Abstract
Myocarditis is an acute or chronic inflammatory disease of the myocardium which can be viral, postinfectious immune or primarily organ-specific autoimmune. Clinical manifestations of acute and chronic myocarditis are extremely varied, ranging from mild to severe. Affected patients may recover or develop (dilated) cardiomyopathy (DCM) with life-threatening symptoms including heart failure, conduction disturbances, arrhythmias, cardiogenic shock or sudden cardiac death.
The diagnosis of myocarditis is a challenging process and not only because of a diverse presentation; other problems are limited sensitivity of endomyocardial biopsies (EMB) and overlapping symptoms. Furthermore, the diagnosis is not well defined. However, early diagnosis is mandatory to address specific aetiology-directed therapeutic management in myocarditis that influences patient morbidity and mortality.
Currently, EMB remains the only way to confirm the presence of a viral genome and other histopathological findings allowing proper treatment to be implemented in cases of myocarditis. Increased recognition of the role of myocardial inflammatory changes has given rise to interest in noninvasive imaging as a diagnostic tool, especially cardiovascular magnetic resonance imaging (CMR). In this review we discuss the current role of CMR in the evaluation of myocarditis-induced inflammatory cardiomyopathies. (Neth Heart J 2009;17:481-6.)
Keywords: myocarditis, cardiac magnetic resonance imaging, cardiomyopathy
Myocarditis is a cardiac disease associated with inflammation and injury of the myocardium.1 The term myocarditis was first introduced in the 19th century. In 1986, in an effort to standardise the diagnostic criteria, a panel consisting of eight cardiac pathologists proposed the Dallas criteria and provided a histopathological categorisation by which the diagnosis of myocarditis could be established.2 However, this classification had several pitfalls, being susceptible to variation in pathological interpretation, sampling error and not considering the exact cause of pathological findings. A clinicopathological classification utilising both histological and clinical features in which four distinct subgroups were subdivided was provided in 1991 and adjusted in 2001 but it has received only limited acceptance. Acute myocarditis (AM) was categorised into a common and a fulminant type depending on whether or not patients received mechanical circulatory support in the management of heart failure. Chronic myocarditis (CM) was subdivided into chronic active and chronic persistent myocarditis. There is consensus that viral infection is responsible for the vast majority of cases in North America and Europe.3,4 Coxsackievirus B3 (CBV3) is considered the dominant viral aetiological agent.5 Other frequently detected viral genomes are enterovirus, adenovirus, parvovirus B19 (PVB19), human herpes virus 6 (HHV6) and Epstein-Barr virus (EBV).6
AM must be considered in patients presenting with recent-onset cardiac failure or arrhythmia, though the onset of clinical symptoms may be vague and clinical features are heterogeneous, ranging from asymptomatic, generalised malaise, acute heart failure, dilated cardiomyopathy (DCM) and even sudden cardiac death, which accounts for up to 20% of cases in young adults.4,7 Important clues to its epidemiology come from routine post-mortem examinations where it is identified in 1 to 9%.8
In contrast, fulminant myocarditis is characterised by a distinct viral prodrome, a rapid onset of symptoms, extensive haemodynamic compromise and marked myocardial inflammation.9 The clinical course is related to the type of virus; with a benign course in PVB19, and a worse progression in the setting of HHV6 with a latent state after primary infection.10 Prognosis in biopsy-proven AM is also related to histological classification and biomarkers with a higher survival rate observed in patients with fulminant myocarditis versus an AM group.11 A high early mortality rate of patients with myocarditis varying from 22% in the acute fulminate group to 50% in the chronic recurrent group has been observed with a high prevalence of late deaths in patients with chronic latent myocarditis.12
CM is a common evolution of AM.13 AM recovers spontaneously within a few weeks to months in up to 50% of patients. Nevertheless, progression of AM to CM or DCM occurs in about 21% of cases by both viral persistence and/or autoimmune self-perpetration.14,15 In CM, a viraemia is frequently absent and intramyocardial inflammation is considerably lower than in patients with AM and thus can be detected only by using sensitive immunohistological techniques and nested polymerase chain rather than histological Dallas criteria. CM can be divided into chronic active myocarditis and chronic persistent myocarditis. In active CM, symptoms of moderate left ventricular dysfunction, ongoing inflammation, myocardial damage and active scar tissue are present (active or borderline myocarditis). Persistent CM often has a normal left ventricular function, atypical chest pain and persistence of inflammation. On occasion, a viral genome can be detected in biopsy sampling. Therefore CM is mainly diagnosed clinically by symptoms of left-sided chronic heart failure, especially idiopathic DCM or myocardial fibrosis. Frequently detected viral genomes in endomyocardial biopsies from patients with clinically suspected myocarditis in the past and DCM are CVB3 and B4, echovirus, adenovirus, PVB19, EBV and HHV6.16
The current diagnostic gold standard for the detection of myocarditis is direct visualisation by autopsy or endomyocardial biopsy with the histological Dallas criteria in conjunction with new tools of immunohistochemistry and viral polymerase chain reaction, according to the 1995 World Health Organisation classification of cardiomyopathies. Although, recent data suggest that current histopathological criteria for myocardial inflammation are not sensitive to identify the populations with viral or autoimmune-related heart compromise.2 Furthermore, interpretation of bioptic findings is subjective due to a considerable interobserver variability, occurrence of complications, and the lack of standardisation in performing the biopsy.7 However, early diagnosis is mandatory to address specific aetiology-directed therapeutic management in AM and CM which influences patients morbidity and mortality. Further, early diagnosis of myocardial involvement may permit earlier onset of heart failure treatment and extend the life span in these patients. New insights into the role of myocardial inflammatory changes have given rise to interest in noninvasive imaging as a diagnostic tool, especially cardiovascular magnetic resonance imaging (CMR). CMR provides the opportunity of anatomical imaging and an accurate assessment of functional parameters, but in this respect what is more important is its ability to characterise tissue. Presence of contrast enhancement (CE) indicates myocardial injury (i.e. scar, fibrosis) and T2-weighted images mark interstitial oedema, known as an integral part of the inflammatory response.
The present review therefore focuses on the current role of CMR in the evaluation of myocarditis and myocarditis-induced cardiomyopathies.
Pathophysiology of myocarditis
In order to better understand the often unpredictable clinical manifestations and progression of viral myocarditis, the pathophysiological process has to be elucidated. Important lessons come largely from studies in animals.17
The actual underlying pathological mechanism remains controversial, though three mechanisms have been proposed. During the first acute phase (days 0 to 3), direct excessive destruction of the myocardium occurs by infiltrating immune cells targeting virusinfected cardiomyocytes, within three days after infection, frequently extending to the remote uninfected region. A high titre of virus is present in the blood. The initial phase frequently passes unnoticed since the initial damage is often prevented by the innate immune response. The second subacute phase (days 4 to 14) develops as a result of autoimmune-mediated destruction of cardiac cells by circulating autoantibodies and/or autoreactive immune cells or by immunemediated obliteration of cardiomyocytes, which is caused by mimicked epitopes shared between viral and cardiac antigens. Finally, in the third chronic phase (days 15 to 90), viral particles are typically absent in blood and peripheral tissues. Viral RNA persistence and immune infiltrates may contribute to long-term tissue degeneration and a typical picture of DCM, and congestive heart failure develops as a result of direct virus-induced cardiomyocyte injury.5,18,19
Myocarditis-induced (dilated) cardiomyopathy
In 20% of cases, acute myocarditis progresses to DCM.20 Evidence suggests that a viral mechanism not only contributes to the acute phase of myocarditis but also to the evolution of ongoing cardiac disease. The persistence of a higher incidence of neutralising antibodies to (coxsackie B) viruses in patients with cardiomyopathy than in age-, sex-, race-, and geographicmatched control subjects pointed to the theory of a viral cause underlying the pathogenesis of cardiomyopathy.19 A substantial proportion (10 to 34%) of DCM patients may in fact suffer from viral myocarditis.9,17 DCM, a major cause of heart failure and cardiac transplantation, is characterised by dilatation and impaired contraction of the ventricles. The clinical manifestation of DCM and a history of acute viral myocarditis does not confirm a CM. Three possible mechanisms for the development of DCM as a consequence of an episode of CBV myocarditis have been described.21 The first proven mechanism is the development of DCM in an acute or subacute timeframe as a direct cytotoxic effect subsequent to an episode of myocarditis. The second potential (hypothetical) mechanism comprises a slow, chronic and continuous destruction of cardiac myocytes or impairment of myocyte function. The third mechanistic possibility is that of DCM developing long after complete resolution of the initial episode of myocarditis. DCM develops as a result of an undefined process in which an episode of remote viral infection renders the healed myocardium more susceptible to the remote development of idiopathic DCM. Mutations in several genes encoding structural proteins of the myocyte can cause DCM, but the gene defects identified so far appear to account for a minority of cases. Recently, increasing evidence has emerged that a substantial portion of patients with myocarditis and DCM represent different stages of an organ-specific autoimmune disease in genetically predisposed individuals.22
CMR in the acute and chronic phase of myocarditis
Recent studies have demonstrated that CMR has shown promising results in the early diagnosis and the follow-up of AM and its subsequent stages. CMR includes several techniques that can be used in various combinations to assess left ventricular (LV) functional parameters, morphology, myocardial perfusion, and myocardial disorders within one examination.23
Besides significantly lower ejection fractions and wall motion abnormalities in patients with AM, two relevant contrast-enhancement CMR (CE-CMR) approaches have been found to be effective in identifying areas of myocardial damage in AM. Myocardial global relative enhancement (gRE) reflects myocardial hyperaemia and increased capillary permeability as features of present inflammation, whereas late CECMR usually indicates irreversible myocardial injury. GRE (T1-weighted imaging pre- and post-contrast, used to calculate gRE from the mean signal intensities (SI) within the manually outlined borders around the LV myocardium and right erector spinae muscle) has been observed significantly more often in myocarditis patients compared with controls.14,24 CE-CMR enables visualisation of myocardial damage in patients with myocarditis after intravenous injection of gadolinium. Due to different wash-in and wash-out kinetics, areas with myocardial changes, such as scarring, fibrosis and oedema, retain gadolinium for prolonged periods. This provides an opportunity to visualise areas of myocarditis defined by histopathology, with a reported sensitivity of 100% and specificity of 90%.25 Presence of late CE is reported in 44 to 95% of patients with myocarditis.10,25 In acute myocarditis, CE is frequently located in the lateral wall originating from the epicardial quartile, though the pattern of myocardial injury is influenced by the virus type.26 According to CE patterns, this technique is also capable of ruling out an ischaemic cause in the differential diagnosis of myocarditis because CE patterns in the setting of ischaemic infarction always include the subendocardial layer of the myocardium (figure 1).4 Enhancement patterns in myocarditis generally exclude the subendocardium with the exception of eosinophilic myocarditis frequently involving the endomyocardium (figure 2).27,28 Further, the type of virus and pattern of myocardial damage are related. CE in the lateral free wall is found in the majority of PVB19 patients, whereas in HHV6 myocarditis, CE frequently is observed in the midwall of the interventricular septum (figure 3).10 Thus CE not only differentiates between myocarditis or infarction, it can also differentiate between various viral origins.
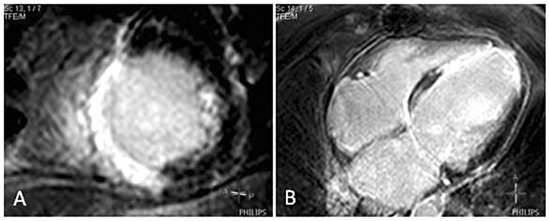
Figure 1.
Ischaemic anteroseptal infarction. A) Short-axis view showing a transmural pattern of contrast enhancement in the anteroseptal and inferoseptal wall. B) Four-chamber view showing a transmural pattern of contrast enhancement in the inferoseptal and apical wall.
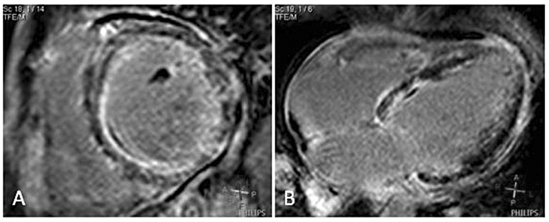
Figure 2.
Eosinophilic myocarditis. A) Short-axis view showing a pattern of subendocardial and midwall contrast enhancement in the anteroseptal and inferoseptal wall. B) Four-chamber view showing subendocardial and midwall contrast enhancement in the inferoseptal wall.
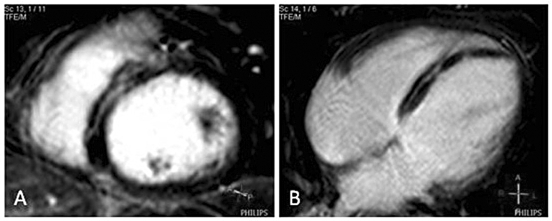
Figure 3.
Myocarditis. A) Short-axis view and four-chamber view showing a pattern of midwall contrast enhancement in the septal wall, B) Four-chamber view showing a pattern of midwall contrast enhancement in the inferoseptal wall.
Another interesting CMR approach in acute myocarditis is T2-weighted imaging, a pulse sequence sensitive to regional or global increases of myocardial water, a substantial feature of the inflammatory response in human myocarditis.15,24 A combined CMR approach using black blood T2-weighted imaging and CE-CMR showed a significantly higher T2 SI in patients with myocarditis.
An alternative acquisition mode for imaging oedema in myocarditis could be the T2-prepared steady state free precession (SSFP) technique which is more reliable for imaging oedema in acute myocardial infarction. It provides fewer artifacts and has better diagnostic accuracy than conventional dark blood acquisitions.29
The parameters described above are also of utmost importance in the clinical follow-up and in the evaluation of the response to the initiated treatment.
MRI can also play an important role in the diagnosis of the chronic phase of myocarditis such as the aetiology of DCM. As noted earlier, CM can be divided into chronic active myocarditis and chronic persistent myocarditis. In patients clinically suspected of CM, viraemia is often absent. Therefore, CM is mainly diagnosed clinically by the presence of chronic heart failure, especially idiopathic DCM or myocardial fibrosis. MRI is capable of accurate detection of myocardial fibrosis in DCM. Global relative enhancement (GRE) may be useful in the noninvasive detection of inflammatory processes of the myocardium. Beside increased oedema ratio from T2-weighted imaging, increased GRE was a common finding in suspected CM that could be confirmed at immunohistological analysis14 CE-CMR identified areas of myocardial inflammation in 70% of patients with biopsy-proven CM presenting with heart failure or ventricular arrhythmias.13 A frequent pattern of CM in CE-CMR is midwall or subepicardial enhancement generally excluding the subendocardium. Evaluated CM subgroups (histological evidence of active myocarditis and borderline myocarditis) reveal different enhancement patterns. In both groups, the group with histological evidence of active myocarditis and the group with borderline myocarditis, a midwall pattern is a frequent finding (figure 4). Though, a pattern of subepicardial CE is only observed in patients with histological evidence of active myocarditis.13 Enhancement patterns seen on CE-MRI may also serve as a map for the exact location to accomplish endomyocardial biopsy if necessary; thus enhancing the diagnostic accuracy of endomyocardial biopsy.
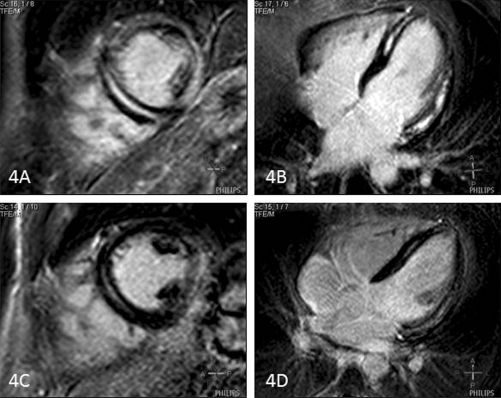
Figure 4 .
Acute and chronic phase of myocarditis in a patient. A, B) Acute myocarditis: short-axis view and four-chamber view showing an extensive pattern of midwall contrast enhancement. Only the basal septal wall and distal anterolateral wall are spared. C, D) Chronic myocarditis: short-axis and fourchamber view showing the same pattern of midwall delayed contrast enhancement with lower signal intensity.
Therapeutic management
The diagnosis of myocarditis has relevant therapeutic implications. Supportive care is the first-line therapy for patients with myocarditis. Treatment of myocarditisinduced cardiac failure includes the standard regimen (including diuretics to lower ventricular filling pressures, an angiontensin-converting-enzyme inhibitor to decrease vascular resistance, and a β-blocker after achieving clinical stability). Arrhythmias should be monitored and treated. Mechanical support with an intra-aortic balloon pump or LV assist device as a bridge to recovery or heart transplantation may be necessary in cases that fail to improve.18
Apart from symptomatic or supportive therapy, additional treatments are being investigated for myocarditis because long-term consequences of myocarditis appear to be related to the activation of cellular and humoral autoimmunity.19 A variety of immunosuppressive agents and intravenous imunoglobulins have caused marked improvement. Interferon-B treatment resulted in elimination of viral genomes (22 of 22 patients) and improved left ventricular function (assessed echocardiographically; 15 of 22 patients) in patients with enteroviral or andenoviral persistence.30 In a series of ten patients with new-onset DCM treated with high-dose immune globulin, echocardiographic left ventricular ejection fraction (LVEF) improved 17 EF units.31 The Marburg registry also favours intravenous immunoglobulin treatment in biopsy-proven adenovirus and parvovirus B19 myocarditis combined with optimal conventional therapy to achieve virus clearance.32 A subset of patients with CM, those expressing class I and II human lymphocyte antigen on cardiac myocytes, may show a response to azathioprine and prednisone.8
Other results did not support routine treatment of myocarditis with immunosuppressive drugs. Additional treatments did not have a real advantage over standard treatment for heart failure; no beneficial effect on the primary endpoint, angiographically determined LVEF, was seen.33 Actually a mild negative influence on left ventricular dimensions was observed.34
There is evidence that intramyocardial viral persistence is associated with progressive ventricular dysfunction, whereas spontaneous viral elimination was associated with a significant improvement in LV function.16 However, another study showed that an advanced New York Heart Association functional class, immunohistologicalsigns of inflammation, and lack of β-blocker therapy, but not histology (positive Dallas criteria) or viral genome detection, are related to poor outcome. With regard to as yet unestablished antiviral or immunosuppressivetreatment strategies, according to these authors it is mandatory to differentiatechronic active viral myocarditis (myocardial viralinfection with cellular inflammation) from postviral autoimmunity and from harmless latent viral persistence without inflammatory infiltrates to address specific aetiologydirected therapeutic management.2
Unfortunately, randomised clinical trials highlighting the clinical importance of CMR visualising the extent of endomyocardial involvement and response of a therapeutic regimen have not been performed. However, a number of cases and case series have been reported, illustrating the usefulness of CE-CMR in the evaluation of the response to the initiated treatment in patients with myocarditis.28,35-37
Conclusion
With current insights into the knowledge of progression of AM to CM or DCM with life-threatening symptoms, we emphasise the importance of a correct diagnosis of myocarditis.
This can be done noninvasively with a high diagnostic accuracy, by using a combined approach with cine CMR, early and late CE-CMR and T2 acquisitions. Evidence of persisting inflammatory activity detected with CMR in AM and CM may have relevant prognostic implications and serve as a powerful tool to triage patients for appropriate treatment. CMR can also monitor the left ventricular functional parameters and the regression of myocardial inflammation in patients undergoing a therapeutic regimen.
We recommend this combined CMR approach in patients suspected of having myocarditis as a valuable noninvasive diagnostic imaging tool in identifying areas of myocardial damage that suggest a myocardial inflammatory process. CMR is also a valuable research tool. The conflicting results of specific treatment, such as antiviral or immunosuppressive medication, need at least to be studied in randomised trials before a final statement can be made on the value of CMR data to visualise the improvement of left ventricular functional parameters and the extent of myocardial involvement.
References
- 1.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4: 411–7. [DOI] [PubMed] [Google Scholar]
- 2.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–5. [DOI] [PubMed] [Google Scholar]
- 3.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–82. [DOI] [PubMed] [Google Scholar]
- 4.Kadalie CT. [MRI in chronic myocarditis]. Z Kardiol 2005;94(Suppl 4):IV/94–IV/96. [DOI] [PubMed] [Google Scholar]
- 5.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55. [DOI] [PubMed] [Google Scholar]
- 6.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–33. [DOI] [PubMed] [Google Scholar]
- 7.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bultmann B, Muller T, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–48. [DOI] [PubMed] [Google Scholar]
- 8.Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48:2085–93. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman EB, Herskowitz A, Rose NR, Baughman KL. A clinicopathologic description of myocarditis. Clin Immunol Immunopathol. 1993;68:191–6. [DOI] [PubMed] [Google Scholar]
- 10.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114: 1581–90. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy RE, III, Boehmer JP, Hruban RH, Hutchkins G.M, Kasper E.K, Hare J.M et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–5. [DOI] [PubMed] [Google Scholar]
- 12.Kodama M, Oda H, Okabe M, Aizawa Y, Izumi T. Early and longterm mortality of the clinical subtypes of myocarditis. Jpn Circ J. 2001;65:961–4. [DOI] [PubMed] [Google Scholar]
- 13.De CF, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. [DOI] [PubMed] [Google Scholar]
- 14.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–9. [DOI] [PubMed] [Google Scholar]
- 15.Zagrosek A, Wassmuth R, Abdel-Aty H, Rudolph A, Dietz R, Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis – a CMR study. J Cardiovasc Magn Reson. 2008;10:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;27; 112:1965–70. [DOI] [PubMed] [Google Scholar]
- 17.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–98. [DOI] [PubMed] [Google Scholar]
- 18.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29:2073–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future. Circulation. 1999;99:1091–100. [DOI] [PubMed] [Google Scholar]
- 20.Noutsias M, Pauschinger M, Kuhl U, Schultheiss HP. [Myocarditis and dilated cardiomyopathy. New methods in diagnosis and therapy]. MMW Fortschr Med. 2002;144:36–40. [PubMed] [Google Scholar]
- 21.Spotnitz MD, Lesch M. Idiopathic dilated cardiomyopathy as a late complication of healed viral (Coxsackie B virus) myocarditis: historical analysis, review of the literature, and a postulated unifying hypothesis. Prog Cardiovasc Dis. 2006;49:42–57. [DOI] [PubMed] [Google Scholar]
- 22.Caforio AL, Iliceto S. Genetically determined myocarditis: clinical presentation and immunological characteristics. Curr Opin Cardiol. 2008;23:219–26. [DOI] [PubMed] [Google Scholar]
- 23.Shehata ML, Turkbey EB, Vogel-Claussen J, Bluemke DA. Role of cardiac magnetic resonance imaging in assessment of nonischemic cardiomyopathies. Top Magn Reson Imaging. 2008;19:43–57. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Aty H, Boye P, Zagrosek, Wassmuth R, Kumar A, Messroghli D, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. [DOI] [PubMed] [Google Scholar]
- 25.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiasdis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–8. [DOI] [PubMed] [Google Scholar]
- 26.Yelgec NS, Dymarkowski S, Ganame J, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol. 2007;17:2211–7. [DOI] [PubMed] [Google Scholar]
- 27.Bohl S, Wassmuth R, Abdel-Aty H, Rudolph A, Messroghli D, Dietz R, et al. Delayed enhancement cardiac magnetic resonance imaging reveals typical patterns of myocardial injury in patients with various forms of non-ischemic heart disease. Int J Cardiovasc Imaging. 2008;24:597–607. [DOI] [PubMed] [Google Scholar]
- 28.Deb K, Djavidani B, Buchner S, Poschenrieder F, Heinicke N, Feuerbach S, et al. Time course of eosinophilic myocarditis visualized by CMR. J Cardiovasc Magn Reson. 2008;10:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–8. [DOI] [PubMed] [Google Scholar]
- 31.McNamara DM, Rosenblum WD, Janosko KM, Trost MK, Villaneuva FS, Demetris AJ, et al. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–8. [DOI] [PubMed] [Google Scholar]
- 32.Karatolios K, Pankuweit S, Maisch B. Diagnosis and treatment of myocarditis: the role of endomyocardial biopsy. Curr Treat Options Cardiovasc Med. 2007;9:473–81. [DOI] [PubMed] [Google Scholar]
- 33.McNamara DM, Holubkov R, Starling RC, Dec GW, Mc Manus BM, Billingham ME, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–9. [DOI] [PubMed] [Google Scholar]
- 34.Mason JW, O'Connell JB, Herskowitz A, Yang E, Mc Connell M.V. Berry C.J, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–75. [DOI] [PubMed] [Google Scholar]
- 35.Allanore Y, Vignaux O, Arnaud L, Puechal X, Pavy S, Dubo D, et al. Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Ann Rheum Dis. 2006;65:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenster BE, Chan FP, Valentine HA, Yang E, Mc Connell MV, Berry CJ, et al. Images in cardiovascular medicine. Cardiac magnetic resonance imaging for myocarditis: effective use in medical decision making. Circulation. 2006;113:e842–e843. [DOI] [PubMed] [Google Scholar]
- 37.Dill T, Ekinci O, Hansel J, Kluge A, Breidenbach C, Hamm CW. Delayed contrast-enhanced magnetic resonance imaging for the detection of autoimmune myocarditis and long-term follow-up. J Cardiovasc Magn Reson. 2005;7:521–3. [DOI] [PubMed] [Google Scholar]