Abstract
Background. In idiopathic dilated cardiomyopathy (IDC) an imbalance between myocardial oxygen consumption and supply has been postulated. Subclinical myocardial ischaemia may contribute to progressive deterioration of left ventricular function. The relation between regional myocardial perfusion reserve (MPR) and contractile performance was investigated.
Methods. Patients with newly diagnosed IDC underwent positron emission tomography (PET) scanning using both 13N-ammonia as a perfusion tracer (baseline and dypiridamole stress), and 18F-fluorodeoxyglucose viability tracer and a dobutamine stress MRI. MPR (assessed by PET) as well as wall motion score (WMS, assessed by MRI) were evaluated in a 17-segment model.
Results. Twenty-two patients were included (age 49±11 years; 15 males, LVEF 33±10%). With MRI, a total of 305 segments could be analysed. Wall motion abnormalities at rest were present in 127 (35.5%) segments and in 103 (29.9%) during dobutamine stress. Twenty-one segments deteriorated during stress and 43 improved. MPR was significantly higher in those segments that improved, compared with those that did not change or were impaired during stress (1.87±0.04 vs. 1.56± 0.07 p<0.01.)
Conclusion. Signs of regional ischaemia were clearly present in IDC patients. Ischaemic regions displayed impaired contractility during stress. This suggests that impaired oxygen supply contributes to cardiac dysfunction in IDC. (Neth Heart J 2009;17:470-4.)
Keywords: idiopathic dilated cardiomyopathy, positron emission tomography, cardiac magnetic resonance imaging, endothelial function, ischaemia
Idiopathic dilated cardiomyopathy (IDC) is a disease characterised by dilated, poorly contracting ventricles, in the absence of coronary artery disease or primary valvular disease, or other specified causes of myocardial dysfunction. The initial injury is often thought to be a myocarditis or intoxication, but this is seldom proven. When present, the disease usually becomes progressive with increasing symptoms of congestive heart failure.1 It ultimately leads to premature death due to intractable heart failure or to sudden death due to intractable arrhythmias. In this way, IDC is associated with excess mortality and a high rate of heart transplantation indications.
The mechanisms of the autonomic progressive deterioration of cardiac function are poorly understood. Viral persistence,2 abnormal hormonal activity,3 and genetic factors4 have been proposed as causal in this autonomic progression. Interestingly, it has been shown that endothelial function is impaired in patients with IDC,5 although the epicardial coronary arteries do not show significant abnormalities. Also myocardial perfusion abnormalities have been found.6 Myocardial ischaemia as a cause of the progression of the disease could be caused by microvascular abnormalities, endothelial dysfunction, autonomic dysregulation of the microvasculature, and high intramural pressure, due to increased Laplace wall stress.7 This may cause myocardial stunning or hibernation, and give rise to myocardial fibrosis, which promotes further left ventricular dysfunction and dilatation.8
We have previously found ischaemia-like myocardial contractile responses during dobutamine stress echocardiography in patients with DCM.9 Furthermore, we have observed a relation between ischaemia and left ventricular (LV) dysfunction10 and a decreased myocardial perfusion reserve (MPR) in patients with IDC.11 In addition, low MPR was paralleled by high LV systolic wall stress.11
In the present study we further explore the role of subclinical myocardial ischaemia as can be assessed by PET. The main objective was to substantiate whether areas with a decreased flow reserve also show ischaemiarelated changes in wall motion during dobutamine stress. Theoretically, such mismatch is accessible for therapy with a β-blocker or another ischaemia-alleviating agent. Therefore, we performed this study in patients with newly diagnosed IDC before starting β-blocker therapy. A PET scan with rest and stress 13N ammonia perfusion as well as viability with 18F-fluorodeoxyglucose (FDG) was compared with wall motion data at rest and during dobutamine stress MRI.
Methods
Study group
Patients with newly diagnosed IDC and mild congestive heart failure were eligible for the study. All patients underwent coronary angiography, which showed normal epicardial coronary arteries. The diagnosis of IDC was based on a combination of clinical variables (LV dilation, global decreased contraction patterns) and the absence of epicardial coronary artery disease, and the absence of other clear causes of cardiomyopathy (significant valvular disease, alcohol abuse, use of cardiotoxic agents in the past, such as adriamycin, or active myocarditis). None of the patients were on β-blocker therapy at the start of this study. PET as well as MRI scanning was performed before initiation of β-blockers. Other medication such as angiotensin-converting enzyme (ACE) inhibitors, and diuretics were allowed. Patients with systemic hypertension (systolic blood pressure >150 mmHg, diastolic blood pressure >95 mmHg), diabetes mellitus, hypercholesterolaemia (>6 mmol/l) or any other systemic illness were excluded. All patients gave written, informed consent, and the protocol was approved by the Institutional Review Board.
Protocol for PET imaging
Patients underwent dynamic rest 13N-ammonia, 13N-ammonia dipyridamole stress and gated 18FDG PET imaging using a one-day protocol, as described previously.11 Briefly, PET studies were performed after patients had discontinued vasoactive medication for five plasma half-lives and had refrained from caffeinated beverages for a minimum of 12 hours before the studies. Imaging was performed in the supine position with a Siemens ECAT 951 positron camera (Siemens CTI, Knoxville Tennessee, USA), measuring 31 planes simultaneously over a length of 10.8 cm. Measured resolution of the system was 6 mm at full width half maximum. Data were automatically corrected for accidental coincidence and dead time. Patients were positioned with the help of a rectilinear scan. Photon attenuation was measured using a retractable external ring source filled with germanium-68/gallium-68. Dipyridamole perfusion imaging was performed infusing dipyridamole (0.56 mg/kg in 4 minutes). Imaging was started by injecting 400 MBq of 13N-ammonia 6 minutes after the start of dipyridamole infusion and continued for 15 minutes.
To stimulate 18FDG uptake, patients were given 75 g of glucose orally just before scanning or were given 500 mg acipimox (Nedios, Byk, the Netherlands) orally to lower circulating free fatty acids12 90 minutes before the scanning procedure. To prevent side effects of acipimox (e.g. skin rash), 250 mg of aspirin was administered orally five minutes before acipimox intake. After completion of the 13N-ammonia data acquisition, 200 MBq of 18FDG was injected intravenously, followed by a PET dynamic acquisition procedure. The total 18FDG PET acquisition time was 40 minutes, with the last 20 minutes acquired in gated mode with 16 frames per cardiac cycle. The length of each gate was based on the current RR interval. The RR interval was allowed to vary ±10%. Data were corrected for attenuation using the transmission scan and were reconstructed using filtered back-projection (Hann filter: 0.5 pixels/cycle).
Analysis of PET data
From the PET data, dynamic parametric polar maps were constructed.11 Myocardial blood flow data were corrected for partial volume effect and spill over and quantified by the Hutchins model.13 Briefly, myocardial and blood time-activity curves derived from regions of interest over the heart and ventricular chamber are fitted using a three-compartment model for 13N-ammonia, yielding rate constants for tracer uptake and retention. Rest, stress, and perfusion flow reserve (dipyridamole/rest ratio) was calculated by dividing the dipyridamole 13N-ammonia stress study with the 13N-ammonia rest study. In brief, dipyridamole 13N-ammonia stress was expressed in ml/min/100 g myocardial tissue and divided by 13N-ammonia rest ml/min/100 g myocardial tissue, resulting in a ratio without a unit. Data analysis of 18FDG uptake was performed with a PATLAK analysis.14
Calculation of the extent of mismatch areas (viable myocardium) and match areas (nonviable myocardium) was calculated from these data. The last frames (20 minutes acquisition time) of the dynamic gated FDG PET studies were summed and transformed into static studies and used for further data analysis with the help of the quantitative gated SPECT (QGS) programme.15 Based on the gated FDG images, left ventricular endsystolic (LVESV) and end-diastolic volumes (LVEDV) as well as left ventricular ejection fraction (LVEF) were computed.
Protocol for MRI imaging
DSMR was performed at a 1.5 Tesla MRI System (Magnetom Sonata; Siemens Medical Solutions; Erlangen; Germany) according to a standard dobutamine-atropine stress protocol (5, 10, 20, and 30 μg/kg/min dobutamine) until 85% of the agepredicted target heart rate was achieved. Imaging at each stress level was performed in one long-axis and three short-axis views and contiguous short-axis views at peak stress using a segmented steady-state free precession sequence (TR 3 ms, TE 1.5 ms, FA 60°, 15 views per segment). Myocardial segments (17-segment model) were evaluated by a panel of three observers (DL, PvdV, RAT). Wall motion score for each individual segment were given: 1 = normal, 2 = hypokinetic, 3 = akinetic, 4 = dyskinetic. Myocardial ischaemia was defined by new or worsening wall motion abnormalities in at least one myocardial segment during dobutamine stress.
Results
Patients
Between May 2003 and March 2007 a total of 30 eligible patients (age 50±13; 21 males) were selected for this study. Eight of them declined informed consent or had an indication for ICD implantation, the remaining 22 patients are subjects of this study (age 49±11; 15 males. Left ventricular ejection fraction before inclusion was 33±10% (measured with either echocardiography, radio-isotope scintigraphy, or MRI). Five patients were judged to have heart failure NYHA class I, 16 class II, and one class III. The majority of patients were treated with an ACE inhibitor or angiotension receptor blocker (ARB); half of the patients were receiving diuretics (table 1).
Table 1 .
Baseline data of included patients (n=22).
| Sex (M/F) | 15/7 |
| Age (years) | 49±11 |
| BMI (kg/m2) | 27.6±4.1 |
| Systolic blood pressure (mmHg) | 135±22 |
| Diastolic blood pressure (mmHg) | 86±13 |
| Heart rate (beats/min) | 72±11 |
| Atrial fibrillation (n) | 1 |
| LVEF (%) | 33±10 |
| LVEDD (mm) | 63±11 |
| LVESD (mm) | 52±14 |
| Haemoglobin (mmol/l) | 9.0±0.7 |
| Creatinine (μmol/l) | 91±23 |
| VO2max (ml/min/kg) | 25.3±8,7 |
| Smoking | 3 |
| NYHA class | |
| - I | 5 |
| - II | 16 |
| - III | 1 |
| - IV | 0 |
| Aspirin | 3 |
| Coumarin | 3 |
| ACE inhibitor | 15 |
| Angiotension receptor blocker | 4 |
| Calcium antagonist | 3 |
| Digoxin | 3 |
| Cordarone | 2 |
| Statin | 2 |
| Diuretics | 11 |
LVEF=left ventricular ejection fraction, LVEDD=left ventricular end-diastolic volumes, LVESD=left ventricular left ventricular end-systolic volumes.
Global analysis
MRI showed a wall motion score of 29±8 at rest. During dobutamine stress a wall motion score (WMS) of 28±9 was found. All but one patient had one or more segments with wall motion abnormalities at rest. During dobutamine stress, WMS improved in ten patients, indicating stunning/hibernation. In three patients WMS during dobutamine decreased, indicating ischaemia.
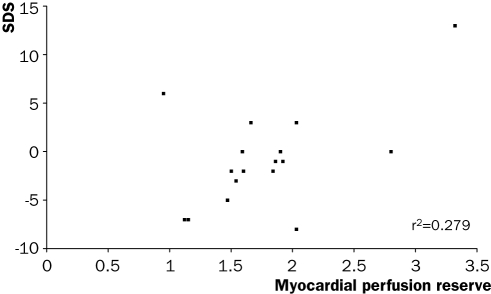
We assessed LV geometry and function with gated FDG PET scanning. LV volumes were greatly increased (LVEDV 220±90 ml and LVESV 167±90 ml), and LVEF was reduced. Myocardial perfusion at rest was 55±15 ml/min/100 g (normal values 148±3516 and during stress 97±34 (normal values 292±95). MPR was 1.8±0.6 (normal >2). No relation was observed between myocardial perfusion and WMS, either at rest or during stress. However a significant correlation was found between global MPR and the summed difference score during dobutamine stress MRI (figure 1).
Figure 1 .
Relation between global myocardial perfusion reserve as measured with PET and the summed difference score (SDS) with dobutamine stress (p=0.029).
Per segment analysis
With MRI, a total of 305 segments could be analysed. Wall motion abnormalities at rest were present in 127 (35.5%) and in 103 (29.9%) during dobutamine stress. A mean WMS per segment at rest of 1.7±0.9 was found. During dobutamine stress this was 1.6±0.9. A total of 21 segments deteriorated during dobutamine stress and 43 improved.
With PET the mean MPR per segment was 1.8±0.7. In a total of 29 segments perfusion decreased during dipyridamole PET imaging. Mismatching defects suggestive of ischaemia in combination with viability were found in 83 segments. A low perfusion as well as low FDG uptake accounted for 33 (nonviable) segments. The remainder of the segments showed normal perfusion as well as FDG uptake (n=241).
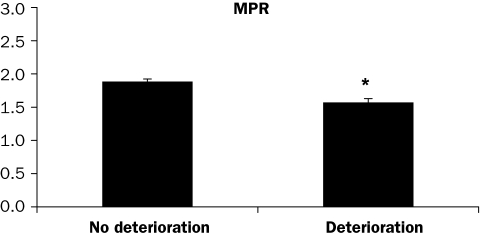
Segments with deteriorating WMS during dobutamine were compared with segments without deterioration, and it was observed that MPR was lower in the deteriorating segments, which can be considered suggestive for ischaemia (figure 2). Three of the 21 segments in which wall motion deteriorated during dobutamine stress showed a perfusion/viability mismatch during PET.
Figure 2 .
Myocardial perfusion reserve (MPR) assessed with PET (rest-dipyridamole 13N-ammonia stress) in myocardial segments with and without wall motion deterioration during dobutamine stress MRI. *p<0.01.
Discussion
In myocardial disease, such as hypertrophic and dilated cardiomyopathies, microvascular dysfunction has been described previously. In hypertrophic cardiomyopathy, the dysfunction is not confined to areas of myocardial hypertrophy. Intramural arterioles show a thickening of the medial layer and to a lesser extent of the intima.17 In dilated cardiomyopathy, we have previously shown that regions with a lower MPR are present.11 Furthermore, we found that global systolic wall stress correlated negatively with MPR. In the present study we further evaluated the relation between MPR and wall motion abnormalities. A significantly lower MPR was found in segments with a deteriorating wall motion during dobutamine stress. These findings are in concert with a study measuring coronary flow reserve invasively.18 In that study also a correlation between regional flow reserve and regional contractile function was found.
There are several explanations for the presence of ischaemia in IDC, even in the absence of significant epicardial coronary artery disease. First, IDC is characterised by alterations in the interstitium and accumulation of collagen, the extent of which has been correlated to the degree of LV dysfunction.19 It could be postulated that fibrosis imposes a barrier for oxygen diffusion through the myocardium and thus interferes with the perfusion or perfusion reserve. In the latter study no such correlation has been found. Furthermore, the diffusion of oxygen to the cardiomyocyte is hampered by the increase in cardiomyocyte diameter and the absence of compensatory angiogenesis, as reviewed in de Boer et al.7
In addition to the myocardial and interstitial changes, also endothelial dysfunction appears to play a pivotal role. Endothelium-dependent vasodilator response to acetylcholine is blunted in resistance17,20 as well as epicardial vessels of IDC patients.21 Endothelial dysfunction may be an explanation for the segmental abnormal MPR in combination with myocardial dysfunction in our study. Disturbed endotheliumdependent dilatation may account for a decreased perfusion reserve. On the one hand, endotheliumderived NO is important in the regulation of vascular tone. On the other hand, it has been postulated that physiological NO concentrations improve calcium influx in atrial myocytes22 and increase contractile function in isolated rat cardiomyocytes.23 Alterations in basal NO production may therefore have an impact on vascular as well as contractile function.
Nuclear and other imaging techniques in patients with heart failure are important for choosing the correct treatment modality such as cardiac resynchronisation therapy. In addition noninvasive imaging methods may identify factors influencing the response of CRTtreated patients.24
In summary, our data comparing wall motion changes during stress MRI with PET data on segmental myocardial perfusion show that the observed regional ischaemia in IDC is severe enough to be associated with contractile dysfunction. We postulate that therapies aimed at improving myocardial oxygen supply in IDC may be of benefit.
Acknowledgements
This study was supported by the Netherlands Heart Foundation grant 2001B099
References
- 1.Van den Broek SA, van Veldhuisen DJ, de Graeff PA, Landsman ML, Hillege H, Lie KI. Comparison between New York Heart Association classification and peak oxygen consumption in the assessment of functional status and prognosis in patients with mild to moderate chronic congestive heart failure secondary to either ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;70:359–63. [DOI] [PubMed] [Google Scholar]
- 2.Fujioka S, Kitaura Y, Ukimura A, et al. Evaluation of viral infection in the myocardium of patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;36:1920–6. [DOI] [PubMed] [Google Scholar]
- 3.Volterrani M, Giustina A, Manelli F, Cicoira MA, Lorusso R, Giordano A. Role of growth hormone in chronic heart failure: therapeutic implications. Ital Heart J. 2000;1:732–8. [PubMed] [Google Scholar]
- 4.Kamisago M, Sharma SD, DePalma SR, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–96. [DOI] [PubMed] [Google Scholar]
- 5.Treasure CB, Vita JA, Cox DA, et al. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–9. [DOI] [PubMed] [Google Scholar]
- 6.Juilliere Y, Marie PY, Danchin N, et al. Radionuclide assessment of regional differences in left ventricular wall motion and myocardial perfusion in idiopathic dilated cardiomyopathy. Eur Heart J. 1993;14:1163–9. [DOI] [PubMed] [Google Scholar]
- 7.de Boer RA, Pinto YM, van Veldhuisen DJ. The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. MicroCirculation. 2003;10:113–26. [DOI] [PubMed] [Google Scholar]
- 8.Ingwall JS, Nascimben L, Gwatmey JK. Heart failure: is the pathology due to calcium overload or to mismatch in energy supply and demand? In: Gwatmey JK, Briggs MG, Allen PD, editors. Heart Failure: Basic and clinical aspects. New York: Marcel Dekker, 2008:667–700. [Google Scholar]
- 9.de Jong RM, Cornel JH, Crijns HJ, van Veldhuisen DJ. Abnormal contractile responses during dobutamine stress echocardiography in patients with idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2001;3:429–36. [DOI] [PubMed] [Google Scholar]
- 10.van Veldhuisen DJ, van den Heuvel AF, Blanksma PK, Crijns HJ. Ischemia and left ventricular dysfunction: a reciprocal relation? J Cardiovasc Pharmacol. 1998;32 Suppl 1:S46–S51. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel AF, van Veldhuisen DJ, van der Wall EE, et al. Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:19–28. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Hawkins RA, Huang SC, et al. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-d-glucose studies. J Nucl Med. 1991;32:733–8. [PubMed] [Google Scholar]
- 13.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–42. [DOI] [PubMed] [Google Scholar]
- 14.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. [DOI] [PubMed] [Google Scholar]
- 15.Blanksma PK, Willemsen ATM, Meeder JG, et al. Quantitative Myocardial Mapping of Perfusion and Metabolism Using Parametric Polar Map Displays in Cardiac Pet. J Nucl Med. 1995;36:153–8. [PubMed] [Google Scholar]
- 16.de Vries J, DeJongste MJ, Jessurun GA, Jager PL, Staal MJ, Slart RH. Myocardial perfusion quantification in patients suspected of cardiac syndrome X with positive and negative exercise testing: a [13N]ammonia positron emission tomography study. Nucl Med Commun. 2006;27:791–4. [DOI] [PubMed] [Google Scholar]
- 17.Canetti M, Akhter MW, Lerman A, et al. Evaluation of myocardial blood flow reserve in patients with chronic congestive heart failure due to idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:1246–9. [DOI] [PubMed] [Google Scholar]
- 18.Skalidis EI, Parthenakis FI, Patrianakos AP, Hamilos MI, Vardas PE. Regional coronary flow and contractile reserve in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:2027–32. [DOI] [PubMed] [Google Scholar]
- 19.Knaapen P, Gotte MJ, Paulus WJ, et al. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology. 2006;240:380–8. [DOI] [PubMed] [Google Scholar]
- 20.Holdright DR, Clarke D, Fox K, Poole-Wilson PA, Collins P. The effects of intracoronary substance P and acetylcholine on coronary blood flow in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 1994;15:1537–44. [DOI] [PubMed] [Google Scholar]
- 21.Mathier MA, Rose GA, Fifer MA, et al. Coronary endothelial dysfunction in patients with acute-onset idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1998;32:216–24. [DOI] [PubMed] [Google Scholar]
- 22.Kirstein M, Rivet-Bastide M, Hatem S, Benardeau A, Mercadier JJ, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J Clin Invest. 1995;95:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. [DOI] [PubMed] [Google Scholar]
- 24.Ypenburg C, van der Wall EE, Schalij MJ, Bax JJ. Imaging in cardiac resynchronisation therapy. Neth Heart J. 2008; 16:S36–S40. [PMC free article] [PubMed] [Google Scholar]