Abstract
An 83-year-old female patient with symptomatic atrial fibrillation was referred to the Department of Cardiology for a scheduled electrocardioversion. Because of a junctional escape rhythm after the electrocardioversion she received a DDD pacemaker which was complicated by dyspnoea and ST-segment elevations in the inferior and precordial leads. Because of suspicion of an acute myocardial infarction she was transferred to a PCI centre. The coronary angiogram showed no abnormalities. In the initial phase, an echocardiogram was performed. The echocardiogram showed apical akinesis and a reduced left ventricular function. During follow-up left ventricular function improved and was completely normal nine weeks after the event. The clinical picture was interpreted as a takotsubo cardiomyopathy after a pacemaker implantation. (Neth Heart J 2009;17:487-90.)
Keywords: takotsubo cardiomyopathy, apical ballooning, pacemaker implantation
Apical ballooning or takotsubo cardiomyopathy is a new and rare phenomenon which is mainly seen in elderly women. It is characterised by chest pain, dyspnoea, ECG changes and normal or slightly elevated heart enzymes without coronary artery lesions. Echocardiography or left ventricle angiography shows a takotsubo-like dilatation of the left ventricle. The prognosis is favourable. We present a Dutch case of takotsubo cardiomyopathy after pacemaker implantation.
Case
An 83-year-old female patient was seen at the Department of Cardiology with symptomatic atrial fibrillation. Besides frequent episodes of rapid atrial fibrillation with an upper rate of 150 beats/min, there were several periods of long RR intervals with a longest interval of 2.7 sec. Prior to admission she complained of dyspnoea and oedema. This improved after initiation of diuretic treatment, ACE inhibition, β-blockade and oral anticoagulation. The echocardiographic findings before admission showed a left ventricular ejection fraction of 55%, a mild tricuspid valve insufficiency and a moderate mitral valve insufficiency with left atrial enlargement.
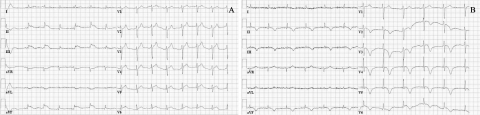
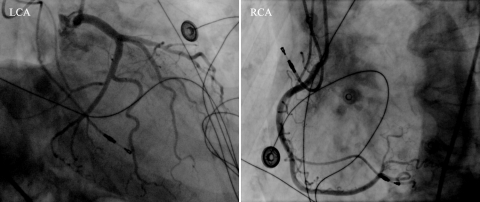
Upon arrival to the hospital she had mild oedema and sometimes she felt dizzy. Except for slight peripheral oedema, the physical examination revealed no abnormalities. Blood pressure was 100/60 mmHg and she had an irregular pulse of 120 beats/min. A 12-lead ECG showed an atrial fibrillation of 118 beats/min. The chest X-ray showed cardiac enlargement and no further abnormalities. An electrocardioversion was scheduled. After cardioversion, the rhythm changed to junctional escape rhythm followed by sinus bradycardia. Because of symptomatic sick sinus syndrome the patient received a DDD pacemaker under local anaesthesia. During the pacemaker implantation, there were no complications, but after the implantation she complained about dyspnoea and a pneumothorax was suspected. A chest X-ray did not show a pneumothorax. The ECG now showed ST-segment elevations primarily in the inferior and precordial leads (figure 1A). An inferior myocardial infarction was suspected. She was transferred to a PCI centre for a primary percutaneous transluminal coronary angioplasty. Surprisingly, the coronary angiogram was completely normal (figure 2). The echocardiogram showed apical akinesia, apical ballooning and a reduced left ventricular function.
Figure 1 .
A) The ECG after the pacemaker implantation with ST-segment elevation mainly in leads II, III and aVF. B) The ECG two days later with negative T waves in II, III and aVF and more prominent in the leads V2 to V6.
Figure 2 .
Coronary angiography revealed normal findings. Left coronary angiogram (LCA): left main coronary artery, the left anterior descending artery and the circumflex artery and their branches. Right coronary angiogram (RCA): the right coronary artery and its branches.
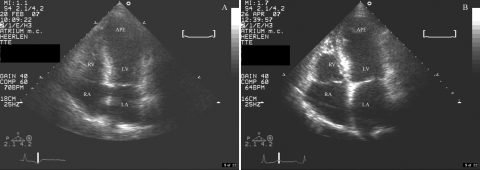
A few days later another echocardiogram was performed. This showed apical akinesia, a left ventricular ejection fraction of 40% and a small thrombus in the apex of the left ventricle (figure 3A). The heart enzymes were slightly elevated. The electrocardiogram two days after the implantation showed negative T waves in leads II, III, aVF and more prominent in the precordial leads V2 to V6. During admission there were no complications and at discharge she was in a good clinical condition. About nine weeks after discharge the echocardiogram showed a normal left ventricular function with an ejection fraction of 50 to 55% (figure 3B). Our clinical diagnosis was transient apical ballooning syndrome or takotsubo cardiomyopathy after pacemaker implantation.
Figure 3 .
A) A systolic apical fourchamber view. Echocardiography shows apical ballooning of the left ventricle. B) An apical four-chamber view about nine weeks after discharge. The apical ballooning is gone. RA=right atrium, RV=right ventricle, LA=left atrium, LV=left ventricle, APE=apex.
Discussion
Takotsubo cardiomyopathy is a new and rare phenomenon with transient dilatation of the left ventricle while sparing the base. It is mainly seen in female patients aged between 50 and 80 years of age.1 Initially described in the 1990s in Japan, this reversible cardiomyopathy is characterised by a distinctive shape observed on left ventriculography, described as apical ballooning. This shape is similar to a Japanese takotsubo pot with a narrow neck and round bottom used by fishermen to trap octopus. The clinical presentation is that of acute coronary ischaemia generally preceded by a stressful emotional, physical, or psychological event such as the death of a loved one. This accounts for this syndrome's other clinical titles, namely broken heart syndrome or stress cardiomyopathy. Symptoms include chest pain, dyspnoea, and, in rare cases, syncope. Cardiac enzymes are elevated, as is brain natriuretic peptide (BNP). The most common ECG finding is anterior ST elevations, but ST depression and T-wave inversions are also observed. Echocardiography reveals mildly to severely decreased LV function with anteroapical akinesis or dyskinesis. Mitral regurgitation caused by systolic anterior wall motion of the mitral valve, LV outflow tract obstruction, and thrombus formation can also be observed. Magnetic resonance imaging (MRI) shows mid to apical LV dyskinesis without delayed gadolinium. The exact aetiology is still unknown and the syndrome is able to mimic an acute myocardial infarction. One of the theories which has been proposed is myocardial stunning due to enhanced sympathetic activity in physical or emotional stress.2
The Mayo Clinic proposed the following criteria for the clinical diagnosis of apical ballooning: transient hypokinesia, akinesia or dyskinesia of the middle segments of the left ventricle with or without apical involvement, wall movement abnormalities in a larger area than the area which is supplied by the related coronary artery, absence of coronary artery disease or angiographic evidence of an acute plaque rupture, ECG abnormalities (ST-segment elevations or T-wave inversion) or an elevated troponin T, absence of a recent significant head injury, intracranial haemorrhage, pheochromocytoma, myocarditis or hyperthrophic cardiomyopathy.3
Possible complications are lung oedema (20%), cardiogenic shock, respiratory insufficiency and arrhythmias.1 In rare cases rupture of the left ventricle has been seen. Echocardiography reveals apical ballooning of the left ventricle and hyperkinesia of the basal segments.2 The mid ventricle is often affected as well.1 The prognosis is favourable2 and the mortality rate is very low, probably 1 to 2%.3
We report a Dutch case of transient apical ballooning after a pacemaker. Chun et al. also reported a transient left ventricular apical ballooning syndrome as a complication of permanent pacemaker implantation in a 77-year-old Hawaiian female patient who was admitted to the hospital for severe bradycardia. She had a heart rate in the low 40s and marked first-degree AV block. Echocardiography showed a normal left ventricular function with an ejection fraction estimated to be over 60%. Because of a Mobitz I block and heart rates in the low 30s a pacemaker was implanted two days later. Prior to the implantation systolic blood pressures were above 100 mmHg. The implantation itself was without complications but 12 hours after it she developed a precipitous fall in blood pressure with systolic pressures as low as 70 mmHg. Cardiac enzymes were mildly elevated and echocardiography was performed to rule out pericardial effusion, which was not seen. It revealed severe left ventricular anteroapical and inferolateral akinesis, basal hyperkinesis and an ejection fraction of 20%. She had no occlusion or significant stenosis of the coronary vessels. During admission she remained asymptomatic. Blood pressure improved gradually with volume replacement and was above 90 mmHg at discharge two days later. Six weeks after discharge there was an almost complete recovery of the left ventricular function with an ejection fraction estimated to be 50%. She met all of the diagnostic criteria for transient left ventricular apical ballooning syndrome proposed by the Mayo Clinic.4
Kurisu et al. reported two cases of persistent left ventricular dysfunction in takotsubo cardiomyopathy after pacemaker implantation, both in elderly patients who received a dual-chamber pacemaker for complete atrioventricular block. The first one was an 89-year-old female patient with hypertension and left hemiparesis who was admitted with dizziness. The ECG showed a sinus rhythm with a complete atrioventricular block at a rate of 38 beats/min. The left ventricular function was normal with an ejection fraction of 62%. She received a dual-chamber pacemaker. The implantation itself was without complications but ten minutes after the operation she developed chest discomfort with significant ST-segment elevations in leads I, aVL and V2 to V6. There was no significant coronary artery disease but left ventriculography revealed akinesia of the mid-to-distal portion of the left ventricle with an ejection fraction of 38%. Because of congestive heart failure she received furosemide. Heart enzymes were elevated. Echocardiography two months later showed persistent akinesia of the mid-to-distal portion of the left ventricle. There was resolution of the ST-segment elevations, but no deep T waves under ventricular pacing.
The second case was a 77-year-old female patient with hypertension and diabetes who was admitted with dyspnoea on effort. The ECG revealed sinus rhythm with a complete atrioventricular block at a rate of 30 beats/min. The left ventricular function was normal with an ejection fraction of 75%. She also received a dual-chamber pacemaker and the implantation was without complications. Three days later she developed orthopnoea with significant ST-segment elevations in leads V2 to V6. Echocardiography showed akinesia of the mid-to-distal portion of the left ventricle with an ejection fraction of 27%. She also had congestive heart failure which required furosemide. Three months later she was re-admitted with orthopnoea. There was no significant coronary artery disease on coronary angiography but left ventriculography revealed persistent akinesia of the mid-to-distal portion of the left ventricle with an ejection fraction of 22%. A follow-up echocardiography four months later also showed persistent akinesia of the mid-to-distal part of the left ventricle. There was resolution of the ST-segment elevations but no deep negative T waves under ventricular pacing.5
Kitaoka et al. reported a takotsubo-like left ventricular dysfunction with delayed recovery of left ventricular shape.6
Our patient presented with symptomatic atrial fibrillation with pauses which was corrected by a scheduled electrocardioversion. She received a DDD pacemaker for a sick sinus syndrome. There were no complications during the implantation itself, but afterwards she complained of dyspnoea. No chest pain was reported. A pneumothorax was ruled out. A 12-lead ECG revealed ST-segment elevations, mainly in II, III and aVF and slightly in the precordial leads. An inferior myocardial infarction was suspected. After transfer to a PCI centre angiography showed no coronary artery disease. Echocardiography revealed apical ballooning with transient left ventricular dysfunction and a small thrombus in the apex of the left ventricle. Kurisu et al. also reported an apical thrombus in a 74-year-old female patient with a takotsubo-like left ventricular dysfunction which caused a transient cerebral ischaemic attack.7 A second ECG two days after the pacemaker implantation showed negative T waves in the inferior lead and more prominently in the precordial leads. The heart enzymes were slightly elevated and no complications were reported during admission. No emotional stress was reported. The physical stress was probably caused by the pacemaker implantation. There were no signs of subarachnoid haemorrhage, pheochromocytoma, myocarditis or hyperthrophic cardiomyopathy. Complications such as cardiogenic shock, respiratory insufficiency, lung oedema and arrhythmias were not reported during admission.1 She did not suffer from a (transient) cerebral ischaemic attack. She does meet the criteria of left ventricular apical ballooning syndrome which have been proposed by the Mayo Clinic, however is not entirely typical.
There are several similarities between the cases of Kurisu et al., the case of Chun et al. and our case. They are all elderly women with arrhythmias or conduction disorders who received a pacemaker. Coronary artery disease was insignificant or absent. The left ventricular ejection fraction was normal prior to the pacemaker implantation. In both Chun's case and ours, the patient was asymptomatic and the left ventricular function recovered almost completely.
There are also several differences between the cases. The cases of Kurisu et al. revealed ST-segment elevations in the leads I, aVL and V2 to V6 and no negative T waves. Our case showed ST-segment elevations predominantly in the leads II, III and aVF followed by negative T waves in the same leads and more prominent in the precordial leads. The akinesia in Kurisu's cases was persistent in contrast to Chun's and our case.
Conclusion
Initially the dyspnoea was possibly suggestive of pneumothorax, which was ruled out by a chest X-ray. A subsequent ECG suggested an inferior infarction but the slightly elevated enzymes were inconsistent with an acute infarction. Moreover the apical ballooning was disproportionate to the slight elevations of the cardiac enzymes. A myocardial infarction was therefore unlikely. There was no emotional stress reported. Our case is consistent with previous reports of transient left ventricular apical ballooning syndrome after pacemaker implantation and we agree with Chun et al. that this syndrome should be considered to be a potential complication of pacemaker implantation.4
References
- 1.Ako J, Sudhir K, Omar Farouque HM, Honda Y, Fitzgerald PJ. Transient left ventricular dysfunction under severe stress: brainheart relationship revisited. Am J Med. 2006;119:10–7. [DOI] [PubMed] [Google Scholar]
- 2.Stöllberger C, Finsterer J, Schneider B. Tako-tsubo-like left ventricular dysfunction: clinical presentation, additional cardiac and non-cardiac diseases and potential pathomechanisms. Minerva Cardioangiol. 2005;53:139–45. [PubMed] [Google Scholar]
- 3.Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation. 2007;115:e56–e59. [DOI] [PubMed] [Google Scholar]
- 4.Chun GS, Kwok V, Pang DK, Lau TK. Transient left ventricular apical ballooning syndrome (takotsubo cardiomyopathy) as a complication of permanent pacemaker implantation. Int J Cardiol. 2007;117:e27–e30. [DOI] [PubMed] [Google Scholar]
- 5.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Takaki H, et al. Persistent left ventricular dysfunction in takotsubo cardiomyopathy after pacemaker implantation. Circ J. 2006;70:641–4. [DOI] [PubMed] [Google Scholar]
- 6.Kitaoka T, Ogawa Y, Kato J, Shiokosha T, Ota T, Harada T, et al. Takotsubo-like left ventricular dysfunction with delayed recovery of left ventricular shape: a case report. J Cardiol. 2006;47:197–205. [PubMed] [Google Scholar]
- 7.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, et al. Left ventricular apical thrombus formation in a patient with suspected tako-tsubo-like left ventricular dysfunction. Circ J. 2003;67:556–8. [DOI] [PubMed] [Google Scholar]