Abstract
Background and Purpose
Notch signaling activity regulates arteriogenesis. Presenilin 1 (PS1) mediates Notch signaling activity via cleavage of Notch, liberating Notch intracellular domain (NICD). We tested the hypothesis that simvastatin enhances arteriogenesis after stroke by increasing PS1 activation of the Notch signaling pathway.
Methods
Rats were subjected to middle cerebral artery occlusion (MCAo) and treated with or without simvastatin (1 mg/kg) starting 24 hours after stroke and daily for 7 days; they were euthanized 14 days after stroke. Immunostaining, Western blot, and real-time polymerase chain reaction assays were performed.
Results
Simvastatin significantly increased arterial diameter, density, and vascular smooth muscle cell proliferation, and upregulated PS1, Notch1, and NICD expression in the ischemic border tissue and in the cerebral arteries compared with MCAo control rats, respectively. However, simvastatin did not increase arteriogenesis, PS1, and NICD expression in sham control animals. To investigate the mechanisms of simvastatin-induced arteriogenesis, primary cerebral artery cultures were used. Rats were subjected to MCAo and treated with or without simvastatin daily for 7 days. The cerebral arteries derived from these stroke rats were cultured in matrigel and treated with or without a γ40-secretase inhibitor II, which blocks Notch signaling activity, inhibiting NICD production. Arterial cell migration was measured. simvastatin treatment significantly increased arterial cell migration compared to control MCAo artery, whereas inhibition of Notch signaling activity by the γ40-secretase inhibitor II significantly attenuated simvastatin-induced arterial cell migration.
Conclusions
These data indicate that simvastatin increases arteriogenesis after stroke, and that simvastatin upregulation of PS1 expression and Notch signaling activity may facilitate an increase in arteriogenesis.
Keywords: arteriogenesis, Notch signaling, presenilin 1, simvastatin, stroke
Stroke patients with higher density of cerebral blood vessels fare better and survive longer than those with lower vascular density.1,2 Arteriolar collateral growth (arteriogenesis) and new capillaries (angiogenesis) support restored perfusion in the ischemic border after mini-stroke and promote long-term functional recovery.2 Arteriogenesis is an important process for adapting preexisting vessels into functional collateral conduits for delivery of oxygen-enriched blood to tissue distal to occlusion of a large, peripheral conduit artery.3 Thrombolysis treatment has a greater clinical impact in patients with better collateral supply after stroke.4 Therefore, stimulating arteriogenesis in particular may provide a new treatment strategy for patients with stroke.
The Delta/Notch signaling pathway plays an important role in the regulation of arteriogenesis and angiogenesis.5 Notch signaling is required for arterial identity by suppressing the venous fate in developing artery cells.6,7 Several members of the Notch family are specifically expressed by arterial endothelial cells, including Notch1, Notch4, and Notch Ligand Delta-like 1 and Delta-like 4.8 Delta-like 1 is essential for postnatal arteriogenesis.9 Delta-Like 4 induces endothelial cell ephrinB2 gene expression and arterial endothelial cell differentiation.10
HMG-CoA reductase inhibitors (statins) are a class of drugs originally used to lower cholesterol. Statins also possess cholesterol-independent benefits. They increase vascular endothelial growth factor expression,11 endothelial nitric oxide synthase activity in the ischemic brain after stroke,12 angiogenesis,11 and collateralization in coronary artery disease patients.13 Delayed (24-hour) treatment of experimental stroke with a statin significantly improves functional outcome but does not decrease lesion volume compared to animals with nontreated stroke.11,14 The functional benefit derived from delayed 24-hour treatment of stroke with simvastatin is attributed to neurorestorative, and not neuroprotective, effects. Statins also increase subventricular zone presenilin 1 (PS1) expression and Notch signaling activity, and promote neurogenesis after stroke.15 Recently, a clinical study found that statin-treated patients had significantly higher collateral artery scores than nonstatin users during acute ischemic stroke.16 However, whether a statin administered 24 hours after stroke regulates arteriogenesis in the ischemic brain and the mechanisms of statin-induced arteriogenesis have not been investigated. In this study, we tested the hypothesis that simvastatin increases PS1 expression and Notch signaling activity in cerebral arteries and promotes arteriogenesis in the ischemic brain after stroke.
Materials and Methods
Animal Middle Cerebral Artery Occlusion Model and Statin Treatment
Adult male Wistar rats (Jackson Laboratory, Bar Harbor, Maine) weighing 270 to 300 grams were used in all experiments. Transient right middle cerebral artery occlusion (MCAo) was induced for 2 hours by advancing a 4-to-0 surgical nylon suture (18.5 to 19.5 mm) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA.17 Sham-operated rats underwent the same surgical procedure without suture insertion. A dose of 1 mg/kg simvastatin administered starting 24 hours after stroke and daily for 7 days after stroke improves functional outcome in rats;14 therefore, this dose was used in this study. MCAo and sham-operated rats were gavaged starting 24 hours after surgery with saline for control or simvastatin (1 mg/kg, Merck & Co, Inc) daily for 7 days. To identify newly formed DNA in ischemic brains, rats received injections of bromodeoxyuridine (BrdU; Sigma Chemical, 100 mg/kg in 0.007N NaOH physiological saline) intraperitoneally starting 1 day after MCAo and subsequently daily for 14 days. One set of rats was euthanized 14 days after MCAo for immunostaining (n=9/group), whereas another set of rats (n=5/group) was euthanized 8 days after MCAo to isolate brain tissue and cerebral arteries for the in vitro arteriogenesis assay, Western blot, and real-time polymerase chain reaction (PCR) analysis.
Immunohistochemical Staining
Rats were euthanized 14 days after stroke. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. A standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6-μm-thick sections were cut from the block. Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against α-SMA (mouse monoclonal IgG 1:800; Dako), PS1 (mouse monoclonal IgG 1:500; Abcam), NICD (rabbit polyclonal IgG 1:1000 dilution; Abcam), were used respectively. Control experiments consisted of staining brain coronal tissue sections as outlined, but the primary antibodies were omitted, as previously described.18 The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Double Immunohistochemical Staining
To specifically identify BrdU-reactive cells colocalized with smooth muscle cells (SMCs), double immunofluorescence staining of BrdU/α-SMA was performed. A monoclonal antibody against α-SMA (mouse monoclonal IgG 1:800; Dako) was used, respectively. FITC (Calbiochem) and cyanine-5.18 (CY5; Jackson Immunoresearch) were used for double-label immunostaining. Each coronal section was first incubated with the primary anti-α-SMA with FITC, and was then followed by BrdU antibody, a proliferating cell marker (1:100; Boehringer Mannheim) with Cy5. Control experiments consisted of staining brain coronal tissue sections as outlined but omitted the primary antibodies.18
Arteriolar Density, Diameter, and Perimeter Measurement
α-SMA immunoreactivity was used as a marker to identify arterioles.19 The ischemic border area is adjacent to the ischemic core and identified using hematoxylin and eosin staining (Figure 1). The α-SMA containing thick wall vessels were defined as arterioles, although a more narrow definition requires that arterioles be ≥ 10 μm in diameter.20 Consequently, the density of α-SMA-stained vessels was analyzed with regard to small and large arterioles (≥ 10-μm diameter and round) in the ischemic border area. Five sections from the standard reference coronal section and 8 brain regions (Figure 1) within each section were acquired. The total number of α-SMA–stained short-diameter ≥ 10-μm-thick wall vessels in the 8 regions of ischemic border area was counted using the Micro Computer Imaging Device (MCID) computer imaging analysis system (Imaging Research). The total number of arteries per mm2 area is presented. For arterial diameter measurement, 8 brain regions (Figure 1) were acquired and the α-SMA–stained thick wall vessels and short-diameter ≥ 10-μm arterial diameters were measured in each region using the MCID system and averaged. Because vessel skew may influence arterial diameter but not arterial perimeter, 10 arteries with enlarged perimeters in the ischemic border (Figure 1) were measured. The data are presented as averaged diameter and perimeter in 8 regions.
Figure 1.

Designated boxes identify areas in the ischemic border where immunostaining was analyzed.
NICD and PS1 Expression Quantification
To test whether simvastatin upregulates PS1 and NICD expression, PS1- and NICD-immunostained sections were digitized using a 40× objective (Olympus BX40) via the MCID computer imaging analysis system. NICD-positive endothelial cell numbers were counted in 20 enlarged arteries in the ischemic border. Data are presented as percentage of the number of the NICD-immunoreactive endothelial cells/total endothelial cells, respectively. PS1-positive areas in the ischemic border were digitized under a 40× objective (Olympus BX40) using a 3-CCD (Charge Coupled Device) color video camera (Sony DXC-970MD) interfaced with an MCID computer imaging analysis system. The digitized images were then contrast-enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within the ischemic border.14 The data are presented as a percentage of positive PS1 area in the ischemic border.
Brain Artery SMC Proliferation
Double immunostaining of BrdU with α-SMA was performed to measure vascular SMC proliferation. BrdU immunostained sections were digitized using a 40× objective (Olympus BX40) via the MCID computer imaging analysis system (Imaging Research). BrdU-positive cells within a total of 10 enlarged arteries located in the ischemic border area were counted in each section. Five sections and 8 views (Figure 2G) in each section were counted per rat with the number of BrdU reactive cells averaged, respectively. Data were analyzed in a blinded manner and presented as percentage of the number of the BrdU-immunoreactive SMCs/total SMCs, respectively.
Figure 2.

Simvastatin treatment of stroke increases arteriogenesis and PS1 and NICD expression in the ischemic brain. A, α-SMA immunostaining in MCAo control and simvastatin-treated rats, and arterial density, diameter, and perimeter quantitative data in MCAo or sham-operated rats treated with or without simvastatin (n=8/group). B, Double immunostaining BrdU (FITC, green)/α-SMA (Cy5, red) and quantitative data in MCAo or sham-operated rats treated with or without simvastatin (n=8/group). NICD (C) and PS1 (D) immunostaining MCAo control and simvastatin-treated rats and quantitative data. *P<0.05 compared with MCAo control (n=8/group). Scale bar B and C=50 μm; A and D=100 μm.
Western Blot
Rats were euthanized 8 days after stroke. Protein was isolated from the ischemic brain tissue and arteries with TRIzol (Invitrogen) following standard protocol. Protein concentrations were determined by a DC protein assay kit (Bio-Rad). Protein samples were electrophoresed on gradient sodium dodecyl sulfate-polyacrylamide gel (Bio-Rad) and subsequently electrotransferred to nitrocellulose membranes. Membranes were treated with blocking buffer (5% skimmed milk in 25 mm Tris-HCl pH 8.0, 125 mm NaCl, 0.1% Tween 20) for 1 hour at room temperature, followed by incubation with primary antibodies for anti-β-actin (1:2000; Sigma), anti-PS1 (1:500 dilution), anti-Notch1 (1:500 dilution), and anti-NICD (1:1000 dilution) for 16 hours at 4°C. The membranes were washed with blocking buffer without milk, and then incubated with horse-radish peroxidase-conjugated secondary antibody in blocking buffer.
Primary Cerebral Artery Culture and Measurement
To elucidate the mechanisms underlying simvastatin mediation of arteriogenesis, a primary cerebral artery culture model was used.21 Rats were subjected to 2-hour MCAo or were sham-operated, and were treated with or without simvastatin (1 mg/kg) daily for 7 days. Rats were euthanized 8 days after stroke. Because a portion of the MCA is damaged after MCAo, it cannot be used to perform a cell migration assay. The portions of the MCA that were not damaged show no significant difference in arterial cell migration compared to the posterior cerebral artery derived from rats with strokes (data not presented). Therefore, posterior cerebral artery culture was used to measure arterial cell migration. The posterior cerebral artery was surgically removed and washed twice in 1× phosphate-buffered saline to remove blood. The posterior cerebral artery was cut and placed in Matrigel. The experimental groups include arteries from the following: (1) nonsimvastatin-treated sham-operated rats; (2) arteries from simvastatin-treated (1 mg/kg) sham-operated rats; (3) nonsimvastatin-treated MCAo rats; (4) simvastatin-treated (1 mg/kg) MCAo rats; and (5) simvastatin-treated (1 mg/kg) MCAo rats cultured and treated with a γ40-secretase inhibitor II (100 μmol/L; Calbiochem) for 7 days. The γ40-secretase inhibitor II blocks β-amyloid precursor protein cleavage and S3 cleavage of the Notch receptor, which inhibit amyloid precursor protein cleavage and Notch signaling activity, respectively.22 Although the γ-secretase inhibitor does not selectively block Notch signaling activity, amyloid precursor protein is primarily expressed in astrocytes and neurons, whereas the Notch receptors are primarily expressed in arteries.8 Therefore, the γ-secretase inhibitor was used to block Notch signaling activity in the arterial cell culture model. Arterial cultures were allowed to grow for 7 days before being photographed, and the 10 longest distances of outgrowth were measured under 4× magnification, processed with the MCID Analysis 7.0 program, and averaged.
Real-Time PCR
Ischemic brain tissues were harvested and total RNA was isolated from treated cells with TRIzol (Invitrogen), following a standard protocol.23 Quantitative PCR was performed using the SYBR Green real-time PCR method on an ABI 7000 PCR instrument (Applied Biosystems) using 3-stage program parameters provided by the manufacturer, as follows: 2 minutes at 50°C, 10 minutes at 95°C, and then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Each sample was tested in triplicate, and analysis of relative gene expression data using the 2−ΔΔCT method was performed. The following primers for real-time PCR were designed using Primer Express software (ABI): Notch1 forward, CTG TAC AGA GGA TGT GGA CGA A; Notch1 reverse, TGA CAC ACA CAC AGT TGT AGC C; PS1 forward, GAG GAA GAC GAA GAG CTG ACA T; PS1 reverse, GAA GCT GAC TGA CTT GAT GGT G; GAPDH forward, AGA ACA TCA TCC CTG CAT CC; GAPDH reverse, CAC ATT GGG GGT AGG AAC AC.
Statistical Analysis
One-way ANOVA and the least significant difference analysis after post hoc test were used after the homogeneity of variance test for testing the data of arterial density, diameter, PS1, NICD, and Notch1 expression in the ischemic brain in vivo and arterial cell migration in vitro. The data are presented as mean ± SE; P<0.05 is considered significant. Independent samples t test was used for testing gene and protein expression measured by real-time PCR and Western blot between the 2 groups treated with or without simvastatin.
Results
Simvastatin Treatment of Stroke Increases Arteriogenesis and PS1 and NICD Expression in the Ischemic Brain
To test the effect of simvastatin on arteriogenesis, and on PS1 and NICD expression after stroke, BrdU/α-SMA, α-SMA, PS1, and NICD immunostaining was performed on cerebral tissue 14 days after stroke. Figure 2 shows a significant increase in arterial density, diameter, and perimeter (Figure 2A), and BrdU-positive vascular SMC expression (Figure 2B) in the ischemic border compared with the control MCAo rats. Figure 2 also shows that simvastatin treatment of stroke rats also significantly increases expression of PS1 (Figure 2B) and NICD (Figure 2C) in the ischemic border compared to the control MCAo rats (P<0.05). However, there are no differences in arterial diameter, density, perimeter, and BrdU, PS1, and NICD expressions between sham-operated animals treated with or without simvastatin.
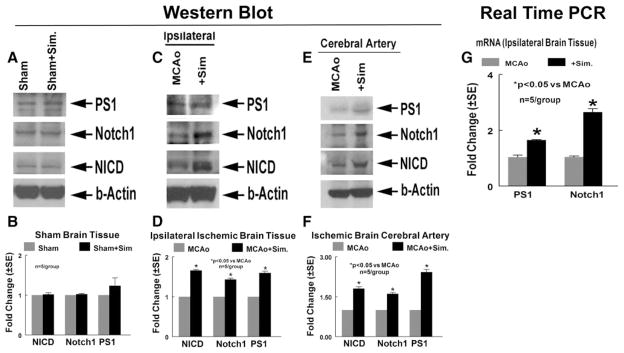
In addition, to confirm the immunostaining data and evaluate ischemic brain tissue and cerebral artery PS1, Notch1, and NICD expression, Western blot and real-time PCR assays were performed. Rats were subjected to 2-hour MCAo or underwent sham operation and were treated with or without simvastatin (1 mg/kg) starting 24 hours after MCAo and continuing daily for 7 days. Rats were euthanized 8 days after MCAo or sham-operation, and ischemic brain tissue and cerebral artery were isolated for Western blot and real-time PCR assay. The Western blot data (Figure 3A–F) show that simvastatin increased ischemic brain tissue (Figure 3C,D) and cerebral artery (Figure 3E,F) protein expression of Notch1, NICD, and PS1 compared to control MCAo rats, respectively. However, there was no difference in protein expression between sham-operated control animals treated with or without simvastatin (Figure 3A,B). The real-time PCR data also show that simvastatin treatment of stroke increased PS1 and Notch1 gene expression in the ischemic brain compared to MCAo control (Figure 3G). These data indicate that simvastatin treatment of stroke increases arteriogenesis and upregulates PS1 expression and Notch signaling activity in the ischemic brain.
Figure 3.
Simvastatin regulates Notch1 and PS1 gene and protein expression in the ischemic brain and cerebral artery measured by Western blot and real-time PCR. Western blot assay is shown. PS1, Notch1, and NICD protein expression in sham-operated rats brain tissue (A), MCAo rat ischemic brain tissue (B), and ischemic brain cerebral artery (C). D to F, Quantitative data of Western blot analysis. G, Gene expression in the ischemic brain tissue treated with or without simvastatin measured by real-time PCR (n=5/group).
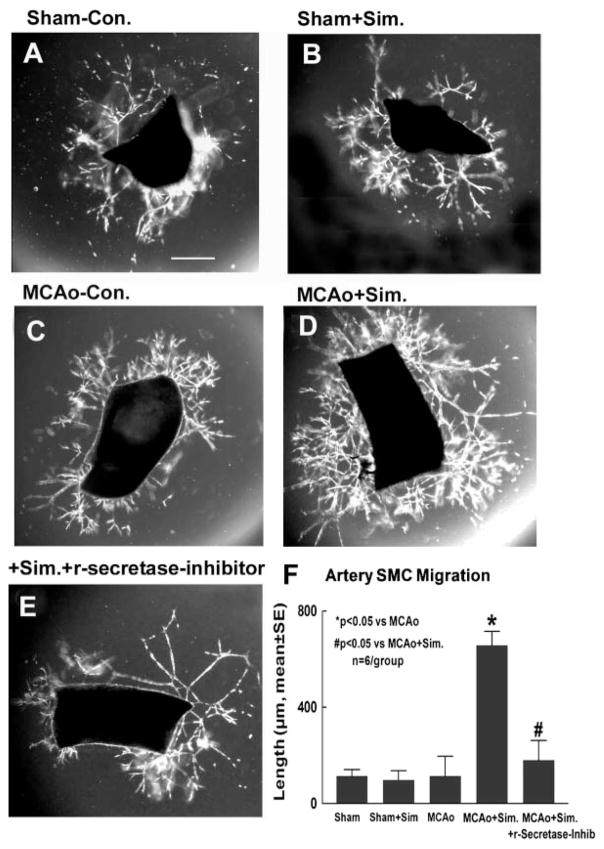
Simvastatin increases cerebral artery SMC migration, and inhibition of Notch signaling attenuates simvastatin-induced arteriogenesis (Figure 4).
Figure 4.
Cerebral artery migration assay. A, Sham-operated nontreatment for control. B, Sham-operated simvastatin-treated artery. C, MCAo nontreatment for control. D, MCAo simvastatin-treated artery. E, MCAo simvastatin-treated artery plus γ-secretase inhibitor. F, Migration quantitative analysis (n=6/group). Scale bar A=100 μm.
The stages of arteriogenesis consist of arteriolar thinning, followed by transformation of SMCs from the contractile into the proliferative and synthetic phenotype.24 Endothelial cells and SMCs proliferate, and SMCs migrate and form a neointima.24 To test whether simvastatin regulates arteriogenesis, arterial cell migration was measured in primary artery culture. SMC migration is most prominent during the second wave (7 to 14 days) of arterial cell migration.21 Therefore, SMC migration was measured at 7 days after arterial culture. Figure 4 shows that treatment of stroke rats with simvastatin significantly increased arterial SMC migration (Figure 4D,F) compared to control MCAo rats (Figure 4C,F; P<0.05), but not in sham-operated control (Figure 4A,B,F). Treatment with the γ-secretase inhibitor significantly attenuated the simvastatin-induced arterial SMC migration (Figure 4E,F; P<0.05). These data imply that Notch signaling activity induces simvastatin-induced arteriogenesis.
Discussion
In this study, we investigate the effect of simvastatin on arteriogenesis after stroke. We found that simvastatin promotes arteriogenesis and PS1 and NICD expression in the ischemic border and in the cerebral arteries after stroke compared to MCAo control in vivo. We also found that inhibition of Notch signaling activity by a γ-secretase inhibitor attenuates simvastatin-induced arteriogenesis in vitro.
Simvastatin Promotes Arteriogenesis After Stroke
Regulation of cerebral blood flow is critical for the maintenance of neural function.25 The formation of new blood vessels via angiogenesis as well as the development of collaterals from preexisting vessels arteriogenesis are of importance in the pathophysiology of vascular disease. The arteriogenesis response consists of the formation of new arterioles, which presumably occurs when preexisting capillaries acquire smooth muscle coating, and these newly formed or preexisting arterioles transform into channels with larger diameters.26,27 In addition, vascular SMC, contractile and matrix-producing support cells that are associated with arteries and, less prominently, with veins, play critical roles in vascular maturation and arteriogenesis.28 Simvastatin treatment of stroke significantly increases cerebral arterial density, diameter, and vascular SMC proliferation in the ischemic brain. Simvastatin also promotes cultured ischemic cerebral artery sprouting and artery SMC migration in vitro. These data indicate that simvastatin treatment promotes arteriogenesis in the ischemic brain after stroke.
Notch Signaling Activity Mediates Simvastatin-Induced Arteriogenesis After Stroke
Functional studies in mice, fish, tumor models, and cell culture systems have shown that the angiogenic growth of the blood vessel network, the proliferation of endothelial cells, and the differentiation of arteries and veins are controlled by Notch signaling.29 Notch signaling is an ancient intercellular signaling mechanism that plays a myriad of roles in the regulation of artery/vein differentiation in endothelial and vascular SMCs, regulation of blood vessel sprouting and branching, and the differentiation and physiological responses of vascular SMCs.30 Mutations in Notch result in alterations in many tissues, such as defects in the main trunk of the anterior cardinal vein, lack of intersomitic vessels, and collapsed dorsal aorta.31 Notch1 and Hey1/Hey2 knockout mice do not express the arterial endothelial markers in the remaining large arteries.32 Notch signaling is essential for arterial versus venous cell fate decisions. Our data show that simvastatin increases Notch1 expression in the ischemic cerebral arteries compared to control MCAo rats. Interaction of Notch with ligands triggers a sequence of proteolytic cleavages that release NICD to the nucleus.33 Notch transmits the signal, dependent on its own glycosylation and cleavage by γ-secretase. The γ-secretase cleaves the transmembrane domains of several integral membrane proteins involved in vasculogenesis. PS1, as the active site component of γ-secretase,34 is required in the processing of Notch signaling activity.35 Nuclear translocation of NICD is markedly reduced in PS1-deficient cells, and is restored by PS1 transfection.36 Mice with a null mutation of the PS1 gene die early after birth because of multiple structural defects, such as significant skeletal defects, brain hemorrhages, and impaired neurogenesis.37 The phenotype of PS1 knockout mice resembles that of Notch1 knockout mice.38 Our data show that simvastatin treatment increases PS1 expression in the ischemic brain, induces Notch signaling activity, and increases NICD expression in the ischemic brain after stroke. Inhibition of γ-secretase significantly decreased Notch signaling activity and attenuated simvastatin-induced artery SMC migration. These data indicate that PS1 promotes Notch signaling activity, which may mediate simvastatin-induced arteriogenesis after stroke. Other pathways may also play a role in simvastatin-induced arteriogenesis, such as endothelial nitric oxide synthase or Rho-kinase.39–41 However, in this study, we focused on the Notch signaling effect. The effects of endothelial nitric oxide synthase and Rho-kinase on simvastatin-induced arteriogenesis warrant testing.
In summary, we have found that treatment with simvastatin starting 24 hours after experimental stroke increases Notch1, PS1, and NICD expression, as well as promotes arteriogenesis in the ischemic brain. Simvastatin-induced upregulation of PS1 expression and Notch signaling activity may facilitate an increase in arteriogenesis after stroke.
Acknowledgments
The authors thank Cynthia Roberts and Qinge Lu for technical assistance.
Sources of Funding
This work was supported by NINDS RO1 NS047682, AHA grant 0750048Z, and NINDS grant PO1 NS23393.
Footnotes
Disclosures
None.
References
- 1.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 2.Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- 3.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, Hahner LD, Cummings ML, Kitchens RL, Marcel YL, Rader DJ, Shaul PW. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-b type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin North Am. 2005;15:553–573. doi: 10.1016/j.nic.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZJ, Shirakawa T, Li Y, Soma A, Oka M, Dotto GP, Fairman RM, Velazquez OC, Herlyn M. Regulation of notch1 and dll4 by vascular endothelial growth factor in arterial endothelial cells: Implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 7.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 8.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 9.Limbourg A, Ploom M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, Drexler H, Limbourg FP. The notch ligand delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007 doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 10.Iso T, Maeno T, Oike Y, Yamazaki M, Doi H, Arai M, Kurabayashi M. Dll4-selective notch signaling induces ephrinb2 gene expression in endothelial cells. Biochem Biophys Res Commun. 2006;341:708–714. doi: 10.1016/j.bbrc.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and bDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (Hmg)-Coa reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pourati I, Kimmelstiel C, Rand W, Karas RH. Statin use is associated with enhanced collateralization of severely diseased coronary arteries. Am Heart J. 2003;146:876–881. doi: 10.1016/S0002-8703(03)00413-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zacharek A, Li A, Cui X, Roberts C, Lu M, Chopp M. Atorvastatin promotes presenilin-1 expression and notch1 activity and increases neural progenitor cell proliferation after stroke. Stroke. 2008;39:220–226. doi: 10.1161/STROKEAHA.107.490946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovbiagele B, Kidwell CS, Saver JL. Expanding indications for statins in cerebral ischemia: A quantitative study. Arch Neurol. 2005;62:67–72. doi: 10.1001/archneur.62.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin d1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980–1971. [DOI] [PubMed] [Google Scholar]
- 19.Ho TK, Rajkumar V, Black CM, Abraham DJ, Baker DM. Increased angiogenic response but deficient arteriolization and abnormal microvessel ultrastructure in critical leg ischaemia. Br J Surg. 2006;93:1368–1376. doi: 10.1002/bjs.5496. [DOI] [PubMed] [Google Scholar]
- 20.Kellar RS, Landeen LK, Shepherd BR, Naughton GK, Ratcliffe A, Williams SK. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation. 2001;104:2063–2068. doi: 10.1161/hc4201.097192. [DOI] [PubMed] [Google Scholar]
- 21.Saward L, Zahradka P. Coronary artery smooth muscle in culture: Migration of heterogeneous cell populations from vessel wall. Mol Cell Biochem. 1997;176:53–59. [PubMed] [Google Scholar]
- 22.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the s3 cleavage of notch. EMBO Rep. 2001;2:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 25.Pratt PF, Medhora M, Harder DR. Mechanisms regulating cerebral blood flow as therapeutic targets. Curr Opin Investig Drugs. 2004;5:952–956. [PubMed] [Google Scholar]
- 26.Buschmann I, Schaper W. The pathophysiology of the collateral circulation (arteriogenesis) J Pathol. 2000;190:338–342. doi: 10.1002/(SICI)1096-9896(200002)190:3<338::AID-PATH594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001;49:543–553. doi: 10.1016/s0008-6363(00)00206-6. [DOI] [PubMed] [Google Scholar]
- 28.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: Similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roca C, Adams RH. Regulation of vascular morphogenesis by notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 30.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 31.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The notch target genes hey1 and hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science. 2006;314:1414–1415. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Presenilin, notch, and the genesis and treatment of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:11039–11041. doi: 10.1073/pnas.211352598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 36.Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proteolytic release and nuclear translocation of notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci U S A. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and cns defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 38.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 39.Wu XQ, Cai WJ, Luo XG. Expression of eNOS in the endothelial cells during the arteriogenesis of pig hind-limbs. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:63–65. [PubMed] [Google Scholar]
- 40.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deindl E. Arteriogenesis: A focus on signal transduction cascades and transcription factors. Thromb Haemost. 2007;98:940–943. [PubMed] [Google Scholar]