 D302. The funding organisations had no involvement in study design, collection, analysis and interpretation of data, in the writing of this paper and in the decision to submit the paper for publication.
References
- 1.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–9. [DOI] [PubMed] [Google Scholar]
- 2.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–10. [DOI] [PubMed] [Google Scholar]
- 3.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–6. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–91. [DOI] [PubMed] [Google Scholar]
- 6.Elliott PM, Gimeno JR, Thaman R, et al. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart. 2006;92:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large nonreferral-based patient population. Circulation. 2000;102:858–64. [DOI] [PubMed] [Google Scholar]
- 8.Miller MA, Gomes JA, Fuster V. Risk stratification of sudden cardiac death in hypertrophic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2007;4:667–76. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–713. [DOI] [PubMed] [Google Scholar]
- 10.Elliott PM, Poloniecki J, Dickie S, et al. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–8. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 12.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. [DOI] [PubMed] [Google Scholar]
- 13.McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart. 1997;77:130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niimura H, Bachinski LL, Sangwatanaroj S, et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248–57. [DOI] [PubMed] [Google Scholar]
- 15.van Langen I, Birnie E, Leschot NJ, Bonsel GJ, Wilde AA. Genetic knowledge and counselling skills of Dutch cardiologists: sufficient for the genomics era? Eur Heart J. 2003;24:560–6. [DOI] [PubMed] [Google Scholar]
- 16.Remme WJ, McMurray JJ, Hobbs FD, et al. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J. 2008;29:1739–52. [DOI] [PubMed] [Google Scholar]
- 17.Southwell C, Moallem M, Auckley D. Cardiologist's knowledge and attitudes about obstructive sleep apnea: a survey study. Sleep Breath. 2008;12:295–302. [DOI] [PubMed] [Google Scholar]